REGULATORY MUTANTS
AT
THE
hisl
OF
CAROL LAX, SEYMOUR FOGEL AND CAROLE CRAMER Department of Genetics, University of California, Berkeley, Cdifornia 94720Manuscript received February 25, 1978 Revised copy received September 7, 1978
ABSTRACT
The hisl gene in Saccharomyces cereuisiae codes for phosphoribosyl trans- ferase, a n allosteric enzyme that catalyzes the initial step in histidine bio- synthesis. Mutants that specifically alter the feedback regulatory function were isolated by selecting hisl prototrophic revertants that overproduce and excrete histidine. The prototrophs were obtained from diploids homoallelic for
hid-7 and heterozygous for the flanking markers thr3 and argb. Among six independently derived mutant isolates, three distinct levels of histidine excre- tion were detected. The mutants were shown to be second-site alterations mapping a t the his1 locus by recovery of the original auoxtrophic parental alleles. The double mutants, HZSI-7e, are dominant with respect to catalytic function but recessive in regulatory function. When removed from this hisl-7
background, the mutant regulatory site (HZSI-e) still confers prototrophy but not histidine excretion. To yield the excretion phenotype, the primary and altered secondary sites are required in cis array. Differences in histidine excre- tion levels correlate with resistance to the histidine analogue, triazoalanine.
I N eukaryotes, catabolic and synthetic pathways are genetically controlled by general as well as by pathway-specific genic systems. Control genes with gen- eral effects regulate several pathways simultaneously (
REILLY
and SHERMAN1965;
WOLFNER
et al. 1975; GREER and FINK 1975), while pathway-specific controls may be exerted by genes coding for enzymes that catalyze a particular step in a given pathway (DORFMAN, GOLDFINGER andBERGER
1969; THURIAUXet al. 1972). Such bifunctional enzyme systems with both catalytic and regula-
tory properties have been termed “autogenous” by GOLDBERGER (1974), who described some twenty autogenously regulated enzymes in prokaryotes and eukaryotes.
In Saccharomyces, several loci that code for first enzymes in various metabolic pathways have been implicated in specific pathway control. These enzymes dis- play certain similarities. They are all multisubunit allosteric enzymes, they are inhibited by their pathway end product, or products if involved in a branched pathway, and they regulate the expression of other genes in the same pathway to a greater or lesser extent. The yeast loci coding for these first enzymes as described above include ilvl, arom4, trp2, and h i d . Respectively, these code for threonine deaminase (4.2.1.16) in the isoleucine-valine pathway ( MAGEE and
HEREFORD 1969; THURIAUX et al. 1971; BOLLON aad
MAGEE
1971, 1973; DEThis work supported by Public Health Service grants RGI-GM17317 and 5T01-GM00367.
364 C. LAX, S. FOGEL A N D C. CRAMER
ROBICHON-SZULMAJSTER and MAGEE 1968;
MAGEE
and DE ROBICHON-SZULMAJ- STER, 1968a, 1968a,b phospho-2-keto3 deoxyheptonate aldolase(DAHP
syn- thetase, 4.1.2.15) in tyrosine metabolism (MEURIS 1974),
anthranilatesynthetase in tryptophan biosynthesis ( SCHURCH, MIOZZARI and HUTTER 1974) and phos- phoribosyl transferase (2.4.2.1 7) in the histidine pathway (FINK 1964, 1965;KORCH
1970, 1973; RASSE-MESSENGUY and FINK 1974). Of these four genes, three,hid,
trp2, and ilvl, map in the right arm of chromosomeV
(MORTIMERand HAWTHORNE 1973). Whether or not this proximity represents mere coinci- dence remains to be determined. The map position of arom4 is currently unknown.
In yeast and other microorganisms, regulatory mutations in genes coding for the first enzyme in a metabolic pathway are often selected on the basis of resis- tance to analogues or insensitivity to high concentrations of pathway end prod- ucts (BECHET, GRENSON and WAIME 1970; BUSSEY 1970; UMBARGER 1971;
RASSE-MESSENGUY and FINK 1974; SCHURCH, MIOZZARI and HUTTER 1974;
MEURIS 1974). However, resistance to analogues may often reflect mutations in other loci. Of special consequence are those loci concerned with diminished nutrilite uptake, such as amino acid permeases (SURDIN et al. 1965;
BUSSEY
andUMBARGER 1970;
RYTKA
1975;CRABEEL
and GRENSON 1970; GRENSON, Houand CRABELL 1970) or general amino acid control genes (WOLFNER et al. 1975). Analogue-resistant mutants are sometimes difficult to isolate because wild-type strains may exhibit resistance. Furthermore, their genetic analysis may also pose technical problems, since the growth conditions required to detect analogue resis- tance may limit the inclusion of auxotrophic genetic markers ( RASSE-MESSENGUY
and FINK 1974; SCHURCH, MIOZZARI and HUTTER 1974). Selection techniques dependent on resistance to end-product feedback inhibition are similarly non- specific and frequently result in the recovery of mutations in genes other than those coding for first enzymes (MEURIS 1974). Selection on the basis of feedback resistance may also have limited utility because wild-type strains may be insen- sitive to high concentrations of end products ( RASSE-MESSENGUY and FINK 1974). The present study involves genetic analysis of another class of regulatory mutations at the
hisl
locus that specifies the first enzyme in histidine biosynthe- tic pathway in yeast. The procedure for the detection of regulatory mutations entails the recovery of histidine excreting prototrophic revertants from diploids homoallelic for particular auxotrophic his1 mutants. The metabolite excretion phenomenon (HOLDEN 1962;DEMAIN
1966; DEMAIN and BIRNBAUM 1968) and amino acid excreting revertants from auxotrophic mutants have been previously reported in other systems (CHAMPNEY and JENSEN 1970). Analogue-resistant mutants that excrete the end products of their respective pathways are also known (KASHMIRI and GROSS 1970; SATYANARAYANA, UMBARGER and LINDEGREN1968; RASSE-MESSENGUY and FINK 1974).
hisl
with the parental mutant site. The second site is denoted
by
the symbol e. These excreter strains are resistant to triazolalanine, and preliminary biochemical evi- dence indicates that they produce altered enzymes that are feedback resistant tohistidine
(ROCKMILL,
personal communication).MATERIALS A N D ME T HOD S
Strains: All HISI-7-e excreter alleles in the present work originated as prototrophic histidine- excreting segregants of independently derived revertants isolated from a single hybrid, L A B .
The hybrid was homoallelic for hid-7, which is a UV-induced mutant, isolated from the wild- type strain X2180 by R. K. MORTIMER. Prototrophic excreter alleles designated HISI-l-e were also recovered from independent prototrophic revertants of the HISI-I-e haploid strain LA 350-25B. h i d - I , received from D. HAWTHORNE, is also UV induced. Various his1 tester strain% including alleles hid-204, 1-315, and 1-270, were obtained from the Berkeley stock collection. These alleles, along with various HISI-7w and hid-7-c alleles derived from LA 8, have been described previously (FOGEL,, LAX and HURST 1978; LAX and FOGEL 1978). HISI-7-w alleles are essentially indistinguishable from wild type. hid-7-c alleles are double-site mutants comple- mentary to hid-7. Both types are derived from hid-7 (LAX and FOGEL 1978; FOGEL, LAX and HURST 1978). G. R. FINK contributed his4-290, as well as TRAl-17. The TRAI-17 mutant, characterized by resistance to the histidine analogue triazolalanine, maps within or near hisl and leads to histidine excretion (RASSE-MESSENGUY and FANK 1974). The terminology for genetic symbols is that proposed by the Nomenclature Committee f o r Yeast (PLISCHKE et al. 1976). In all experiments, strain X2180 provided the standard HIS1 allele. The following haploid deriva- tives were the source of HISI-I-e and HISI-7-e alleles:
Strain: Genotype:
hom3 HISI-I-el9 trp2 adel hom3 HIS1-1-e23 trp2 adel HIS1-74117 arg6 adel hom3 NISI-74117 t r p l adel
hom3 HISI-7-eI19 trp2 adel
adel
trp2 adel leu1
trp2 adel
LA350-25B-19 a- -0
LA350-25B-23 a -0
LA8-117-1 IC a -0
LA8-117-6B a- -0
LA8-119-15D a -0
LA8-119-17D ff -0
LA8-122-3B a -0
LA8-122-12D a- --0
LA8-125-5C a -0
LA8-125-18B a-
LA8-132-ID a - -0
LA8-132-2C a- -0
LA8-13 7-3C a -
LA8-137-9A a- -0
_-
ura3 hom3 HISI-7-ell9
HIS1-7-el22
ura3 HIS1-7-eI22
--
-
--
adel leul
- -
HIS1-7-e125 arg6
trp2
_-
adel ura3 H I M - 7 4 1 25adel leul
___ __- hom3 HIS1-7-eI32 arg6
w a 3 hom3 HISI-7-el32 arg6 trp2 adel
_-
adel leu1
_ _ _ -
hom3 HISI-7-ei37 arg6
366 C. LAX, S . FOGEL A N D C. CHAMER
Some of the diploids used i n this study are listed below; others are noted in the text:
Hybrid: Genotype:
LA8 LA162 LA1 74 LA1 76 LA1 78 LA191
LA 1 92
LA193 LA1 94 LA1 95 LA196 a
-
a: a - a! a - a! a - a: a - a: a - a! a_
a: a - a:ura3 hom3 h i s 1 3 arg4
+
+
+
hisl-7+
trp2+
hom3 HIS1-7-el32 argb+
adel adel
adel
~-
-
ura3
+
HISl-7-el22+
trp2+
h o d HISl-7-el17 argd trp2ura3
+
HISl-7-el22+
irp2+
+
HIS1-7-el17 argd+
,.
_ _ _ _ _ ~ _ _+
adel adel adel -__+
leu1+
-- "urn3 hom3 HISl-7-el32 argb trp2
hom3 HISl-7-el17 argd
+
adel
adel
+
-
+
HIS1-7-e137
+
trp2H I S I - 7 4 1 7 argd
adel
adel leu1
-
+
+
hom3 HISl-7-ell9 trp2
+
adel- +
+
+
HISl-7-el22 trD2
+
-t+
adel+
___
a hom3 HISI-7-el25 trp2 adel
- _ _ -op-p-
_-_
oi
+
+
+
+
a hom3 HIS1-7+132 argb adel
a:
+
+
+
+
- _ _ ___
a hom3 HISl-7-el37 argd
-
01
+
+
+
a HISI-7-el37 argb
- -~
LA21 7
a!
+
+
leu1
+
leu1+
adel leu1
+
+
adel leu1
+
+
___
___
--_
__Media: Strains were routinely grown on complex complete medium, GNA. Auxotrophic markers were scored on a series of plates containing synthetic media, SC, each deficient f o r a single nutrilite. Sporulation was induced by transferring diploid cells from GNA t o potassium acetate medium, KAc. GNA, SC, and KAc media are described elsewhere (FOGEL, LAX and
HURST 1978). All experiments were performed a t 30".
Triazolalanine sensitivity: Sensitivity to the histidine analogue triazolalanine was tested by
procedures given by ROTH (1970) and WOFNER et al. (1975). Strains t o be tested were grown overnight in liquid SC medium. The cells were washed and streaked in a radial pattern from the center to the edge of a minimal agar plate containing proline (0.12%) as the sole nitrogen source. A triazolalanine solution (40 pl of a 10 mg/ml) was placed on a centrally positioned sterile filter paper disc. Plates were incubated for 48 h r and the extent of inhibition of growth from the center was determined. Wild-type, X2180 and TRAI-17 strains were routinely in-
Histidine-excretion defecfion procedure: Yeast strains to he tested for histidine excretion were grown overnight on GNA agar plates. These were replica-plated to histidine omission medium previously sprayed to confluence with a suspension of a yeast strain auxotrophic for histidine. The histidine requiring diploid strain LA299 was homoallelic for his4-290. Since the mustant his4- 290 blocks the conversion of histidinol to histidine, the last step in histidine biosynthesis (FINK 1964, 1965), the bioassay estimates the excretion of histidine and not a precursor. The auxo- trophic receptor strains grows as a halo around a prototrophic excretor clone. This was used as
a measure of the histidine released. Excretion was usually scored after 48 or 72 hr. Exceptions are noted i n the text. A maximum of 16 clones per plate was tested to minimize scoring errors due to excretion by adjacent clones. Ambiguities were retested and judgmental errors minimized by randomizing the placement of clones on test plates.
G e n e h ana2ysis: Standard genetic procedures including zygote isolation, tetrad analysis, induced X-ray and UV-mitotic conversion were previously described (FOGEL, LAX and HURST 1978).
RE S UL T S
The recovery of histidine excreter alleles (HISZ-7-e) as spontaneous rever- tants from diploids homoallelic for hisl-7 was described in a previous study
( F OGEL. LAX and
HURST
1978). The notation indicates that the mutants do notrequire histidine, originate from hisl-7, and excrete histidine. The standard wild- type strain, X2180, and strains containing HISI-7-w alleles or leaky hisl-7-c
mutants do not excrete sufficient histidine to yield a positive bioassay. To better understand the excretion phenomenon and to uncover possible genetic and func- tional correlations between alterations at the HIS1 locus and its enzyme product, six independently derived HISI-7-e alleles were studied in detail.
Dominance relationships of HISl-7-e: Though all HISI-7-e/hisZ-7 hybrids
are prototrophic, none excrete histidine. Thus, HISI-7-e alleles are dominant with respect to catalytic function and recessive in excretory function. The reces- sive excretion quality is apparent when any HISI-7-e allele is hybridized with
1
2
3
4
A
B
C
D
HISI-7-ef32
+ +
368 C. LAX, S. FOGEL A N D C. CRAMER
X2180, the original wild-type source of hisl-7. When the two hybrid types
described above are subjected to tetrad analysis, the following segregations are observed. HISl-7-e/hisl-7 hybrids yield two prototrophic excreters and two histidine-requiring segregants. HIS2-7-e/++ hybrids yield two prototrophic excreters and two prototrophic nonexcreter segregants (see Figure I ) .
Characterization of the excretion property: The independently derived
HISl-7-e alleles excrete histidine at different levels. They are unambiguously
classifiable into nonoverlapping classes designated, strong, moderate, and weak with respect to the quantity of excreted histidine. HISl-7-ell9, -el22,-e125,
and -e237 are strong excreters, HISI-7-el32 is moderate and HISl-7-el17 is
extremely weak (see Figure 2).
Several genes other than his1 are implicated in the regulation of histidine
(GREER and
FINK
1975; WOLFNER et al. 1975) and amino acid excretion(HALOS
1975). Hence, it was essential to determine if the observed excretion levels were attributable to specific mutational changes within the his1 locus. Three hybrids involving various HISI-7-e alleles in pairwise combination were constructed. They had the following chromosome V genotypes:
hom3 HISl-7-el32 arg6
f
HISl-7-el224-
4- HISI-7-el22
+
’
hom3 HISI-7-eZ17 arp6..,
’
4- HISl-7-ell7 a ~ g 6
and
.
Respectively, these hybrids represent mod-hom3 HISI-7-el32 are6
erate/strong, strong/weak, and- weak/moderate crosses. They were sporulated and dissected. In all of the I 1 7 unselected tetrads analyzed, the excretion prop- erty segregated in accordance with the excretion levels characteristic of the input parental alleles, i.e., strong/moderate hybrids always segregated two strong and
two moderate spore clones; strong/weak yielded two strong and two weak segre- gants; and weak/moderate gave two weak and two moderate ascosporal clones. Furthermore, markers flanking the specific HISZ-7-e allele exhibited the ex-
1
2
FIGURE 2.-Histidine excretion levels of various mutants. “Strong” includes A2, B1, B2,
pected close linkage to the excretion level. We conclude that the excretion pheno- types are allele specific and map at the HZSI locus,
The six HZSI-7-e alleles were crossed to each other in all painvise combina- tions and the excretion level for each diploid was determined. These results are presented in Table 1. Briefly summarized, the findings are: (a) all diploids excreted histidine, (2) in general, weaker excretion is dominant over stronger excretion, (3) however, in reciprocal crosses two strong/weak hybrids yielded an intermediate response.
Meiotic gene conversion and the recovery of hisl-7 from HISI-7-e: Our previ- ous analysis of hisl-7-c alleles derived from and complementary to hid-7
demonstrated that the events responsible for prototrophy involved intragenic changes at sites other than the original 7 site. We concluded that hisl-7-c alleles retain an unaltered mutant 7 site and that their interallelic complementation responses depend on the occurrence of various nonrandomly distributed second- site alterations, termed c sites (LAX and
FOGEL
1978). Similarly, HZSI-7-ealleles may contain an unaltered hisl-7 mutation, and their excretion pheno- types may be a consequence of the interaction between the 7 site in cis array with a variety of second-site alterations, designated as e sites. With this as a working hypothesis, an experiment was designed to recover hid-7 from
HZSI-7-e. Coincidentally, the experiment could also determine if independent,
phenotypically distinct excreter alleles carry recombinationally separable e
sites.
Our approach utilized the phenomenon of single-site meiotic gene conversion as the basis for separating mutant sites within a locus
(FOGEL
and MORTZMER1969). The following hybrid (LA168) containing two HZSI-7-e alleles exhibit- ing differential excretion phenotypes was constructed:
his1
hom3 7
+
e137+
*
+
7 e117 f arg6* The given order for the excreter alleles is assumed, though probably correct.
TABLE 1
Histidine excretion levels for hybrids of the type HIS1-7-el/HIS1-7-e2
Strong Moderate Weak
mate
HIS-l-7-e2 -e119 -el22 -el 25 -e137 -e132 -e117
mala HISI-7-el
Strong -e1 19 strong strong strong strong moderate weak Strong -e122 strong strong strong strong moderate weak Strong -e125 strong strong strong strong moderate moderate* Strong -e137 strong strong strong strong moderate moderate* Moderate -e132 moderate moderate moderate moderate moderate weak Weak -e117 weak weak moderate* moderate" weak weak
3 70 C. LAX, S. FOGEL A N D C. CRAMER
7 , e117, and e137 are assumed to be distinct, separable genetic alterations. Ordinarily, each tetrad would yield four prototrophic segregants, two strong excreters (HZS1-7-eI37) and two weak excreters (HZSI-7-eI17). Outside marker association would be that expected except for incidental crossing over. However, single-site meiotic gene conversion of e137 to
+
or e117 to+
would yield an auxotrophic hid-7 segregant, If e117 and e137 representDNA
altera- tions at the same site, then theoretically hid-7 should not be recoverable. The presumptive heteroallelic hybrid was sporulated and subjected to tetrad analysis. I n all, 583 unselected tetrads were analyzed; 578 were normal, as expected. Five exhibited aberrant segregation at his1 and are presented in Table 2.TWO
aberrant tetrads ( 1 and 2) represent co-conversions; one yielded three strong excreters and one weak excreter, while the other contained one strong excreter and three weak excreters. Three auxotrophic segregants, presumably single-site conversions of e117 ++,
i.e., HZSI-7-el17 -+his1-7, were recovered in tetrads3 , 4 and
5.
These presumptive hid-7 auxotrophic segregants were crossed to sev- eral tester strains including hid-7 for allele identification? The complementation pattern and prototrophic mitotic gene-conversion frequencies were compared to those of the hid-7 control crossed with the same hisl alleles. As shown in Table3, the three auxotrophic clones were indistinguishable by all criteria from hid-7.
Taken collectively, the data presented establish that HZS1-7-e117 carries a 7
site mutation; e117 and e137 are distinct and separable second-site alterations;
TABLE 2
hisl
+
7 e117+
argb__-__--__
hom3 7
+
e137+
Conversional ieirads recovered from hybrid LA178
~ ~ ~~~~
Spore clone phenotype* Histidine
Tetrad No hom3 hzd arg6 excretlon level h d genotype Conversional event
1 A
+ + +
B
-
+ -
C
+ + -
D
-
+ +
2 A + + - I -
B
-
+ -
C
+ + -
+ +
D
-
3 A
+ - -
+ +
B
C
-
+ +
D
+ + -
4 a n d 5 A
- - -
+ +
C
+ + -
D
+ + +
-
-
B strong strong weak strong weak weak weak strong-
strong strong weak-
strong weak strong7
+
e137 7+
e137 7 e117+
7+
e1377 e117
+
7 e117+
7 e117+
7,+
e1377 +
+
7
+
e137 7+
e137 7 e117+
7 +
+
7
+
e137 7 e117+
7+
e137co-conversion of
e117
+
++
e137with associated exchangeco-conversion of
+
e137 + e117 +with associated exchangesingle-site conversion of
e117 +
+
without exchangesingle-site conversion of
e117 + +with associated exchange
TABLE 3
HISI-7-e117* HISI-74117 Allele identification+ tests of auxotrophic mutants recovered from LA178,
Mutant
Allele tester
Recovered hisi-7
mutants mutants
hisl-l his1315 hisl-270 hisl-7
hisl-7-c7, 4 6 , 4 7 , -e%, -c30,
4 9 , -clOl, -c107, or -cl35 hisl-7-c75 or hisl-7-cll6
-c57, - ~ 6 5 , -~ 6 7 , -c76, 4 0 , ~ 8 3 ,
P - h P - h
P - m P - m
++
++
P - I ‘ P - I *
f f
* Adapted from LAX and FOGEL (1978).
t
Mitotic conversion frequencies based on eight clones, UV dose 400 ergs per “2.P
-
m = Intermediate revertibility: 20-30 papillae/clone. P-
h = High revertibility:>
100 papillae/clone.++
= Growth indistinguishable from wild type after 24 hr. f =Weak intra- genic complementation after three to four days. P-
1’ = Characteristic homoallelic reversion frequency of hisl-7/hisl-7.and the aberrant tetrads
4
and 5 of Table 2 allow the ordering of e137 distal toeI17. Our conclusions are drawn on the basis of properties previously described for meiotic single-site gene conversion, including fidelity of informational trans- fer in gene conversion, and the finding that the gene conversions are often asso- ciated with reciprocal outside marker exchange (FOGEL and MORTIMER 1969;
HURST,
FOGEL
and MORTIMER 1972; MORTIMER andFOGEL
1974).Meiotic conversioln and the recovery of HISI-e from HISI-7-e: Meiotic single-site gene conversion was also employed for the isolation and recovery of an e site alone in an otherwise standard HZSl gene. This allele is symbolized
HZSI-e (LAX and
FOGEL
1978). The recovery and identification of HZSI-e pre- sents a special problem not encountered during the isolation of hid-7 fromHZSI-7-e. Namely, HZSI-e must be distinguishable from HZS1-7-e, wild type, and hisl-7. We supposed that HZSI-e would be prototrophic, but its excretion phenotype could not be predicted.
Hybrids of the generalized type HZS1-7-e/++ were constructed.
If
the e site alone manifests excretion, then single-site meiotic gene conversion of+
to e or7 to -I- would yield tetrads with 1
+
and 3 excreters, and coconversions of++
to 7 e would yield equivalent results. If HZSI-e is a nonexcreter, then a single- site conversion of 7 to
+
would yield aberrant 3+
and 1 excreter tetrads. This pattern would also be recovered from coconversional events of 7 e to++.
his1
hom3 ‘7 e137’ arg6 adel leu1
f i - i - i-
--
+
f
From hybrid LA 196, with the genotype
3 72 C. LAX, S. FOGEL A N D C. CRAMER
TABLE 4
Ezcrefion properties of segregants from HISl-ei37/HIS1-7-e137 and HISI-e137/+ compared to segreganfs from HIS1-7-e137/+
llistidine excretion levels IIsploid hybridized with:
Ilaploid IVild
Ilybrid Told tetrado spore clone IllS1-7-el37 typo IIISf-ef37 Genotype
0. weak 0 0
+
ef37H I S 4 37 0 weak 0 0
+
ei37strong strong 0 weak 7 ef37 H I S f -7-ef 37
strong strong 0 weak 7 e137
0 weak 0 0
+
ef37HISf -ef 37 0 weak 0 0
+
ef370 0 0 0
+ +
0 0 0 0
+ +
strong strong 0 weak 7 ei37 H l S f -7-d 37 strong strong 0 weak 7 ef37
0 0 0 0
+ +
0 0 0 0
+ +
123
-
-25+
700
+
* 0 indicates no excretion.
each tetrad were backcrossed io HIS1-7-el37 to ascertain the presence of the e
site in an otherwise standard HIS1 gene. Normal 2 +:2 strong excreter segre- gants backcrossed to HISI-7-eZ37 yield 2 nonexcreters and 2 strong excreters, respectively. However, some aberrant tetrads with 3
+:
1 excreter segregantswhen hybridized to HIS1-7-eI37 yield 2 nonexcreters,
1
weak excreter, and Istrong excreter hybrids. The weak excretion response of the third test hybrid is not diagnostic for +/HZS2-7437 or homoallelic HIS2-7-eZ37 diploids. Spore clones exhibiting this atypical response might carry the presumptive
HIS2-e allele. Several segregants of this type were backcrossed to HISI-7-el37
and wild type. The results of these crosses are summarized in Table 4. For com- parison, segregants of HIS1-7-e137/+ are included in the table. HISZ-eI37 is histidine independent by itself, and it does not lead to histidine excretion in the haploid or homozygous diploid state. The feature that differentially distinguishes this allele from wild type is observed when HISI-eZ37 is crossed to HIS1-7-el37.
The resultant diploid is a weak excreter. When wild type is crossed to HZS2-7- ~ 2 3 7 , no excretion is observed. Figure 3 compares the excretion levels of asco- sporal segregants from a HIS1-7-e137/HISI-e237 hybrid. From the overall
FIGURE 3.-Excretion levels of ascosporal dones from a single tetrad of the hybrid
H l S i -7-ef 37/HlSf -ef 37. Note two strong excreters and two wild-type clones. The hybrid is
data, we may conclude that HZSI-7-e alleles embody two components, a 7 site and an e site,
To
yield the excreter phenotype, both the altered secondary site and the original primary mutant site are required in cis array.Triazolalanine (TRA) resistance and HIS1-7-e excreter alleles: ~ S S E -
MESSENGUY
andFINK
(1974) reported TRAI mutants resistant to the histidine analogue, triazolalanine. These alleles map at the his? locus and some, includingTRAI-17, lead to histidine excretion. The sensitivity of HZSI-7-e excreter alleles to triazolalanine was examined relative to the following questions: Are HZSI-7-e
alleles resistant to triazolalanine? Is resistance dominant? Is triazoalanine resis- tance correlated with histidine excretion levels?
Sensitivity to triazolalanine was assessed by the procedure described in MATERIALS AND METHODS. Sensitivity was always compared to the response of
the wild-type haploid strain, 21 80-1B. Resistance was measured by similarity
of response to TRAI-17. These two controls were included in every test. Table
5
summarizes the triazolalanine-resistance response of various haploid and diploid strains. Also included in the table are the histidine excretion properties of these same strains.
In haploid strains, the six NISI-7-e mutants are as resistant as TRAI-17.
However, unlike TRAZ mutants ( RASSE-MESSENGUY and FINK 19741, their
TABLE 5
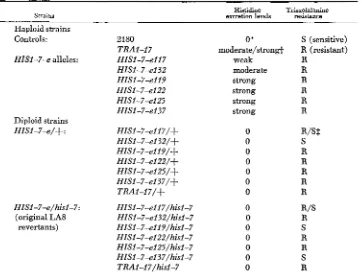
Triazolalanine resistance and histidine excretion of haploid and diploid strains
Strains excrenon levels Histidine Triazolalanine resistance
Haploid strains Controls:
HISI-7-e alleles:
Diploid strains HISl-7-e/+ :
HISl-7-e/hisl-7: (original LA8
revertants)
2180 TRAI-17 HIS1-7-el17 HISI-74132 HISI-7-ell9
HIS1-7-eI25 HISl-7-el37 HIS1-7-el22
HISl-7+117/+ HISl-7-e132/+ HIS1-7-e119/+ HISI-7-el 22/+ HIS1-74125/+ HIS1-7-e137/+ T R A l - l 7 / + HISl-7-el17/hisl-7 HISl-7-e132/hisl-7 HISl-7-ell9/hisl-7 HIM-7-el 22/hisl-7 HISl-7-e125/hisI-7 HISI-7-el37/hisl-7 TRAl-l7/hisl-7
O*
moderate/strong+ weak moderate strong strong strong strong
S (sensitive)
R (resistant) R
R R
R R R
0 R/SS
0 S
0 R
0 R
0 R
0 R
0 R
0 R/S
0 R
0 S
0 R
0 R
0 S
3 74 C. LAX, S . FOGEL A N D C. CRAMER
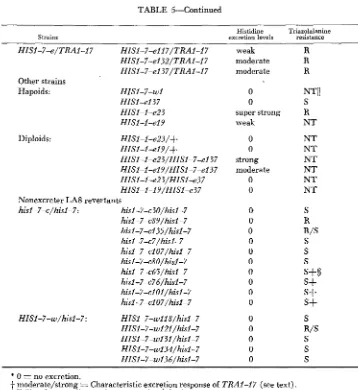
TABLE 5-Continued
Histidie Triazolalanine
resistance
Strains excretion levels
Other strains Hapoids: Diploids: HISI-7-wl HISI-el37 HISI-I-e23 HISI-I-el9 HISI-I-e23/+ HISI-I-e19/+ HISI-I-e23/HISI-7-e137 HISl-l-el9/HISI-7-el37 HISI-I-e23/HISI-e37 HISI-I-19/HISl-e37
Nonexcreter LA8 revertants
hisl-7-c/hisl-7: his1-7-c30/hisl-7 hisl-7-c89/hisl-7 hisl-7-cI35/hisl-7 hisl-7-c7/hisl-7 hisl -7-el 07/hisi-7 his1 -7-c80/hisl-7 hisl-7-c65/hisl-7 hisl -7-c76/hisl-7 hisI-7-dOl/hisl-7 hisI-7-cI07/hisI-7
HISIJ-w/hisI-7: HISl-7-w118/hisl-7
HISI-7-wl2l/hisl-7 HISI-7-w13l/hisI -7 HISI-7-wf 34/hisI-7 HISl-7-wI36/hisl-7 weak moderate moderate 0 0 super strong weak 0 0 strong moderate 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 R R R NTII S R NT NT NT NT NT NT NT S R R/S S S S
s+s
s+
s+
s+
S R/S S S S* 0 = no excretion.
t
moderate/strong = Characteristic excretion response of T R A G I 7 (see text)$ R/S = Intermediate resistance to triazolalanine.
S+ = Very sensitive to triazolalanine.
resistance is not always dominant. The moderate excreter, HIS1-7-el32, crossed to wild type yields a sensitive diploid. The HISI-7-eI17 (weak)/+ hybrid ex- hibits a resistance level intermediate between the sensitive and resistant controls. All strong excreters, HIS1-7-el19, -122, -125, and -137 exhibit dominant resis- tance to the analogue in combination with wild type. Evidently a correlation exists between histidine excretion levels and the dominance of triazolalanine resistance. Yet, there is a differential response in analogue resistance when the four strong excreter alleles are hybridized with the parental hid-7 mutant.
HISZ-7-e122/hisl-7 and HISI-7-e125/hisZ-7 are resistant to triazolalanine. However, HIS1-7-e119/his1-7 and HISI-7-e137/his1-7 are sensitive. Both
hisl
contrast, HZSl-7-e132/+ is sensitive, but HZS1-7-e132/hisI-7 is resistant;
hisl-7 is not testable for sensitivity since it requires histidine.
Several diploid prototrophic revertants of LA8 yielding HZSI-7w or hisl-7-c
alleles were tested for triazolalanine sensitivity. None of these alleles exhibits histidine excretion as haploids. Hybrids obtained from four hid-7-c and four
HZSI-7-w alleles are analogue sensitive. However, four hisI-7-c/hisl-7 diploids are more sensitive than wild type, one hisl-7-c mutant and a HZSI-7-w allele hybridized to hisl-7 are somewhat resistant, and one hisl-c/hisl-7 hybrid shows resistance levels equivalent to TRAI-17. It is important to note that hisl-7-c30
and h i s l - 7 4 9 , previously inseparable by any other criteria
(LAX
andFOGEL
1978) are differentiated by their response to triazolalanine. Apparently, thoughHZSI-7-w and leaky hisl-7-c alleles do not excrete, histidine they may be defec- tive in regulation.
Aside from hisl-7 revertants, Table
5
also lists two spontaneously arising prototrophic excreter alleles recovered from a hisl-l haploid strain. These mu- tants are only partially characterized, but their inclusion in the table emphasizes that excreter alleles are readily recoverable from haploid strains and from his1alleles other than hisl-7
(FOGEL,
LAX
andHURST
1978). hisl-7 and hisl-1differ markedly. Localized in the proximal one-sixth of the gene, the former is highly revertible and complements many his1 mutants. The latter, positioned in the distal one-sixth, has a significantly lower revertibility and does not com- plement any other hisl allele tested (KORCH 1970; KORCH and SNOW 1973;
FOGEL,
LAX
andHURST
1978). Nevertheless, levels of triazolalanine resistance and histidine excretions are similar in HZSI-I-e and HZSI-7-e mutants.TRAl and HIS1-7-e compared: The selection procedures for isolating T R A l
mutants and HZSI-7-e mutants are different. T R A l mutants were selected from a haploid wild-type strain S288c on the basis of resistance to triazolalanine
(RASSE-MESSENGUY and
FINK
1974). HZSl-7-e alleles were recovered as proto- trophic excreter segregants from ascus dissections of homoallelic hisl-7 rever- tants. hisl-7 was induced in X2180, a derivative of S288c. HZSI-7-e alleles differ from wild type by at least two base-pair substitutions. Whether T R A lmutants are double-site mutants is unknown. Yet, HZSl-7-e and T R A l alleles are similar. They map near or in the hisl locus and display triazolalanine resis- tance. HZSI-7-e mutants and several T R A l alleles lead to histidine excretion. Accordingly, we considered the relationship between T R A l and HZSI-7-e alleles in a comparison that examined analogue resistance and histidine-excretion patterns.
The excretion physiology for HISI-7-e alleles and TRAI-17 is different. Excreter mutants reach maximum excretion levels after 48 to 72 hr, and strong, moderate and weak levels typically remain differentiable. In a comparable period, TRAI-17 is a weak excreter, but reaches moderate to strong level after 96 to 120 hr. Meiotic segregants from TRAl-l7/HISI-7-e hybrids often display modified excretion phenotypes. It is unknown whether the difference is mutant specific or attributable to genetic background. This question is considered in the
376 C. LAX, S. FOGEL A N D C. CRAMER
TRAl/wild-type (21 80) diploids are consistently triazolalanine resistant, as given in Table
5 (RASSE-MESSENGUY
andFINK
1974).Our
four strong excreter alleles hybridized with wild type are also triazoalanine resistant. The weak excreter/wild-type hybrid is only somewhat resistant, and the moderate excreter allele hybridized with wild type is completely sensitive. Concerning triazoalanine resistance, HZSI-7-e alleles exhibit a greater range of responses (see Table5)
than theT R A l
mutants described by RASSE-NIESSENGUY andFINK
(1974).HZSI-7-e alleles crossed to hisl-7 show varying degrees of resistance. Only TRAI-17/his1-7 was tested and the hybrid is resistant.
T R A l mutants were reported dominant in their histidine excretion property when hybridized with S288c (RASSE-MESSENGUY and
FINK
1974; FINK, personalcommunication). However, when combined with our laboratory wild-type strain (2180), TRAI-I7 does not excrete histidine, though excretion is measured com- parably in both laboratories. This discrepancy remains unresolved. No other TRAl mutants were tested against our wild-type strain. All hybrids of the type HZSI-7-e/+, HZS1-7-e/his1-7, and TRA1-17/his1-7 fail to excrete histidine. TRAZ-17 was hybridized with a weak, a moderate and a strong excreter. All of these hybrids excrete histidine. The level of excretion mimics the pattern of heteroallelic HZSI-7-e hybrids, i.e., the excretion level of the diploid is that of the lesser excreting parent. Unfortunately, the segregation patterns of TRA1-17/ HZSI-7-e hybrids yielded inconclusive results. All tetrads yielded four excreting
spore clones, but the histidine excretion levels were not clearly 2: 2 representa- tives of the parent alleles. A rationale for this effect will be presented in the
DISCUSSION. However, it is reasonable to conclude that TRAI-17 and the
HZSI-7-e mutants are allelic.
How many distinct excreter alleles? The recovery of two auxotrophic hid-7 ascosporal clones from the heteroallelic hybrid, LA1 78,
hom3 7
+
e137+
+
7 e117+
arg6’
was described earlier. The two hid-7 mutants were generated as single-site conversions of e117 -+ -I-, associated with an exchange of the flanking markers.
unique second-site alterations. Table
6
presents a comprehensive summary of the various excreter alleles and their distinguishing properties.DISCUSSION
One approach to obtaining mutants defective in feedback sensitivity is to iso- late end-product excreting revertants from catalytically deficient mutants. This procedure allowed us to detect genetic alterations at
his1 (HISZ-e
mutants) that are readily distinguishable from wild type. We demonstrated that excreter strains are in fact double mutants carrying second-site alterations separable from the original mutant site. The double mutants are defective in enzyme regulatory properties. That only particular alleles produce excreter revertants suggests that the primary mutant lesions in such auxotrophic mutants may alter the con- formation of both the catalytic and regulatory sites. The various excreter isolates are not simply repeated selections of a single intragenic event. Independent excreter alleles are quite different from each othw in both excretion levels and resistance to triazolalanine. At least three of the six randomly chosen HZSI-7-ealleles examined in this study represent unique mutational events.
The prototrophic nature of the HZSI-eI37 allele may yield a n insight into an interesting anomaly. In the RESULTS section, some discrepancies were noted in the behavior of hybrids involving related wild-type strains used in different laboratories. TRAI-17 was dominant in excretion when hybridized to its parent strain, S288c (RASSE-MESSENGUY and
FINK
1974). Yet, when TRAI-17 is crossed to X2180, a Berkeley derivative of S288c, a nonexcreter diploid is ob- tained. Thus, TRAI-17 excretion is recessive, relative to X2180. Since theTRAI-17/HISZ-e137 and HZSI-7-e137/HISI-e137 both excrete histidine, it is conceivable that the difference in excretion properties of TRAI-17/+ (S288~) and TRA1-17/+ (X2180) is the consequence of the S288c wild-type strain accumulating “e-like” sites at hid. Alternatively the “e-like” sites may have been lost in the X2180 strains.
Similar problems involving strains originating from different laboratories were also encountered in the analysis of hybrids that combine TRAI-17 and various excreter alleles. Though the 121 tetrads analyzed from these hybrids yielded only histidine-excreting segregants, several excretion levels were noted that were not allele specific (see RESULTS). The variability in excretion levels probably indicates the segregation of several heterozygous modifier genes.
There is a correlation between HIS-7-e haploid excretion levels and the corresponding HIS1-7-e/+ hybrid‘s level of triazolalanine resistance. Though
HZS1-7-e/+ hybrids do not excrete histidine, they are likely to have elevated internal histidine pools. We expect that histidine excretion levels and TRA- resistance levels will be proportional to the intracellular histidine levels.
TABLE 6 Summary of HISI-7-e alleles histidine excretion responses
n
Ilistidine excretion levels 2N = IfIS1-7-e hybridized to: IIlSI-7-e allele IN+
hisf-7 TRAI-17 HISf-el37 HIS1-7-e117 HISl-74fl7 weak 0 0 weak 0 weak HISl-7-el32 moderate 0 0 moderate 0 weak HIS1 -7-ell 9 strong 0 0 NT weak weak HIS1 -74137 strong 0 0 moderate weak moderate HISI-7-el22 strong 0 0 NT 0 weak HISI-7-el25 strong 0 0 NT weak moderate Triazolalanine resistanceI" ?I
1N
+
hisl-7
TRAl-17
0 0 M r
R R/S R/S R R S R R > NT
3
R R Sn
R R S R R R R NT 0 R R R NT$
2N = HIM-7-e hybridized to:B 0
might be expected that they are also feedback resistant. Preliminary studies with enzyme preparations from weak and strong excreter strains indicate that both are feedback insensitive to histidine (
ROCKMILL,
personal communication). Selection techniques based on analogue resistance have yielded recessive feed- back-insensitive mutants in first enzymes of other biosynthetic pathways in yeast ( SCHURCH, MIOZZARI andHUTTER
1974). In contrast, the previously reported feedback-insensitive mutants at hisl are dominant (RASSE-MESSENGUY andFINK
1974). The fact that some HISI-7-e alleles, hybridized to wild type, are tri- azolalanine sensitive raises the possibility that in addition to feedback irregulari- ties, excreter alleles may be altered in another capacity.
It has been suggested that phosphoribosyl transferase may perform another essential, yet unknown, function. This notion stems from the conspicuous ab- sence of nonsense mutants at the his1 locus (KORCH 1970;
KORCH
and SNOW 1973). Conceivably, the phosphoribosyl transferase subunit is part of an enzyme aggregate that has an additional essential function associated with it (KORCH 1970). Alternatively, the lack of nonsense mutants may indicate that the hislgene and an adjacent essential gene are transcribed as a polycistronic message. In any event, the unknown function implied above cannot be provided by the addition of exogenous histidine. Yet, the function may be related to histidine biosynthesis through enzymes such as histidyl-tRNA synthetase or histidine permease.
When HISI-7-e, HISI-7-w and hisl-7-c alleles are hybridized to hisl-7,
some diploids of each class are sensitive to triazolalanine, but others are resistant. This observation, along with the tentative conclusion that all hisl-7 revertants include the 7 site, suggests an overlapping rather than discrete distributions of
w , c, and e sites. The nonrandom distribution of c sites and the likelihood of c-site clusters at the proximal segment of the gene near hid-7 and the distal portion near hisl-I have been presented (LAX and
FOGEL
1978). We suppose that e sitesare similarly clustered. Our data bearing on the sequence of three e sites are consistent with clusters.
If we equate resistance to feedback inhibition at the enzymatic level with his- tidine excretion and triazolalanine resistance at the cellular level, we may pose the question, “What might clustering mean in terms of the specified gene prod- uct?” The model presented by KORCH (1973) suggests that both feedback and catalytic sites of phosphoribosyl transferase occur at the point of contact between aggregated subunits. Thus, each subunit has two regions where mutational altera- tions might affect subunit aggregation, catalysis, and feedback inhibition simul- taneously. The following evidence suggests that the hisl-l and h i s 1 3 mutant sites define these two regions: (1) Revertants of hisl-7 restore catalytic function with or without the restoration of normal feedback function. (2) hisl-7-c mu-
tants map in two regions, one close to hisl-I and one close to hisl-7 (LAX and
380 C. LAX, S. FOGEL A N D C. CRAMER
c135/hisl-7 is slightly
TRA
resistant; hisl-7-c65/hisl-7 is extremelyTRA
sensitive. (6) HZSl-7-wl2l/hisl-7 is slightly
TRA
resistant.Clearly, further biochemical studies are required.
A
critical analysis of the effects of different allele combinations on the hybrid enzyme’s catalytic and feedback properties could lead us to correlate genetic structure with enzyme function. By assaying the activity of other histidine biosynthetic enzymes; it should be possible to determine what effects regulatory mutant combinations exert on the repression of the histidine pathway.We anticipate that analyses of prototrophic alleles at the enzymatic level along with an in vitro complementation study of auxotrophic alleles will elucidate the peculiar and novel properties of the the his1 locus. These properties include a complex complementation map (KORCH and SNOW 1973), the reversional po-
larity of the proximal and distal gene segments
(FOGEL,
LAX
and HURST 1978),
the possible clusters of w , c, and e sites, and the absence of nonsense mutants (KORCH
1970).The excellent technical assistance of KARIN LUSNAK is acknowledged. We also wish to thank DANIEL MALONEY, AMAR KLAR, MICHAEL FREELING, and ROBERT K. MORTIMER for helpful suggestions and a critical review of the manuscript.
LITERATURE CITED
BECHET, J., M. GRENSON and J. M. WAIME, 1970 Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cereuisiae. Eur. J. Biochem. 12 : 31-39.
BOLLON, A. P. and P. T. MAGEE, 1971 Involvement of threonine deaminase in multivalent re- pression of the isoleucine-value pathway in Saccharomyces cereuisiae. Proc. Natl. Acad. Sci.
U.S. 68: 216912172.
-,
1973 Involvement of threonine deaminase in repression of the isoleucine-value and leucine pathways in Saccharomyces cereuisiae. J. Bacteriol. 113 :1333-1344.
BUSSEY, H., 1970 Simple selection for end product inhibitor-insensitive mutants in yeast. J.
BUSSEY, H. and H. E. UMBARGER, 1970 Biosynthesis of the branched-chain amino acids in yeast: a trifluoroleucine resistant mutant with altered regulation of leucine uptake. J. Bacteriol. 103 : 286-294.
CHAMPNEY, W. S. and R. A. JENSEN, 1970 Metabolic influences in tyrosine excretion in Bacil- lus subtilis. J. Bacteriol. 104: 351-359.
CRABEEL, M. and M. GRENSON, 1970 Regulation of histidine uptake by specific feedback inhibi- tion of two histidine permeases in Saccharomyces cereuisiae. Eur. J. Biochem. 14: 197-204.
DEMAIN, A. L., 1966 Industrial fermentations and their relation to regulatory mechanisms. Adv. Appl. Microbiol. 8 : 9-27.
DEMAIN, A. L. and J. BIRNBAUM, 1968 Alteration of permeability for the release of metabolites
from the microbial cell. Curr. Top. Microbiol. Immunol. 46: 1-25.
DORFMAN, B-Z., B. A. GOLDFINGER and M. BERGER, 1969 Partial reversion in yeast: genetic evidence for a new type of bifunctional protein. Science 168: 1482-1484.
FINK, G. R., 1964 Gene enzyme relations in histidine biosynthesis in yeast. Science 146: 525-
527.
-
,
1965 Geneenzyme relationships in histidine biosynthesis in yeast. Ph.D. thesis, Yale University, New Haven, Connecticut.hisl
FOGEL, S., C. LAX and D. D. HURST, 1978 Reversion at the hisl locus of yeast. Genetics 90: 489-500.
FOGEL, S. and R. K. MORTIMER, 1969
Natl. Acad. Sci. U.S. 62: 96103.
Informational transfer in meiotic gene conversion. Proc.
GOLDBERGER, R. F., 1974 Autogenous regulation of gene expression. Science 183: 810416. GREER, H. and G. R. FINK, 1975 Isolation of regulatory mutants in Saccharomyces cereuisiae.
pp. 247-272. In: Methods of Cell Biology XI: Yeast Cells, edited by D. M. PRESCOTT, Aca- demic Press, Inc., New York.
GRENSON, M., C. Hou and M. CRABELL, 1970. Multiplicity of the amino acid permeases in
Saccharomyces cerevisiae. J. Bacteriol. 103 : 770-777.
HALOS, S . DE LA CRUZ, 1975 Cysteine Mutants in Saccharomyces cereuisiae. Ph.D. thesis, Uni- versity of California, Berkeley, California.
HOLDEN, J. L., 1962 The composition of microbial amino acid pools. In: Amino Acid pools, edited by J. T. HOLDEN, Elsevier, New York.
HURST, D. D., S. FOGEL and R. K. MORTIMER, 1972 Conversion associated recombination in
yeast. Proc. Natl. Acad. Sci. U.S. 69: 101-105.
Mutations affecting the regulation of production of the enzymes of leucine synthesis in Neurospora. Genetics 64: 42340.
Genetic and biochemical studies of complementation in the first gene of
histidine biosynthesis in Saccharomyces cereuisiae. Ph.D. thesis. University of California, Davis, California.
--
, 1973 Allelic complementation in the first gene for histidine bio- synthesis in Saccharomyces cerevisiae. 11. Complementation mapping of mutants and a subunit model of the enzyme. Genetics 74: 307-329.KORCH, C. T. and R. SNOW, 1973 Allelic complementation in the first gene for histidine bio- synthesis in Saccharomyces cereuisiae. I. Characteristics of mutants and genetic mapping of alleles. Genetics 74: 287-305.
KASHMIRI, S. V. S. and S. R. GROSS, 1970
KORCH, C. T., 1970
LAX, C. and S. FOGEL, 1978 Novel interallelic complementation at the hisl locus of yeast.
The regulation of isoleucine-valine bio- synthesis in Saccharomyces cereuisine: 2. Identification and characterization of mutants lack- ing the acetohydroxyacid synthetase. Eur. J. Biochem. 3: 502-506. - , 1968b The
regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae: 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur. J. Biochem. 3: 507-511. MAGEE, P. T. and L. M. HEREFORD, 1969 Multivalent repression of isoleucine-valine biosynthe-
sis in Saccharomyces cereuisiae. J. Bacteriol. 98: 857-862.
MEURIS, P., 1974 Feedback insensitive mutants of the gene for the tyrosine-inhibited DAHP synthetase in yeast. Genetics 76: 735-744..
MORTIMER, R. K. and S. FOGEL, 1974 Genetical interference and gene conversion. pp. 263-275. In: Mechanisms in Recombination, edited by R. F. GRELL, Plenum Press, New York. MORTIMER, R. K. and D. C. HAWTHORNE, 1973 Genetic mapping in Saccharomyces IV Mapping
of temperature-sensitive genes and use of disomic strains in locating genes. Genetics 74: 33-54.
Genetic mark- ers and associated gene products in Saccharomyces cerevisiae: pp. 765-832. In: Handbook
of Biochemistry and Molecular Biology, 3rd edition. Edited by G. D. FASSMAN, Chemical Rubber Co., Cleveland, Ohio.
Genetics 90: 501-516.
MAGEE, P. T. and H. DE ROBICHON-SZULMAJSTER, 196th
382 C . LAX, S. FOGEL A N D C . CRAMER
RASSE-MESSENGUY, F. and G. R. FINK, 1974 Feedback-resistant mutants of histidine biosynthe- sis in yeast: pp: 85-95. In: Genes, Enzymes and Populations. Edited by A. M. SRB, Plenum Press, New York.
Glucose repression of cytochrome C synthesis in cytochrome- deficient mutants of yeast. Biochem. Biophys. Acta 9 5 : 640-651.
The regulation of isoleucine-valine biosynthesis in Saccharomyces cereuisiae: 1. Threonine deaminase. Eur. J. Biochem. 3: 492-501.
ROTH, J. R., 1970 Genetic techniques in studies of bacterial metabolism: pp. 3-35. In: Methods in Enzymology, vol. 17A, edited by H. and C. W. TABOR, Academic Press, New York. RYTKA, J., 1975 Positive selection of general amino acid permease mutants i n Saccharomyces
cereuisiae. J. Bacteriol. 121: 562-570.
SATYANARAYANA, J. H. E., G. UMBARGER and G. LINDEGREN, 1968 Biosynthesis of branched- chain amino acids in yeast: regulation of leucine biosynthesis in prototrophic and leucine auxotrophic strains. J. Bacteriol. 96: 2018-2024.
Regulation of tryFotophan biosynthesis in
Saccharomyces cereuisiae: Mode of action of 5-methyl-tryptophan and 5-methyl-tryptophan- sensitix e mutants. J. Bacteriol. 117: 1131-1 140.
Proper- ties and genetic control of the amino acid permeases in yeast. Biochem. Biophys. Acta 107:
546-566.
Genetic fine struc- ture and function of mutants at the ilul-gene locus of Saccharomyces cereuisiae, Mol. gen. Genetics 112: 60-72.
Regulation of the carbamoylphosphate synthetase belonging to the arginine biosynthetic pathway of Sac-
charomyces cereuisiae. J. Mol. Biol. 67: 277-257.
Metabolite analogs as genetic and biochemical probes: pp. 119-140. In: Advances in Genetics, vol. 16, edited by E. W. CASPARI, Academic Press, New York. Integration of amino acid biosynthe- sis into the cell cycle of Saccharomyces cereuisiae. J. Mol. Biol. 96 : 273-290.
Corresponding editor: R. E. ESPOSITO REILLY, C. and F. SHERMAN, 1965
ROBICHON-SZULMAJSTER, H. DE and P. T. MAGEE, 1968
SCHURCH, A., J. MIOZZARI and R. HUTTER, 1974
SURDIN, Y., W. SLY, J. SIRC, A. M. BORDIS and H. DE ROBICHON-SZULMAJSTER, 1965
THURIAUX, P., M. MINET, A. M. A. TEN BERGE and R. F. ZIMMERMAN, 1971
THURIAUX, P., F. RAMOS, A. PIBRARD, M. GRENSON and J. M. WAIME, 1972
UMBARGER, H. E., 1971