Reciprocal Recombination and the Evolution
of
the Ribosomal Gene Family
of Drosophila melanogaster
Scott M. Williams,**'
James A. Kennison,t'2 Leonard G. Robbins* and Curtis Strobeck*
*Department ofZoology and tDepartment of Genetics, University ofAlberta, Edmonton, Alberta T6G 2E9; and 'Department o f
Zoology and Genetics Program, Michigan State University, East Lansing, Michigan 48824
Manuscript received August 19, 1988
Accepted for publication March 15, 1989
ABSTRACT
T h e role of reciprocal recombination in the coevolution of the ribosomal RNA gene family on the X and Y chromosomes of Drosophila melanogaster was assessed by determining the frequency and nature of such exchange. In order to detect exchange events within the ribosomal RNA gene family, both flanking markers and restriction fragment length polymorphisms within the tandemly repeated gene family were used. T h e vast majority of crossovers between flanking markers were within the ribosomal RNA gene region, indicating that this region is a hotspot for heterochromatic recombina- tion. T h e frequency of crossovers within the ribosomal RNA gene region was approximately 1 0-4 in both X / X and X / Y individuals. In conjunction with published X chromosome-specific and Y chromo- some-specific sequences and restriction patterns, the data indicate that reciprocal recombination alone cannot be responsible for the observed variation in natural populations.
M
ULTIGENE families in eukaryotic organisms are known to be much more homogeneous within a species than would be expected if the mem- bers of the family were evolving independently (HOOD, CAMPBELL and ELGIN, 1975; ZIMMER et al. 1980; DOVER 1982; ARNHEIM 1983). T h e greater than expected homogeneity often includes members of a gene family on different chromosomes-homol- ogous and nonhomologous (TARTOF and DAWID1976; ARNHEIM et al. 1980; WORTON et al. 1988), raising the question of how genes on different chro- mosomes evolve in concert when the transfer of infor- mation is presumably limited. Homogeneity of the members of a multigene family, however, requires that some information be transferred among all chro- mosomal locations of the family. At least two mecha- nisms may account for this transfer of information: (1) unequal homologous exchange (or exchange be- tween homologous regions of different chromosomes) (OHTA 1980; ARNHEIM et al. 1980; DOVER et al. 1982; DOVER 1982; COEN and DOVER 1983; ARNHEIM 1983; GILLINGS et al. 1987) and (2) interchromosomal gene conversion (FOGEL and MORTIMER 1969; HOOD, CAMPBELL and ELGIN 1975; FOGEL et al. 1978; DOVER
1982; DOVER et al. 1982). T o determine how each of these mechanisms might be involved in gene family evolution, it is necessary t o experimentally test the role of each mechanism.
T h e ribosomal RNA (rDNA) gene family of Dro-
' Present address: Department of Biology, Boston University, 2 Cum- Present address: Laboratory of Molecular Genetics, Building 6, Room mington Street, Boston, Massachusetts 02215.
31 1. NICHD, NIH, Bethesda, Maryland 20892. Genetics 122: 617-624 (July, 1989)
sophila melanogaster offers an excellent system for the study of gene family evolution on different chromo- somes. It consists of approximately 250 tandemly re- peated genes located in the heterochromatin of the X and Y chromosomes (RITOSSA 1976). T h e X-linked and Y-linked rDNA arrays are in general similar in sequence, yet have some discernible differences (TAR- TOF and DAWID 1976; YAGURA, YAGURA and MURA- MATSU 1979; INDIK and TARTOF 1980; COEN, THO- DAY and DOVER 1982; WILLIAMS et al. 1987). This paper addresses the role of reciprocal recombination in the evolution of the rDNA gene family of D. melanogaster. Using genetic markers flanking the rDNA regions of both chromosomes and molecular markers within the rDNA, we have estimated the frequencies of both X-X and X-Y exchange within the rDNA tandem arrays. T h e data indicate that the patterns of variation within and between the rDNA arrays on the two sex chromosomes cannot be ex- plained solely as a product of reciprocal recombina- tion. At least one other mechanism must, therefore, be responsible for the pattern of rDNA variation on the two chromosomes. However, it is possible that X- Y recombination is important in transferring genetic information from the Y chromosome to the X chro- mosome array.
MATERIALS AND METHODS
Females heterozygous for this Dp(l;l)sc'" du lication chro- mosome (carrying the marker mutations y wh
'fear
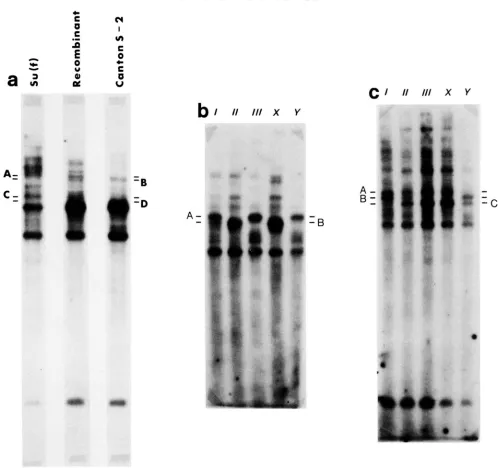
s u m in the left arm) and a Canton S X chromosome (carrying the marker mutations y w h a 2 f ) were crossed to y a c v f s u m males. Two different Canton S X chromosomes both marked w i t h y whRZfwere used. Each X chromosome stock used was derived from a single male shortly before the start of the experiments. All copies of each parental X chromosome were therefore isogenic. On subsequent analysis it was de- termined that the two Canton S X chromosomes had iden- tical rDNA restriction patterns. Recombinant chromosomes were detected as y y ( t h e y phenotype results from thes u o genotype) or y + J males or females (Figure la). All recombinants were made into balanced stocks and subse- quently crossed to a Df(l)bb158 chromosome with the marker mutation y for the molecular analyses. Df(l)bb158 is deficient for 82% of the X heterochromatin, and is deleted for all of the ribosomal genes (LINDSLEY and ZIMM 1987). Thus, flies bearing any recombinant chromosome and Df(l)bbl58 contain ribosomal DNA only from the recom- binant chromosome. DNA was extracted from 50-150 mg of adult flies by the procedure of ISH-HOROWICZ et al. (1 979), except that the DNA was pelleted in a centrifuge at 10,000 x g for 10-20 min instead of being spooled out. The DNA was then digested with either Hind111 or Hind111 and EcoRI. Reaction conditions were as prescribed by the manufacturer (Bethesda Research Labs). Digested DNA samples were then run on 0.6% agarose gels, transferred to nitrocellulose and hybridized with 92P-labeled pDmrY22c DNA as previously described (WILLIAMS, DESALLE and STRORECK 1985). Since the DNA was extracted from adult flies, restriction pattern differences reflect genomic differ- ences and should be unaffected by stage of tissue specific replication of the rDNA (ENDOW and GLOVER 1979).
The clone of a complete ribosomal gene repeat, pDmr- Y22c, was kindly provided by I. DAWID. The rDNA from the parental X chromosomes differed with respect to their banding patterns after digestion with these restriction en- zymes. In addition to some common bands seen in both chromosomes, there are bands diagnostic of each chromo- some (Figure 2a).
We emphasize that the number of rDNA crossovers as determined by molecular analysis is only a minimal estimate of exchange in the rDNA. Not all exchanges within the rDNA can be resolved by the molecular markers used. Only exchange products that have rDNA variants unique to both parental chromosomes can be detected by molecular analysis as recombinants, whereas all single crossovers will be de- tected by the exchange of flanking markers. The actual frequency of within-rDNA crossovers must lie between the
r
bC
FIGURE 1 .-Schematic representation of crosses and recombi- nant chromosomes. X-X recombination, (a) shows the parental fe- male genotype diagrammed on the left. On the right are the two reciprocal products of an exchange event in the rDNA region. The wild-type (+) us. mutant allele for the s u m locus and the presence (shown as y+) or absence of Dp(I;I)scv' were used to detect the exchange events. X-Y recombination, (b and c) show the parental males and exchange products for crosses I and 11, respectively. The parental males in panel b carry a Y chromosome with the markers
By on the long (YL.) arm and y+ on the short (YS) arm. In panel c, the parental males bear an X chromosome with the mutant alleley' on the left arm, and Dp(l;l)scv' (shown asy+) on the right arm. For
all X and Y chromosomes in this figure, the rDNA regions are shaded.
frequency estimated by exchange between the visible out- side markers and the frequency of crossovers between di- agnostic molecular markers within the rDNA itself.
- C
within the rDNA regions was performed as described above for the X-X exchange events (Figures 2b and 2c).
Exchange between the rDNA cistrons of the X and Y chromosomes yields two types of recombinant chromo-
somes. The first type contains the X euchromatin and the
long arm of the Y chromosome (designated X . Y L ) and be-
haves as a normal X chromosome in terms of sex determi-
nation and other genetic properties. It should be recovera- ble from rDNA exchanges in natural populations. The other type of recombinant chromosome (designated YSX.) contains
the short arm of the Y chromosome appended to the centric
heterochromatin (including the centromere) of the X chro-
mosome. YSX. lacks the genes located on the long arm of the Y chromosome that are required for male fertility (STERN
1929). In natural populations, YSX. recombinants would be found only in sterile males. Thus, the YSX. chromosome is a n evolutionary dead end. T h e frequency of X-Y exchange
important for the evolution of the rDNA should, therefore, only be calculated from the X . Y L chromosomes recovered.
RESULTS
X-X recombination: Of a total of 56,256 progeny
recovered, only 16 were recombinants between the s u o locus and the duplication marking the right arm (Table 1). Of the 16 recombinants between the flank- ing markers, 13 could be shown to result from ex- change between the rDNA of the two parental chro- mosomes using restriction fragment length polymor- phisms. T h e frequency of X-X exchange in the rDNA region is, therefore, between 2.3 X and 2.8 X
lop4 (the estimates from the molecular and genetic analyses, respectively).
X-Y
recombination: T h e total number of progeny scored and the number of exchanges between the genetic markers flanking the rDNA in the Y chromo- some are shown in Table 1. Of a total 82,595 progeny scored, 17 recombinants were crossovers between kl-I and ks-I on the Y chromosome and the centric heterochromatin of the X chromosome. No significant difference was found in the frequencies of exchange in cross I and cross 11
( x 2
= 0.8765, 1 d.f., 0.1<
P<
0.5). Thus, presence of the transposed X chromosome fragments on the BsYyC chromosome or on the
Dp(1 ;I)sc"' chromosome does not affect recombina- tion in the rDNA region. Two of the exchange prod-
shown to be exchanges between rDNA cistrons (Table 1). T h e frequency of all X-Y exchanges is, therefore, between 1.3 X 1 0-4 and 2.1 x 1 0-4 and the frequency of X-Y products that are recoverable in natural popu- lations (X.YL chromosomes) is between 8.5 X and
In addition to the recombinants shown in Table 1, three other exceptional progeny were recovered (two from cross I and one from cross 11). One of the exceptional offspring from cross I carried a BSYyf chromosome that had lost the Bs marker. The other exception from cross I carried an element resulting from exchange between k l - I and the bb locus of the Y chromosome and an unknown chromosome (the ele- ment is BSKL.bb-). If the exchange that produced this element was between the rDNA regions on the X and Y chromosomes, this product would be a dicentric chromosome. It is more likely that the exchange in- volved the small fourth chromosome (PARKER 1967). T h e exceptional offspring from cross I1 carried a chromosome lacking the majority of the paternal X
chromosome, but still bearing bb+ and Dp(1 ;I)sc". N o evidence of Y chromosome fertility factors could be detected in this chromosome.
In neither the X-X nor the X-Y exchange experi- ments was there any indication of clustering of cross- overs. Thus, the recombination events are probably meiotic.
1.1 X
DISCUSSION
Recombination and rDNA Evolution 62 1
1980), as well as the distribution of moderately-repet- itive DNA inserted in the 28 S genes (WELLAUER, DAWID and TARTOF 1978). One class of inserts, called Type 1, are found only in the X-linked rDNA genes (WELLAUER, DAWID and TARTOF 1978). In addition, X-linked rDNA spacers are significantly more similar to each other than Y-linked rDNA spacers are to each other (WILLIAMS et al. 1987). T h e pattern of gross similarity in restriction site maps, with diagnostic dif- ferences between the X and Y chromosome rDNA arrays, implies that, although some isolation between the two rDNA arrays exists, some mechanism must keep the genes on the X and Y chromosomes at least marginally similar. Natural selection may be partially responsible for this observation (TARTOF and DAWID
1976), but it is unlikely to be the sole mechanism since natural selection appears to be operating much more strongly on X-linked rDNA arrays (WILLIAMS et al. 1987). This leaves the mechanisms included in the term molecular drive (DOVER 1982).
One of the mechanisms proposed to homogenize tandemly repeated sequences is unequal reciprocal recombination (SMITH 1976, PERELSON AND BELL 1977; HOOD, CAMPBELL and ELCIN 1975; DOVER et
al. 1982; COEN and DOVER 1983; GILLINCS et al.
1987). In order to assess the importance of reciprocal recombination for the evolution of rDNA sequences in Drosophila, we estimated the frequency of recom- bination between rDNA regions in both X-X and X-Y individuals. Previous estimates of rDNA exchange in Drosophila have not been designed to recover all of the recombinant types (STERN 1929; SCHALET 1969; MADDERN 198 1; HAWLEY and TARTOF 1983), have often used chromosomes of inverted sequence or un- known rDNA provenance (NEUHAUS 1937; LINDSLEY 1955), and have failed to directly compare X-X and X-
Y recombination frequencies. Our experiments were designed to recover all recombinant types from both X-X and X-Y events (even those that are not recovera- ble in natural populations) and to compare, as directly as possible, recombination in XlX and XIY individuals. T h e use of visible genetic markers flanking the rDNA regions on both the X and Y chromosomes allows us to detect and recover all single crossovers within the rDNA regions. Because the exchange of flanking markers could also result from exchange in the het- erochromatin flanking the rDNA regions, the fre-
quency of exchange of flanking markers is a maximum estimate of rDNA recombination frequency. T h e use of molecular markers within the rDNA regions allows us to differentiate most crossovers in the rDNA from crossovers proximal or distal to the rDNA. T h e fre- quency of crossovers between the restriction fragment length polymorphisms within the rDNA region is, however, a minimum estimate of the frequency of rDNA recombination. As the majority of flanking
marker exchange could be shown to be between rDNA cistrons, there is little difference between the minimum and maximum estimates of rDNA recom- bination.
These experiments also map the regions of hetero- chromatic exchange in the X and Y chromosomes more accurately than previous studies. From the size of the rDNA repeat and the number of copies per chromosome it can be estimated that the rDNA region comprises approximately 5% of the Y chromosome and 25% of the X heterochromatin (HILLIKER, APPELS and SCHALET 1980). T h e distribution of radiation- induced X-Y translocations agrees with these physical estimates (KENNISON 1981). Seven percent of the ra- diation-induced exchanges were between the closest loci flanking the Y chromosome rDNA, and approxi- mately 17% were within the X chromosome rDNA. T h e location of spontaneous exchanges between the X and Y chromosomes are decidedly nonrandom. T h e vast majority, and possibly all, of the X-Y and proximal X-X exchanges are within the rDNA regions. Al- though much of the non-rDNA Y and X heterochro- matin are not homologous (HILLIKER and APPELS 1982), which would indicate that recombination
would have to be between the rDNA of these two chromosomes, the non-rDNA of the two X chromo- somes are homologous. This suggests that rDNA ex- change is not random breakage and rejoining in a translocation-like event, but is the result of either a specific recombination system for the exchange of rDNA or a specific response of the rDNA to the general recombination machinery. For the sponta- neous X-Y exchanges reported here, 100% (17117) were between the Y chromosome loci that flank the rDNA region, significantly different from the
7%
seen for radiation-induced exchanges. At least 11 out of the 17 were within the X chromosome rDNA itself. T h e distribution of spontaneous X-X exchanges is also significantly different from the 17% within the rDNA seen for the induced exchanges. At least 13 of the 16 crossovers were within the rDNA. Taken together these results clearly indicate that the rDNA is a hot- spot for heterochromatic recombination, as suggested by MADDERN (1 98 1).Although the frequency of X-X crossovers between the rDNA cistrons is not significantly different from the total frequency of X-Y crossovers in the same region
( x 2
= 0.8699, 1 d.f., 0.2<
P<
0.5, from the flanking marker data), the frequency of recovered X.YL chromosomes was significantly lower( x 2
=chromosomes can spontaneously lose the
YL
arm, yielding cytologically normalX
chromosomes. This has been observed in some X .YL
chromosomes kept in the laboratory (GILLINGS et al. 1987). Therefore, the ease of transfer of rDNA sequences is ordered (in decreasing likelihood) from oneX
chromosome to another, from aY
chromosome to anX
chromosome, and from anX
chromosome to aY
chromosome. Transfer from aY
chromosome to anotherY
chro- mosome via reciprocal recombination will be about as difficult as movement from anX
array to aY
array because this can be accomplished only via anX.YL
intermediate. This is consistent with the presence of type I inserts in 28 S genes ofX
chromosomes but notY
chromosomes. T h e inference would be that type I inserts arose in theX
chromosome and rarely, if ever, were transferred to theY
chromosome, whereas the type I1 inserts originated on theY
chromosome and were able to spread to the X linked arrays.Unlike information transfer, homogenization re- quires multiple recombination events among all chro- mosomes. T h e model of OHTA and DOVER (1 983) can be used as a framework for interpreting our data in terms of these repeated events. Their model analyzes the effects of rate differences of asymmetric gene conversion within one chromosome and asymmetric gene conversion between homologous and nonho- mologous chromosomes. It may also be used to ap- proximate symmetric events and reciprocal recombi- nation with minor modification (OHTA and DOVER
1983). With this in mind, the frequencies we meas- ured can be interpreted using their model in the following way: (1)
X-X
recombination can be viewed as analogous to one of their gene conversion events. Using OHTA and DOVER’S terminology,X-X
reciprocal recombinants are symmetrical and terminal with re- spect to the rDNA; (2) eachX-Y
crossover can be viewed as one half of one of their conversion events because, as discussed above, it takes two events to transfer a block of information from one intact X chromosome to an intactY
chromosome (a productFRANKHAM, personal communication). Only if the
YL
arm is deleted at a very high frequency could the observed similarity of rDNA variation on theX
and Ychromosomes be explained as a consequence of recip- rocal recombination. This does not seem likely be- cause after almost 10 yr only 5 of 17
X.YL
chromo- some containing lines lost all or part of theYL
(GILL- INGS et al. 1987). We would, therefore, expect very different patterns of variation inX
andY
chromosome rDNA arrays if recombination of the type we meas- ured were the major mechanism for the evolution of Drosophila rDNA.Since reciprocal recombination by itself cannot yield the observed pattern of Drosophila rDNA vari- ation, another mechanism must be considered. T h e mechanism most commonly invoked is gene conver- sion (asymmetrical exchange of information). How- ever, depending on the exact mechanism of conver- sion, it alone may also be insufficient to explain natural variation. For example, if we assume: (1) that the recombinant chromosomes we recovered were the products of conversion events that were resolved as crossovers; and
(2)
that the probability of resolving conversion events as crossovers is the same for bothX-X
andX-Y
events, then we can reach the following conclusions about gene conversion and rDNA evolu- tion. If flanking marker crossovers occur in approxi- mately 50% of conversion events (FOGEL and MORTI- MER 1969; FOGEL, MORTIMER and LUSNAK 198 l), the effective frequency ofX-X
exchange would be twice that ofX-Y
exchange. This is based on the fact thatX-
Recombination and rDNA Evolution
between different X arrays. This is clearly not the case.
It is unlikely that either gene conversion or recip- rocal recombination alone is responsible for molecular structure of the rDNA cluster. Gene conversion even with accompanying crossing over would yield homo- geneity, and crossing-over alone would yield diver- gence. However, a combination of the two could produce the level and type of variation observed. T h e ratio of conversions to crossovers needed to produce the observed pattern of variation depends on how often recombination complexes are resolved as cross- overs with no conversion. It may also be worth consid- ering that heteroduplexes are certainly much shorter than the length of the rDNA cluster and conversion will only transfer information within these short seg- ments while crossovers will transfer much larger por- tions of the rDNA. Alternatively, a high frequency of double crossovers (high negative interference) may be important. Since our data demonstrate that the rDNA is a recombinational hotspot in a recombination-less region the possibility exists that rDNA recombination is not conventional. Double recombinants may, there- fore, occur relatively frequently. Because we could not detect these events in our study, we cannot address this possibility directly.
It is clear that knowing the frequency of events is not sufficient (although it is necessary) for understand- ing the importance of a given mechanism in the evo- lution of a gene family. It is also necessary to know the exact mechanism that underlies the observed events. How heteroduplexes are resolved will cer- tainly affect how a family evolves.
We thank J. CORREIA for technical assistance and P. CLUSTER, G . DOVER, G . B. GOLDING, D. JOHNSON and R. RASOOLY for reading and commenting on earlier versions of this paper. I. DAWID pro- vided the rDNA clone, pDmrY22c. M. DRYGAS-WILLIAMS helped with the graphics. This work was supported by Alberta Heritage Foundation for Medical Research Fellowships (to S.M.W. and J.A.K.), Natural Science and Engineering Council (Canada) grants to C.S. and to M. A. RUSSELL.
LITERATURE CITED
ARNHEIM, N., 1983 Concerted evolution of multigene families, pp. 38-68 in Evolution of Genes and Proteins, edited by M. NEI and R. K. KOEHN. Sinauer, Boston.
ARNHEIM, N., M. KRYSTAL, R. SCHMICKEL, G . WILSON, 0. RYDER and E. ZIMMER, 1980 Molecular evidence for genetic ex- changes among ribosomal genes on nonhomologous chromo- somes in man and apes. Proc. Natl. Acad. Sci. USA 74: 7323- 7327.
COEN, E. S., and G. A. DOVER, 1983 Unequal exchanges and the coevolution of X and Y rDNA arrays in Drosophila melanogaster. Cell 33: 849-855.
COEN, E. S., J. M. THODAY and G . DOVER, 1982 Rate ofturnover of the structural variants in the rDNA gene family of Drosophila melanogaster. Nature 295: 564-568.
DOVER, G . A., 1982 Molecular drive: a cohesive mode of species evolution. Nature 299: 1 1 1 - 1 17.
DOVER, G . A., S. BROWN, E. S. COEN, J. DALLAS, T . STRACHAN and M. TRICK, 1982 T h e dynamics of genome evolution and
species differentiation, pp. 343-372 in Genome Evolution, edited by G. A. DOVER and R. B. FLAVELL. Academic Press, New York.
ENDOW, S. A,, and D. M. GLOVER, 1979 Differential replication of ribosomal gene repeats in the polytene nuclei of Drosophila melanogaster. Cell 17: 597-605.
FOGEL, S., and R. K. MORTIMER, 1969 Information transfer in meiotic gene conversion. Proc. Natl. Acad. Sci. USA 62: 96-
103.
FOGEL, S., R. MORTIMER and K. LUSNAK, 1981 Mechanisms of meitotic gene conversion, or “wandering on a foreign strand,” pp. 289-339 in The Molecular Biology ofthe Yeast Saccharomyces: L$e Cycle and Inheritance, edited by J. N. STRATHERN, E. W. JONES and J. R. BROACH. Cold Spring Harbor Laboratory Press,
Cold Spring Harbor, N. Y .
FOGEL, S., R. K. MORTIMER, K. LUSNAK and F. TAVARES,
1978 Meiotic gene conversion: a signal of the basic recombi- nation event in yeast. Cold Spring Harbor Symp. Quant. Biol.
GILLINGS, M. R., R. FRANKHAM, J. SPEIRS and M. WHALLEY,
1987 X-Y exchange and coevolution of the X and Y rDNA arrays in Drosophila melanogaster. Genetics 116 24 1-25 1 .
HAWLEY, R. S., and K. TARTOF, 1983 T h e effect of mei-41 on rDNA redundancy in Drosophila melanogaster. Genetics 104:
HILLIKER, A. J., a nd R. APPELS, 1982 Pleiotropic effects associ- ated with the deletion of heterochromatin surrounding rDNA on the X chromosome of Drosophila. Chromosoma 8 6 469- 490.
HILLIKER, A. J., R. APPEIS and A. SCHALET, 1980 T h e genetic analysis of Drosophila melanogaster heterochromatin. Cell 21:
HOOD, L., J. H. CAMPBELL and S. C. R. ELGIN, 1975 Th e o rg a- nization, expression, a nd evolution of antibody genes and other multigene families. Annu. Rev. Genet. 9 305-353.
INDIK, 2. K., and K. D. TARTOF, 1980 Long spacers among ribosomal genes of Drosophila melanogaster. Nature 2 8 4 477- 479.
ISH-HOROWICZ, D., S. M. PNCHIN, P. SCHEDL, S. ARTAVANIS-TSA- KONIS and M. MIRAULT, 1979 Genetic and molecular analysis of 87A7 and 87C1 heat-inducible loci of D. melanogaster. Cell
JINKS-ROBERTSON, S., a n d T . D. PETES, 1985 High-frequency meiotic gene conversion between repeated genes on nonho- mologous chromosomes in yeast. Proc. Natl. Acad. Sci. USA
KENNISON, J. A., 1981 T h e genetic and cytological organization of the Y chromosome of Drosophila melanogaster. Genetics 98:
KENNISON, J. A., 1983 Analysis of Y-linked mutations to male sterility in Drosophila melanogaster. Genetics 103: 2 19-234.
KLAR, A. J., and J. N. STRATHERN, 1984 Resolution of recombi- nation intermediates generated during yeast mating type switching. Nature 310: 744-748.
KLEIN, H., 1984 Lack of association between intrachromosomal gene conversion and reciprocal exchange. Nature 310: 748- 753.
LINDSLEY, D. L., 1955 Spermatogonial exchange between the X and Y chromosomes of Drosophila melanogaster. Genetics 40:
LINDSLEY, D. L., and E. H. GRELL, 1968 Genetic variations of Drosophila melanogaster. Carnegie Inst. Wash. Publ. 627. LINDSLEY, D. L., and G. ZIMM, 1987 The ge nome of Drosophila
melanogaster. Part 3: rearrangements. Drosophila Inform. Serv. 65.
MADDERN, R. H., 1981 Exchange between the ribosomal RNA 43: 1325-1 342.
63-80.
607-619.
18: 1351-1358.
82: 3350-3354.
91-103.
and Biology of Drosophila, Vol. lb, edited by M. ASHBURNER and E. NOVITSKI. Academic Press, New York.
SCHALET, A., 1969 Exchanges at the bobbed locus of Drosophila melanogaster. Genetics 63: 133-1 53.
SMITH, G. P., 1976 Evolution of repeated DNA sequences by unequal crossover. Science 191: 528-535.
STERN, C., 1929 Untersuchungen uber aberrationen des Y-chro- mosoms von Drosophila melanogaster. Z . Indukt. Abstammungs. Vererbungsl. 51: 253-353.
239: 64-68.
YAGURA, T., M. YACURA and M. MURAMATSU, 1979 Drosophila
melanogaster has different ribosomal sequences on X and Y
chromosomes. J. Mol. Biol. 133: 533-547.
ZIMMER, E. A,, S. L. MARTIN, S. M. BEVERLY, Y. W. KAN and A. C. WILSON, 1980 Rapid duplication and loss of genes coding for the 01 chains of hemoglobin. Proc. Natl. Acad. Sci. USA 77:
2158-2162.