University of Windsor University of Windsor
Scholarship at UWindsor
Scholarship at UWindsor
Electronic Theses and Dissertations Theses, Dissertations, and Major Papers
1-1-1979
Simultaneous heat and mass transfer from a vertical isothermal
Simultaneous heat and mass transfer from a vertical isothermal
surface
surface
A. B. M. Abdul Hye University of Windsor
Follow this and additional works at: https://scholar.uwindsor.ca/etd
Recommended Citation Recommended Citation
Hye, A. B. M. Abdul, "Simultaneous heat and mass transfer from a vertical isothermal surface" (1979). Electronic Theses and Dissertations. 7335.
https://scholar.uwindsor.ca/etd/7335
This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email
2. HYE, A.B.M. ABDUL.
Simulta~eous
heat and mass ... surface. Phd. 1979.
SIMULTANEOUS HEAT AND MASS TRANSFER FROM A VERTICAL ISOTHERMAL SURFACE
by
A. B. M. Abdul Hye
A Dissertation
submitted to the Faculty of Graduate Studies through the Department of Mechanical Engineering in partial fulfillment of the requirements for the
Degree of Doctor of Philosophy at the University of Windsor
2. HYf
National Library of Canada
Bib I i oth~que national e du Canada
CANADIAN THESES ON MICROFICHE
0
THlSES CANAD/ENNES SUR MICROFICHE
"LE OF THESIS/n~£0£LA ~~:~~~~~~S~i=m=~~t~a=n~e~o~u=s~h=e=a~t~a=n~d~m=a=s~s~t~r~a=n~s~f~e~r~~fr~o=m~=a~~~~~~~~~
vertical isothermal surface.
IVERSITY IUNIVERSITE University of Windsor, Windsor, Ontario
3REE FOR WHICH THESIS WAS ~ESENTED/
~AOEPD~LEOUUCEITETHEUWTM~fflTh~~~~~~~-P-h_._D _.~~~~~~~~~~~~~~~~~~~-IR THIS DEGREE CONFERRED/ANNE£ O'OBTENTIDN DEC£ GRAOE _ _ ~M:.:;a""y--=1.,.9._.7""'9..._ _ _ _ _ ~-~~--~----~--ME~SU~~ISOOmOMOUO/MCIT~Mmiu_~---~D~r~·~T~·~W~·~M~c=D~o=n~a=l~d
~---~--~--~---mission is hereby granted to the NATIONAL LIBRARY OF L'autorisation est. par Ia pr~sente, accord~e ~ Ia BIBLIOTHE-NADA to microfilm this thesis and to lend or sell copies QUE NATIONALE DU CANADA de microfilmer cette tMse et the film. de preter ou de vendre des exemplaires du film.
1 author reserves other publication rights, and neither the L'auteur se reserve les autres droits de publication; ni Ia sis nor extensive extracts from it may be printed or other- tMse ni de longs extraits de celle-ci ne doivent etre imprimes e reproduced without the author's written permission. ou autrement reproduits sans f'autorisation ~crite de !'auteur.
"EDIDATE
~
1...-j rn
ABSTRACT
An analysis of simultaneous
diffusion
and
heat
transfer
from a
vertical isothermal flat plate
has been
carried
out.
The analysis
considered only the natural convection flow around
the plate with
uniform velocity of injection of the injected
gas
along
the plate
into
the surrounding air. The temperature of the
plate was
always
higher
than
the ambient temperature.
The analysis was carried out to determine the effect of diffusion
on heat transfer and conversely, the effect of heat transfer on diffusion.
That is,
to
investigate the diffusion thermo effect (DT) or
Dufour
effect
(the
heat flow due to concentration
gradient)
and the thermal
diffusion
effect (TD) or Soret effect (the diffusive flon of
mass
due
to temperature gradient). The property variations due to te
mp
erature
and concentration
were
also considered.
In order to obtain the
analytical
solution, the
governing
equations
(two-dimensional, steady state)
were transformed
by a
p9ly
ing standard
similarity transformations. Standard
perturbation
techni
qu
es
were used
to
obtain infinite sets of ordinary differential equations in order of
increasing
powers
of the expansion
parameter
('blowing
parameter').
The
first three sets of equations
were
solved by Rosenbrock's
optimization
method
of
hill-climbing
for selected
gases as
the
diffusing mediums
and
selected
surface
tem9eratures.
The selected
gases were helium,
air
and
carbon dioxide
and the wall
temperatures
were 310, 330, 350, 370K.
It
was
found that
the
series expansion
was
conver
g
ent
only
for lo
w
injection velocities.
~ithinthese
low injection velocit
y
ran
ges
,
the
thermal diffusion effect was about
2%
of
the
heat transfer rate at the
wal
l.
M
ost
of the heat was transferred
from
the
wa
ll
by
con
duction
and
by
the
effect of diffusion thermo. The effect
of
pro?erty variations on
the heat
transfer
rate due to tem9erature and
concent
ration
vas about
5%.
Consequently,
the thermal diffusion effect
~asneglecte
d
and the
property variations
~ereretained
only
in
the bu
o
yancy force
ter~.T
h
is
~ade
the
equations considerably less comulicated
and decreased
t~erequired computer time. With these simplifications,
the
first five sets
of equations
were
solved.
'!l
i thin the limit
of
the conver
g
ency
of the
•series,
the maximum possible
numerical values
of
the injection velocity
at the
wall were
0
.00375
cm/s for helium,
0.0375
cm/s for air and
0
.075
cm/s
for carbon dioxide
w
ith
the ratio of wall temperature to the
ambient temperature
of
1.1.
The experiment was
done with
an interferometer
~hichemployed two
lasers
of different
wave
lengths. A sintered stainless steel
porous
plate
was
used as the wall. A heated
gas
(helium, air
o
r
carbon dioxide)
was injected from behind the plate. The velocity of injection
was
uniform over the surface of the plate. Under steady state condition,
the
temperatures of the wall and the gas were the same and uniform. From the
interferograms, the temperature and the concentration
distributions
in
the
boundary layer were obtained and
compared
with
the analytical
results.
It was found that
the local
values
of concentration
were within
5%
of the analytical values at different points in
the
boundary layer
except at the wall where
t
he concentration
differed by
30%.
This large
deviation was due to the graphical
extrapolation
method use' to locate
the
correct
position
of the
w
all in
the
interferograms.
The experimental
values of temperature were
w
ithin
5%
of the analytical values. As
air
injection did not involve any
concentration,
the si
3
ultaneous equations
used to calculate temperature
were
simplified. The experimental results
for air injection were
·:.ri
thin
5
%
of the analytical results.
ACKNO'NLEDGEMENT S
The author is
g
rateful to Dr. T.
N.
M
cDonald,
H
ea
d
of t
h
e
Department of Mechanical Engineerin
g
, for
h
is con
t
inuous su
pp
ort
and interest.
The author wishes to express his deep
g
rati
t
u
d
e and sincere
thanks to Dr.
W
.T. Kierkus for his invaluable
g
uidance, help and
encouragement during the course of this study. In particular, t
h
e
author would like to express gratitude for the ent
h
usiastic counsel
given by Professor W.G. Colborne, Dr. C.C. St. Pierre and Dr. J.E.S.
Venart.
Thanks are also due to Messrs. Robert Gasper,
N
azrul Islam,
Thomas Moore, A.K. Sethi for their help and co-operation and to
c.
Cada and Robert Tattersall for their technical assistance in
fabricating the apparatus.
To his wife, the author wish simply to say that there are no
words
w
hich can adequately express his feelin
g
s for the support
she has given to enable him to reach this point.
Acknowledgement is made to the National Re
s
earch Council of
Canada for providing financial support.
T
AB
LE OF CO
N
TE
NT
S
ABSTRACT
ACK
NO'.'/
LEDGE
H
E
N
T
TA
B
LE OF CO
N
TE
N
TS
LIST OF FIGURES
LIST OF TA
B
LES
LIST OF SYMBOLS
CHAPTER
I
I
N
TRODUCTIO
N
II
LITERATURE
S
URVEY
2.1 General
2.2 Free Convection
2.3 Heat and
Ma
ss Transfer
A.
Conden~ation &.Evaporation
B. Suction
& Injection
III THEORY
IV
v
VI
3.1
Thermal Diffusion and
Diffusion Thermo
3.2 The Governing Equations and
Boundary Conditions.
3.3
Transport Properties
METHOD OF SOLUTION
EXPERI
M
E
N
TAL A
R
RA
N
GEMENT
5.1 Major Components
5.2 Temperature
M
easure
m
ent
5.
3
Concentration
M
easure
m
ent
5
.
4
Instrumentation
EX PE
RI >
:Zl",.:'AL
P
J
O
C
ZDUR
E
vi
...
P
age
iii
vv
i
vi
i
i
xi
xi i
VII
METHOD OF
CALCULATION
&
INTERPRETATIO
N
VIII
RESULTS
IX
8
.1
Effects of
D
iffusion
The
rmo
and
Thermal
Diffusion
8.2
Effects of
Different
Property
Ratios
8.3
Extension of Solution
with
Some Simplifications
8.4
Experimental Results and
Comparison
CO
N
CLUSIONS
& RECO!<~lENDATIONS RE?ERETCES
APPE
IDICES
vii
47
47
52
52
63
81
83
LIST
OF F
I
GU:q:ss
P
a
g
e
l
.l
M
o
d
el Studie
d
3
3
.1
C
o
-or
d
inate Syste
m
205.1
G
eneral Arrange
m
ent of E
x
peri
m
ental
App
ara
t
us.
33
5
.2
G
eneral vie
w
of t
h
e experimental set-
u:9
.
34
5.3
D
etails of the porous plate, baffles, t
h
er
m
ocou
p
les.
35
5.4
Optical layout
.
of interfero
m
eter.
37
5
.5
The ca
m
era facing the interferometer.
38
5.6
Test section of the interf
e
rometer.
39
7.1
Determination of fringe shifts.
44
7.2
Interpolation of fringe shifts to same y position.
4
5
8.1
Analytical heat Transfer re.sults for Heli
·
um Injection
48
8.2
8
.3
8
.4
8
.5
8
.6
8
.7
8.8
8
.9
8.10 8.11
8
.12
8
.1
3
Anal
y
tical Heat
Transf~rresults for
co
2
injection
Analytical Heat Transfer results for Air injectio
n
.
Effect of terms on perturbation series solution.
Velocity distribution for helium injection.
Velocity distribution for
co
2injection.
Velocity distribution for air injection.
Temperature distribution for helium injection.
Temperature distribution for
co
2
injection.
Temperature distribution for air injection.
Concentration distribution for helium injection.
Concentration distribution for
co
2injection.
Temperature distribution for helium injection
w
ith
v
=
0.00002m/s.
w
vi
i
i
8
.1
4
8
.15
8
.16
8
.17
8
.1
8
8.19
8
.20
8.21
8.22
8
.23
8
.2
4
8.25
8
.26
8.27
9.1
9.2
9.3
9
•
.5
9
.6
15.1
Concentration distribution for
helium
injection
with
v=
0
.
00002
m/s.
w
Temperature distribution for helium
injection
with
v= 0
.
0000275
m
/s.
w
Concentration
distribution for helium
injection
with
v
=
0.0000275
m/s.
w
Temperature
distribution for helium
injection
with
v
::.0.
0000311
m/s.
w
Concentration distribution
for helium
injection
with
v
w=
0.0000311
m/s.
Temperature distribution for
co
2
injection with v
=
0.000378
m/s.
w
Concentration distribution for
c
o
2
injection with v
=
0.00037
8
m/s.
w
Temperature distribution for
co
2
injection with v
=
0.000507
m/s.
w
Concentration distribution for
co
2
injection with vw=
0.000507
m/s.
Temperature distribution for
co
2
injection with vw=
0.000624
m/s.
Concentration distribution for
co
2
injection with vw-=-
0.000624
m/s.
Temperature distribution for air
injection with v
=
0.000201
m/s.
w
Temperature distribution for air
injection with vw::
0.000273
m/s.
Temperature distribution for air
injection with vw=
0.000316
m/s.
Correlation chart for flowmeters.
Calibration chart for flowmeter - 1.
Calibration chart for
flowmet~r- 2.
Calibration
chart
for flowmeter
-
3.
Calibration chart for flowmeter -
4.
Calibration chart
for flowmeter
-
5.
Red
fringe with
no
heat and mass transfer.
15.2 15.3
15.4
15.5
15.6 15.7 15.8 15.10 15.11 15.12 15.13 15.14 15.15 15.16 15.17
15.18
15.19
15.20
Green
f
ri
ng
e
w
it
h
no
h
ea
t
and
m
ass
t
r
a
ns
f
er.
Red frin
g
e for
h
elium
i
n
j
ec
t
i
o
n
w
i
th
v=
0. 00002m
/ s.
w
Green frin
g
e for
h
elium injection
w
i
t
h
v w=
0 • 0000 2m/ s •
Red frin
g
e for heliu
m
in
j
ection
w
ith
v
w= 0
.0000275m/s.
Green fringe for helium injecti
o
n
w
it
h
Vw= 0.0000275
m/s.
Red fringe for helium injection with
vw
=
0.0000311m/s.
Green fringe for helium injection w
i
th
vw
=
0.0000311m/s.
Red frin
g
e for air injection with
v -::::.0.000201m/s.
w
Green fringe for air injection with
vw
=-
0.000201m/ s.
Red frin
g
e for air injection with
v
= 0.000273
m/s.
wGreen fringe for air injection with
v
=
0.000273m/s.
w
Red fringe for air injection with
v
=. 0.000316m/s.
w
Green fringe for air injection with
v
=
0.000316m/s.
w
Red frin
g
e for
co
2
injection with
v
=
0.000378m/s.
w
Green fringe for
co
2
injection
w
ith
v
=
0.000378m/s.
w
Red frin
g
e for
co
2
injection
w
ith
v -::. 0 • 000 507 m/ s •w
Green fringe for
co
2injection with
v
w=
0.000507m/s.
Red fringe for CO? injection
w
ith
v=-
0.000624m/s.
~w
Green frin
g
e for
co
2
injection
w
ith
v :::
0.000624m/s.
w
X
160
161
162
163
164 165 166 167 168 169 170
171
1.1
6
.1
8
.1
8
.2
9
.1
9
.2
9-3
9
.4
9
-5
11.1
11.2
11.
3
11.
4
12.1
12.2
12.
3
LIST OF
T
A
B
L
E
S
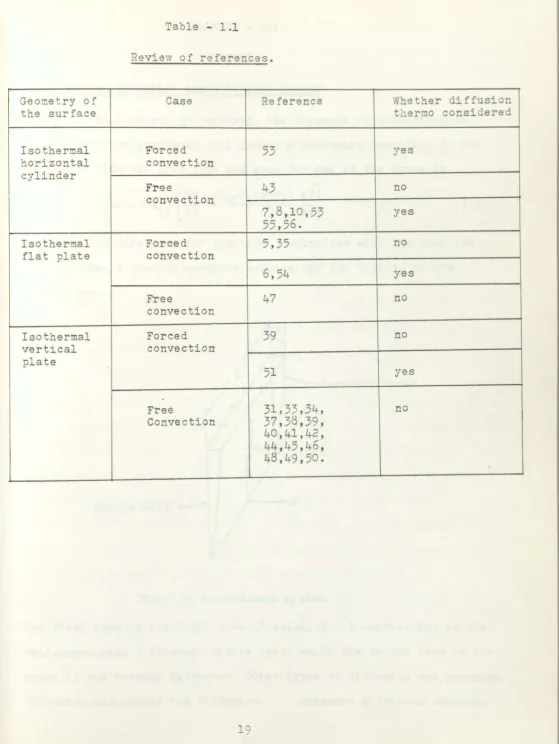
Revi
ew
of ref
e
rences.
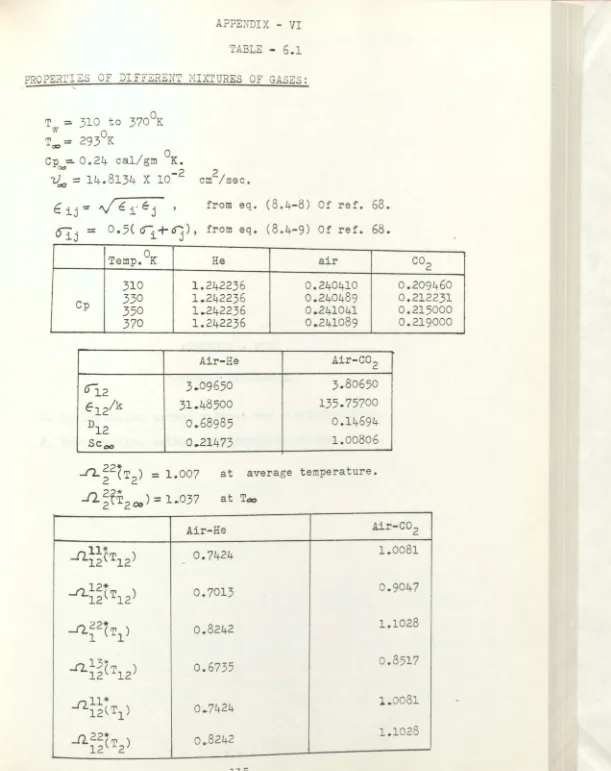
Pro
perties of di
fferent
gases.
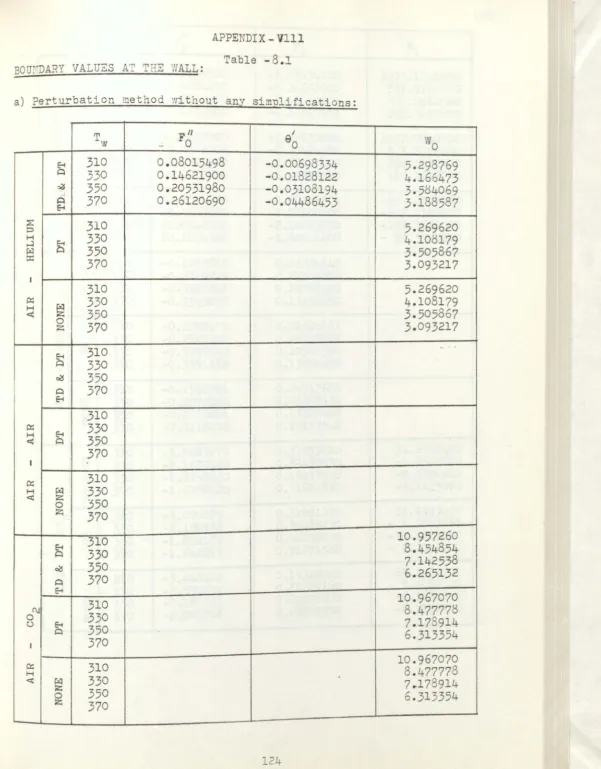
Boundary values at t
he
nall b
y perturbat
i
on
method
w
ithout any sim
plific
at
i
on.
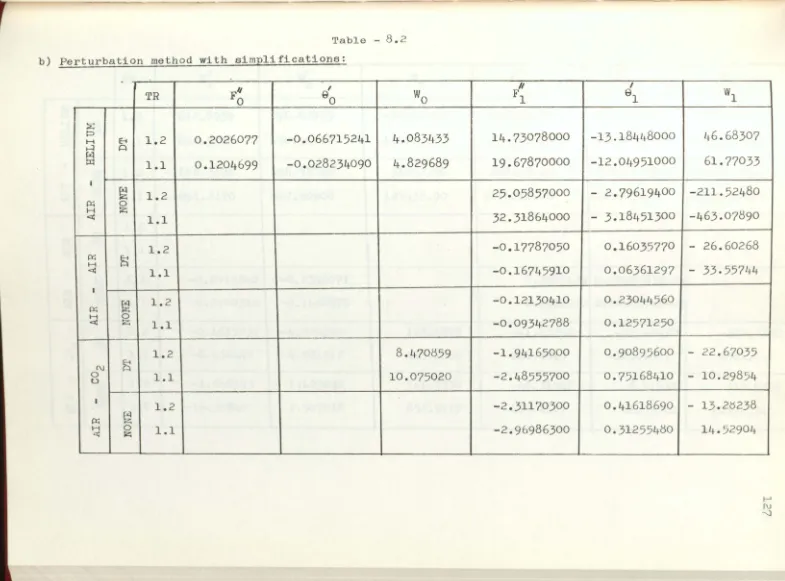
Boundary values at the
w
al
l
b
y per
tur
bation
met
h
od
w
ith si
m
plification.
Calibration for flowmeter-1 f
or dif
f
erent
gases.
Calibration fqr flowmeter-2 for different
gases.
Calibration for flowmeter-3 for different
gases.
Calibration for flowmeter-4 for different gase
s
.
Calibration for flowmeter-5 for different
gases.
Experimental data.
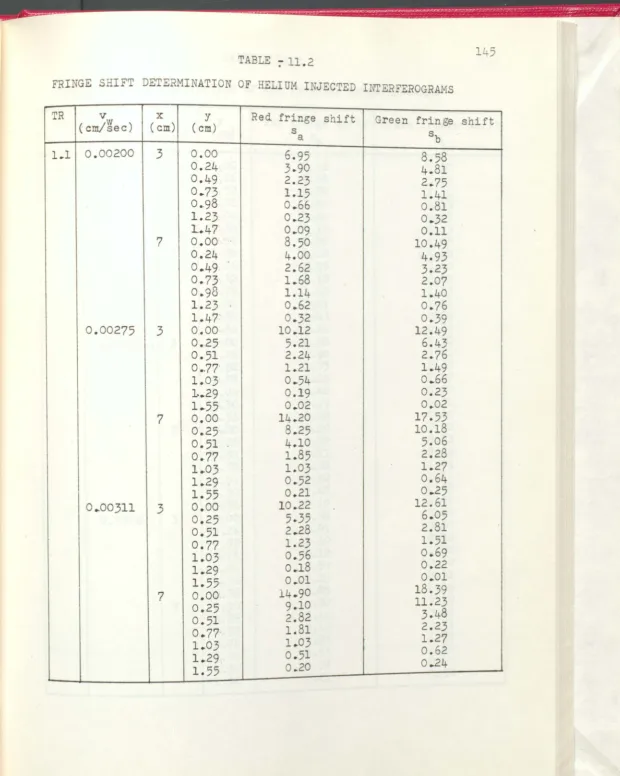
Fringe shift determination of helium injected
interferograms.
Fringe shift determination of air injected
interferograms.
Fringe shift determ
ination of
co
2 injected
-
interfero
grams ..
Index of refraction of different gases.
Index of refraction of different gases at
different temperatures.
Physical constants of different
gases.
xi
Pa
g
e
1
9
11
5
l2W.
127
1
3
6
1
37
138
13
9
1
40
1
4
3
1
45
146
1
4
7
1
4
8
1
49
Symbol
A
Al, A2'
*
Al2' AD B2' B3' c2' c3'
*
cl2 c p c pi Dl2 D. J DT El E2 E cp f I f"
fI I I f F. 1. g Gr GrAB h ji k
LIST OF SP1BOLS
Quantity
A
3, .••• Different Property Constants Property Constants
Different Property Constants Different Property Constants Property Constant
Specific Heat of Mixture at Constant Pressure Specific Heat of Component i at Constant Pressure Coefficient of Binary Diffusion
Nlj-N2j
Abbreviation for Diffusion Thermo.
M
2
/M
1
-l
H2/Ml
(~pl/Cp2)-l
2 Function, F 0(">() -t- F l ("1) ~ -t- F 2(~)
5
I I I 2
Fa+ Fl~
+
F2!,+ ...
.
" " " 2
Fo+Fl_s
+
F2 _$+ ...
.
I 11 I I I I I I 2 FO-t-
FlS
+
F2 ~+
Exterior Force Exerted on a Unit Mass of Component i
Acceleration due to Gravity Heat Transfer Grashof Number Mass Transfer Grashof Number Specific Enthalpy of Mixture
Diffusive mass Flux of Component i
Ther~al Conductivity of Mixture
xii
Units
m
-
3/h
Dimensionless Dimensionless Dimensionless Dimensionless Dimensionless
J/(kg.K)
J/(kg
.
K)
2
m
/s.
m3/mol.
2
J/(s.m .K) Dimensionless Dimensionless Dimensionless Dimensionless Dimensionless Dimensionless Dimensionless 2 m/s .
2
m/s •
Dimensionless Dimensionless
J/kg
.
2
kg/(m
.s)
Symbol k. l
kT
K.
l L Le MH
.
l n. l N. l Nu p Pr qR
Ros
.
J sl' Sc Sh t T TDTR
*
T u v 1.'1. l s2 QuantityThermal Conductivity of Component i
Thermal Diffusion Ratio, ~.x
1
x2
Mass of Component i Created by Chemical Reaction per Unit Volume and Unit Time.
Width of the Porous Plate
Lewis Number, k/(P.Cp.D
12>
Molecular ~eight of the Mixture
Molecular Weight of Component i
Index of Refraction of Component i
Molar Refractivity of Component i
Nusselt Number
Total Pressure
Prandtl Number, ~.Cp/k
Heat Flux per unit Time and Area
Gas Constant of the Mixture
Universal Gas Constant
Fringe Shift of Beam j at any point
Property Constants
Schmidt Number, "l>/D12
Sherwood Number
Time
Tem~erature
Abbreviation for Thermal Diffusion
Absolute Temperature - Ratio, T VI /T~ ...,
Reduced Temperature, kT/ E
Com~onent of Velocity Along x-Direction
Different Property Constants
Component of Velocity Along y-Direction
Hass Fraction,
Pi
/
f
=w
0(•1.)
3
+
'.'11 (1_)3
2+. · ·
xi i i
Units
W/(m.K)
Di::nensionless
3 kg/m .s.
m Dimensionless kg/mol. kg/mol. Dimensionless Dimensionless 2 N/m .
Dimensionless
J/(m2
.s)
J/(kg.K)
J/(mol.i\) Dimensionless Dimensionless Dimensionless Dimensionless s K 2 kg/(m
.s)
Symbol
'
wl II VI l X X. l~
,xk
y
y~ 'yk
~
,zko(l2 f i
"1__,
¢10lf
~p
~
k
A.j AA
1)p
fi
0e
'
e
"
e
Quantity"I' >.e .,, ' 'i? 2
·
"
o
.:;,+
·1,::J+
" " 2
w
05 +
'.V l ,::5+
Distance Along the Surface from the Leading Edge
Mole Fraction of Component i
Pro~erty Constants
Distance Normal to the Surface
Property Constants
Property Constants
Thermal Diffusion Factor(s~ecies l into species 2)
Lennard-Jones Potential Parameter of Component i
Similarity Parameter,
Ax-l/
4
~yyf.
dyViscous Dissipation Function
Stream Function
.
P;
P
00 , Similarly for other ~Blowing Parameter,
vwx
114!(4AlJ
~
)
Boltzmann C~nstant
Wave Length of Light j
Absolute Viscosity of Mixture
Absolute Viscosity of Component i
Kinematic Viscosity
Density of Mixture=
f\
+
f
2Local Density of Component i
Molecular Collision Diameter
x:iv Units Dimensionless Dimensionless m Dimensionless Dimensionless m Dimensionless Dimensionless Dimensionless J Dimensionless J/(kg.m3 ) 2
m
/s.
Dimensionless Dimensionless J/K
it
kg/(m.s) kg/(m.s)m2/s
kg/m3
kg/m
3
Dimensionless
Dimensionless
XV
Symbol Quantity Units
__f2_ l l * ( T*
JL
13**
__12. 22* *12 1 ), 12 (Tl2), 12 (T2)' Different Omega Integrals
to Calculate Molecular Force Constants Dimensionless
Subscript
l Injected Gas 2 Ambient Gas
i ==1,2
0 No Blowing
w
Condition at Halloo Condition at y -oo
m Mixture
a Red Beam from He-Ne Laser b Green Beam from Ar-Ion Laser
j
=a,
bk,n Summation Indices
Superscript
First Differentiation with Res~ect to
Yl
II Double Differentiation with Res:)ect to
' t
I I I Triple Differentiation with Respect to ~
2.
Sean
CHAPTER-I
INTRODUCTION
Diffusion is said to occur in a mixture when there is a relative motion of its components. Such relative motion commonly arises
because of inhomogeneity of composition. In a binary mixture in
which the composition is not uniform the components move in opposite directions, each down its concentration gradient, with the result that,
if the temperature is uniform, the inequalities of composition are
reduced and ultimately removed. Diffusion originating in this way is 'ordinary' or 'concentration' diffusion. This is governed by the
Fick's law of diffusion. Diffusion may, however, have other causes;
in parti~ular, i t may be due to non-uniformity of temperature within a mixture. Diffusion from this cause is called thermal diffusion or Soret effect.
Thermal diffusion consists in a relative motion of the components of a mixture arising from temperature differences within the mixture.
It leads, therefore, in a mixture initially of uniform composition,
to the development of a concentration gradient. Since this concentration ~radient in turn causes ordinary diffusion tending to eli~inate the
gradient, a steady state is possible in Nhich the separating effect of thermal diffusion is balanced by the re~ixing effect of ordinary diffusion. ·~hether or not such a steady state exists, i t is cenerally true that when the temperature of a mixture is not uniform its
2
difference in composition of the mixture in the t~o bul~s is establis~ed.
3ecause the ~olecules of the light gas have more kinetic ener~y and so
they wi l l occupy more s~ace in the up_er bulb. Thermal li~fusion thus
brings about a )artial seuaration of the com~onents of the initially
unif~rn Tixture.
In
1873
Dufour published a paper entitled 'On the diffusion ofgases throu~h porous partitions and the accompanying temperature
changes'. In the experiments t here described, a ~orous vessel, in
which a sensitive thermometer was inserted, was contained within
another vessel. The interior of the porous vessel was in communication
through a glass tube with the atmosphere so that the pressure ~ithin
i t was constant. When this inner vessel vas filled with air, and
hydrogen was circulated in the space between the two vessels, Dufour
observed that as the hydrogen diffused into the inner vessel the
thermometer indicated a fall in temperature. He found a similar effect
when the porous vessel was initially filled with carbon dioxide and
air was circulated about it. Dufour expressed the view that the
temperature changes occur, not throughout the gas but near the surface
of the porous partition. In this way he discovered what has since
come to be called the diffusion thermo effect. or Dufour effect.
The diffusion thermo effect consists in a transport of heat as
part of the process of diffusion in a gas mixture; i t is therefore
associated with the existance of a concentration gradient. The effect
is initially connected with thermal diffusion, the molecular transport
associated with a temperature gradient. Thus thermal diffusion and
diffusion thermo are thermodynamically coupled phenomena. so i f t;·/o
gases initially at the same temperature interdiffuse there is a
transport of heat, with a consequent develo,rnent of a tem~erature
2
difference in composition of the ~ixture in the t~o bul~s is establis~ed.
9ecause the ~olecules of the light gas have more ~inetic ener~y and so they will occupy more S?ace in the upper bulb. Thermal li:fusion thus
brings about a ,ar tial senaration of the co~~onents of the initially unif~rn ~ixture.
• In
1873
Dufour ~ublished a pa?er entitled 'On the diffusion ofgases throuGh porous partitions and the accompanying temperature
changes'. In the experiments there described, a ,orous vessel , in
which a sensitive thermometer was inserted, was contained wi thin
another vessel. The interior of the porous vessel was i~ communication
through a glass tube with the atmosphere so that the pressure ~ithin
i t was constant. When this inner vessel was filled with air, and
hydrogen nas circulated in the space between the two vessels, Dufour
observed that as the hydrogen diffused into the inner vessel the
thermometer indicated a fall in tem~erature. He found a similar effect
when the porous vessel was initially filled with carbon dioxide and
air was circulated about it. Dufour expressed the view that the
temperature changes occur, not throughout the gas but near the surface
of the porous partition. In this way he discovered what has since
come to be called the diffusion thermo effect. or Dufour effect.
The diffusion thermo effect consists in a transport of heat as
part of the process of diffusion in a gas mixture; i t is therefore
associated with the existance of a concentration gradient. The effect
is initially connected with thermal diffusion, the molecular transport
associated with a temperature gradient. Thus ther~al diffusion and
diffusion thermo are thermodynamically coupled phenomena. So i f bto
gases initially at the same temperature interdiffuse there is a
transport of heat, with a consequent develo,rnent of a temperature
In the gas ~ixture, heat is transferred by conduction, co~vection
and diffusion ther~o effect. The effect of radiation is negl igi ble.
If the gas is at a particular temperature, t~e internal energy (heat
content) of the two species are dif~erent and during the ~recess of
diffusion, the two s~ecies will exchange energy. This is called the
heat transfer by interdiffusional convection.
This process of si~ultaneous heat and ~ass trans~er has received
considerable attention over a number of years as i t is closely related
to problems of drying, surface evaporation or condensation, suction or
injection cooling etc. In general, the temperature of the surface can
be higher or lower than that of the surrounding fluid and the direction
of the diffusive flow can be from or toward the surface. The present
study was concerned only with the case when the surface temperature
is higher than the ambient fluid and with the injection of different
gases (helium, air, carbon dioxide) into the boundary layer. The
velocity of the injected gas was uniform over the plate surface. The
process studied was natural convection, two-dimensional, steady state.
Under steady state condition, the temperature of the injected gas and
the wall ~ill be same. The a~bient temperature is considered constant.
The geo~etry of the model studied is shown in Fig. l.l.
Vertical Porous
Wall of Infinite Extent.
Tw _ constant
v
=
constant _ _ __... VI'Injected Gas'
X
y
Fig.l .l: Model Studied.
'Ambient Gas'
T00
=
constantT.;, ). T00
4
Basic
studies
cl
o
sel
y
relate
d
to
t~enresent
~orkwere don
e
by Gross
et
al
1
*
Sziklas
2
,
Go
llnic
k3
and Scott4
~he
re
t~e
effect
on
heat transfer
ra
te
for
various foreign
s
ases
injecte
d
into
the b
oun
dary
layer
th
r
ou
~per~eable
surf
a
ce
were
studied~Results
of
a
later analysis
done by
Baron
5
siowed
that when
a
con
siderably lighter
gas
(heliu~,
hydrogen)
trans
p
ired into
a
heavier one
(air),
the ratio of
heat transfer
fro
m
the surface
decreased
ra'!_Jidly. The
d
ifference
between the
rate
of
h
eat
transfer for
a
single component
g
as
,
and
t~ocomponent
ga
ses (transpiring
gas and
surrounding fluid) increased when the molecul
a
r
weight difference
of the tvro
gases
(transpiring gas and ambient fluid) increased. Recent
6
7
.
8 9
10 11
studies by Sparrow et
al
' ,
Tewflk et al
'
'
'
have
indicate
d
that the effects of thermal diffusion and diffusion ther
m
o
contribute
to the difference.
In the
present
analysis the relative effects of TD and DT on
heat
.
transfer were
analysed
for different blowing
~ases.The variation
of
properties
du
e to temperature and concentration
rasalso
considered.
From the analysis, the temperature
and
concentration
distributions at
different points in the boundary layer
were
calculated.
Experimental
results
obtained
by using a
H
ach-Zehnder
interferometer
were
co
mpared
with the analytical
results.
CHAPI'ER - II
LITERATURE SURVEY
2.1. GENERAL:
12
Nusselt was one of the first authors to investigate
convective mass transfer in relation to an engineering application.
12 13
He ' remarked that under certain circumstances the differential
equations and boundary conditions for heat and mass transfer
respec-tively were mathematically equivalent, so that these processes were
similar. If this similarity holds, then the equations governing mass
transfer may be derived from the equations governing heat transfer
and vice versa. Schmidt1
4
has formulated the similarity theory ofheat and mass transfer for free convection. Nusselt and Schmidt both
developed their similarity relations under the assumption that the
mass flux of a component caused by ordinary diffusion was proportional
to the negative gradient of the mass concentration of the component
considered. This means that they assumed that the diffusive potential
was given by the mass concentration. This assumption had already been
introduced many years earlier by Fick15 and is sometimes called
Fick's diffusion law. Ackermann16 triel to show that for simultaneous
heat and mass transfer i t was better to use the partial pressure as a
diffusive potential. 'rhis point of view has been accepted by Eckert17,
18
19
.
20 21 22
Jakob and Sherwood et al • Later on Spa2..dlng ' ' proposed that
simultaneous heat and mass transfer might be described in a very
convenient way if the fractional mass concentration was used as the
diffusive potential. Merk2
3,
24 used this mass fraction andformulated the basic equations in a general vectorial form which
could be used to analyse any specific problem of simultaneous heat
and mass transfer.
6
Nusselt in his works concluded that the process of heat and mass
transfer were similar in nature because the energy equation and the
diffusion equations were si~ilar in form except that the energy
equation had the Prandtl number, Pr, while the diffusion equation
had the Schmidt number, Sc. Thus the two processes can be analogous
•
only i f the Prandtl number and the Schmidt number ~ere equal in
magnitude, (i.e., the Lewis number, Le, which is the ratio of Pr to
Sc, is equal to unity). If mass transfer occurs, mass is absorbed or
given up by the surface of the ~ass-exchanging body. As a result a
finite velocity perpendicular to the surface exists, which is missing
in pure heat transfer. Nusselt later drew attention to the existence
of this velocity, which is sometimes called the velocity of
Stefan-Nusselt or the convective compensating velocity. The occurance of
this convective compensating velocity in mass transfer process disturbs
the above analogy between mass transfer and pure heat transfer. On the
assumption that Le is unity, i t is easy to relate the heat transfer
to the mass transfer if both process occur simultaneously. ~he
influence of convective velocity on the mass transfer has been
calculated by Schuh2
5;
Eckert et a126; Mickley et al27; Spalding21and Emmons28 , within the range of 0. 6 L :::5c L 2. Merk24 extended
this range using a perturbation method to 0.5L Sc ~oo.
2. 2. FRE:S
co
TiTECTI ON:Eckert 2
9
and Ostrach30 studied the behaviour of free convectionheat transfer. Ustrach considered an isothermal vertical plate with
free convection flow. The body force created by the difference in
density was the driving force for the flow. The flow was found to
be de pendent on the Grasho f number alone. For large Grasho f nuutber,
7
Prandtl's forced flow boundary layer theory. Velocity and temnerature
distributions for
Prandtl
numbers of
0.01~0
.72,
0
.733,
1, 2, 10, 100,
1000
w
ere
com~uted.He
did not consider the effect of variable
~roperties
due to temperature.
Hi
s
works showed the influence
of
Prandtl
nu~beron free
convectio~heat transfer. For the same
temperature difference between the wall and the surrounding, the
heat
transfer rate increases
with
increasing Prandtl number.
2.3.
HEAT&
MASS TRANSFER:A •. CONDENSATIO!'T & EVAPORATION:
Somers3l used evaporation and condensation
processes for the
study of heat and mass transfer in a free convection boundary layer
on a
wetted
isothermal vertical flat plate.
This
problem
presents
itself in the practical case of vaporization cooling
of
equipment
without
forced circulation of the ambient gas. Somers used
an
integral
method
for this
problem
taking into account the non-zero
wall
velocity
arising from the mass transfer.
His
work
was
concerned
with the
combined thermal and diffusive flow that
would
arise adjacent
to
the
vertical plate in a non-saturated atmosphere. The condition of very
small diffusing species concentration
was
used and an analysis
was
carried out for uniform surface temperature and
diffusing
species
concentration. Somers came up
with
a relation between
the
ratio of
heau
transfer and mass transfer as a function ofvfL,e. Somers
did not
consider
the effect of variable
properties
and
the behaviour
of
therT.al diffusion
and
diffusion
t~ermoon
heat
and
mass
transfer.
N
aka
mu
ra3
2
studied
the
si~ultaneous
transf8r of sensible and
latent heat
wh
en
the
materi
al transferred
was water vapor, as the case
in many
a~~licationswhere free convection
rnight
be important.
8
of enthalphy differences. ~ru~amura also neglected buoyancy forcss
due to concentration distributions.
~ath
ers
et al33for~ulated
the problem of simultaneous heat andmass transfer i n ter~s of the boundary layer differential equations
resulting fro~ force-momentu~, energy and chemical species conservation,
at very low concentration. Neglecting inertia effects, the resulting
equations were solved by analog computer for Pr=l.O and Sc=0.5-l0
for the ratio of species and thermal diffusion buoyancy effects of 1.0
and 0.5. Resulting trans~ort information appears to support the ~
factor of Somers31• His
ex~erimental
results withna~thalene
andbenzene diffusing from a sphere in air were found to be 20% a~ay from
the numerical results. The reason for this discre~ancy may be due to his assumution of constant physical ~roperties and exclusion of the
effects of thermal diffusion and diffusion thermo •
.
~!
ilco~4
in his analysis used an integral method with theassumptions of uniform conditions along a surface, arbitrary profiles
of velocity, te~perature and concentration. ~e d8duced the trans~ort
equations vrhich showed the factor
,y"Le.
He compared his results withthe exact results for flows driven entirely by thermal diffusion.
Differencss were ap~reciable and the comparison did not validate the
use of a simple integral method analysis for convection with the
combined effects of convective heat and mass transfer. Also, his
analysis did not include the effects of thermal diffusion and
diffusion thermo.
Li0htfoot3
6
consideredasy:?:~totic
behaviou::- for Sc>)
Pr andGr
>>
GrAB (·.'!here Gr is the thermal Grasho f number and Gr A3 is the masstransfer Grashof nu~ber) in which the ~ass transfer boundary layer
9
substantially
all
convection resulted from
tem~eratureinequalities.
Hi
s
trans~ortrelation for
Nusse
l
t
number
was not
in
good agreement
·:1i
th the
geYJ.e
r
al
result of
'.
'/
ilco
x34
,
'
.vhere
the
limiting
case
.vas
P
r - s c .
Adams and
i'1
cFadden37 reported
experimental
me
asure
m
e
n
ts of
th
e
h
eat
and
m
ass
transfer parameters using a
Ma
c
h
Zehnder
interferometer
during
the su
bl
i
m
ation of
P-dichlorobenzene (Sc
=
2.23)
from
a
heated
vertical surf
a
ce into air. T
h
e
body
force created
by
this heavy organic vapor in the boundary layer opposed the
upward
thermal body force caused by heating the surface. Presentation of
the data in terms of the effective net buoyant force predicted by
Somers3l resulted in experimental data being 10 - 15% lower than
any of the integral method results.
DenBouter et al3
8
reported
an
experimental study of
simultaneous
heat
and mass transfer
by an
electroche
m
ical
me
thod.
A
vertical cop
p
er
,
late
w
as
maintained
at a constant temper
atu
re
in a copper sulphate-sulphuric acid solution.
Measurements
we
re
made w
ith the two cases
wh
en the buoyancy effects
\':e
re aidin
g
and
wh
en op
p
osing.
The
heat and
mass
transfer
para~eters,correl
a
ted
in
terms
of a combined buoyancy effect
agreed
w
ell for
the
effects
of
aiding.
H
owever, for the effects
opposed, the
deviatio~in
the
magnitude
of combined
heat
and mass
transfer
rate
was
m
ore
than
30%. Perhaps even
that level
of
a
g
ree
m
ent
was surprising
since
Pr
~10 and
Sc
::::=
2
000
apply in
t
h
eir stu
dy
.
Manganaro and
Hanna39 d
iscussed
the equations
wh
ic
h
~ould
g
overn si
multan
eous
thermal and
che
mi
cal
S?ecies transport in
a
10
functions of both temperature and composition.
Both forced
and free
convection flows were considered.
fhey
considered large mass transfer
rates
from
the surface in order to rnini::nize
the
effect of chemical
reactions. Practicc:Ily t!r:ts
made-
the
s-olU:
_
t±on.,_,
-
d.i ffi
cult
to
achieve.
The consideration of different properties as arbitrary functions of
temperature showed that property variations were generally more
important for free convection than for forced convection.
Saville and Churchill 40 considered simultaneous
thermal
and
species diffusion caused by buoyancy forces for very low concentration
levels. They used the formulation of Saville and Churchill4l
tor
natural convection adjacent to general
surfaces like horizontal
cylinders and vertical axisymmetric bodies. Attention was restricted
to asymptotic processes in terms of the Prandtl and Schmidt numbers.
The dependence of the transport parameters was
found for Pr =Sc - o ,
for
Pr= sc-
=,
for Pr--o and sc.-oo, and for Pr and sc--oowi th
Sc )) Pr. For Pr= Sc, the processes were a 3ymptotic. But the t'.'IO
processes would be asymptatic onl-y if the effects of
ther~aldiffusion and diffusion thermo were
neglec:e~.So the calculation
of transport parameters only on the basis
of
the values of
Pr
and
Sc
·
camrot' be justified.
Bottemanne
·
42 made a very simple theoretical
in~estigation
of the simultaneous heat and
~asstransfer f0r a vertical isothermal
surface
r
:fith Pr=O.?l and Sc =0.63. He assumed constant properties and
neglected the effects of thermal diffusion
and
diffusion thermo.
His
experimental work 43 with a vertical isothermal cylinder with
s
h
o
w
ed
g
ood agree
m
ent
w
ith his
th
eore
tic
al resu
l
ts
4
2
of
is
oth
er
m
al
v
ertical surface.
H
ere
P
r
w
as ver
y
c
lo
se
to S
c.
5ofo
r so
m
e
oth
er
substance having Sc much differen
t
t
h
an
P
r
, th
e
d
i
ffer
e
nc
e
b
e
t
;
v
een
th
e
experi~entaland t
h
eoretical resu
lt w
ou
ld
increase even if the
effect of thermal diffusion and dif
f
usi
o
n
th
ermo
w
ere
n
o
t
c
o
nsi
d
ere
d
as s
h
own by Lig
h
tfoot3
6.
B. SUCTIO
N
&
I
N
JECTTON:
Smi th35 developed a theory which
. described the e f feet of
diffusion on the dynamic and thermal characteristics of a laminar
boundary layer on a flat plate in steady compressible flow.
F
luid
properties were considered as functions of temperature and
concan-tration of the foreign gas. The diffusion currents due to the
gradients of concentration and temperature were considered.
By
means
of the usual transformations, the system
wasreduced to a set of
.
ordinary differential equations, which in turn were transformed into
a set of integral equations and were solved by successive approximations.
The effects of thermal diffusion and diffusion thermo were neglected
in the whole analysis • Hydrogen and helium, both notable for their
low densities, high heat capacities and high specific heats were
considered
inthe isothermal (low speed) problem and helium alone
was considered in the case of compressible (hypersonic
)
flow. He
f
o
und that helium gas held great promise as a coolant in flight
regimes where aerodynamic heating was important. This result gave a
general idea of the effect of blo\nng even
w
ithout considering t
h
e
ef
f
ect of diffusion thermo.
Baron3 analysed the effect of thermal dif
f
usi
o
n
u
sing t
h
e
12
neglected
the
effect of
diffusion
ther~o.He found that the
effect
of
thermal
diffusion on heat trans
fer was increased
·.nth
tC..e increase
of
injection of helium.
·
rhe
adiabatic
wall
tenper::~.turefor the
·
l!JiJ:ture
boundary layer
was
found to
be
40%
higher whan ther:1al diffusion
:;
as
considered than
when
it was neglected.
Eichhorn
44
,
studied the effects of blowing and suction of a
gas on the free convect
.
ion. flow heat transfer about a
vertical
plate.
The analysis
was
carried out under the condition
that
the buoyancy
forces
were due
only to temperature differences,
with
the mass transfer
entering the
problem
only as a prescribed flow
through the
surface.
He
considered the wall temperature as a function of
the
distance
from
~heleading edge of the plate, i.e.,
Tw -Too= Bx ,
nwhere
B is a
constant.
·In. -order t-e
ha\re:
:
a
similar solution
-.
-Of
.
the laminar boundary layer equations,
he
needed to assume the
blow~ngvelocity (v ) across the plate to be a particular type
w
of the distance along the plate from the leading edge,
of
function
- (n
-1)/4
~.e.,
v
=X •w