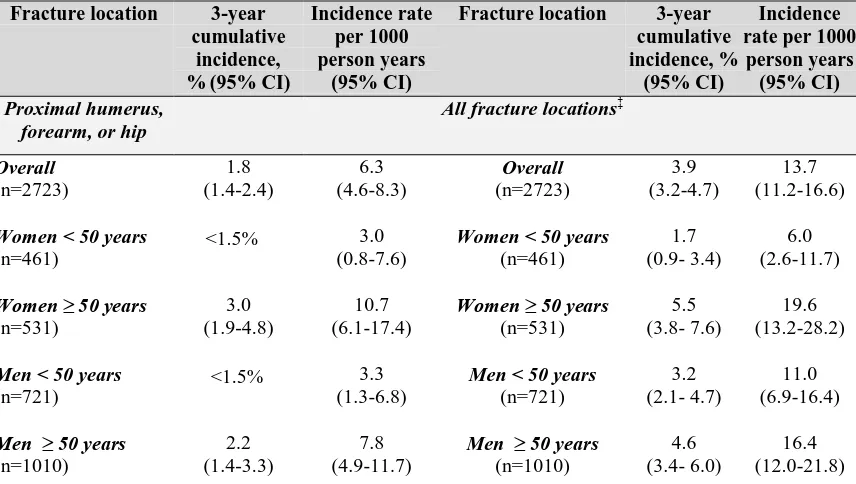
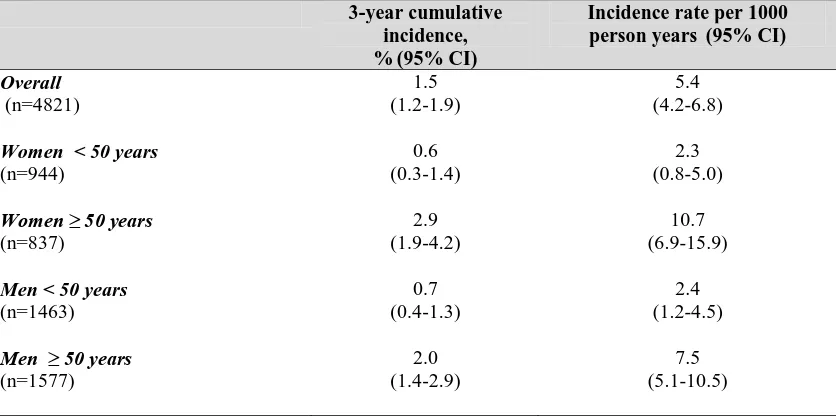
Epidemiology of Fracture in Adults with Kidney Disease
Full text
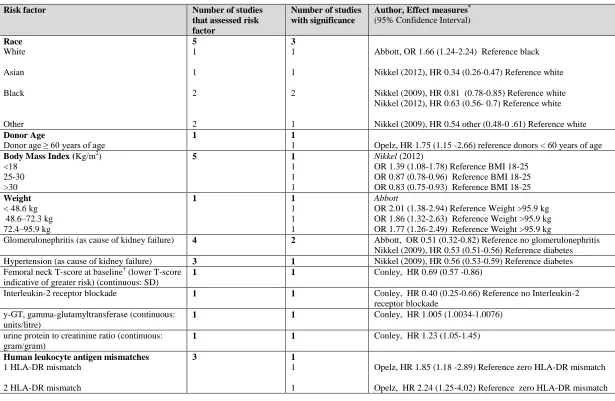
Figure
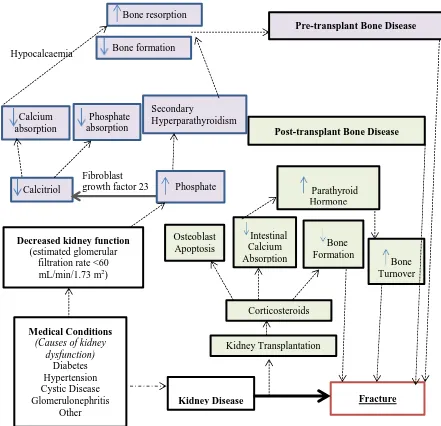
Outline
Related documents
For the purpose of this Proposal, the term Gross Profit shall mean Net Receipts from an event (as defined in the following sentence) less the following items: (1) 3% of Net
Any person who wishes to appeal against his or her assessment is required to file his or her notice of appeal, accompanied by a $100.00 appeal fee which will be returned if the
Theoretically, at least, the mortgage is merely security for the loan and the primary obligation is the personal one on the note.H In addition, the Massachusetts rule does
The nanoparticles were produced in chitosan aqueous solutions by chemical reduction at room temperature, the metals nanoparticles become attached to the polymer, thus providing in
Using the researcher designed and psychometrically robust CALS- Instrument, results revealed core academic language skills to be a significant predictor of reading comprehension,
Dear Brothers and Sisters, I am Alfredo Quintana and I am responsible for dispatch at the Santa Cruz office and I am the Business Agent that covers the area of Santa Cruz
In short, besides attacks based on statistical frequency, an adversary can use algebraic identities to determine the encryption of certain values and then leverage this to build
With a protected bitstream file, the decryption key for data processing can remain protected, as it will only be exposed within the FPGA. Three cryptography schemes will be analysed
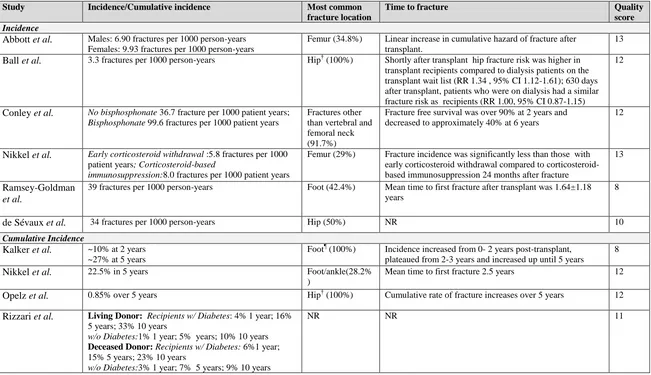
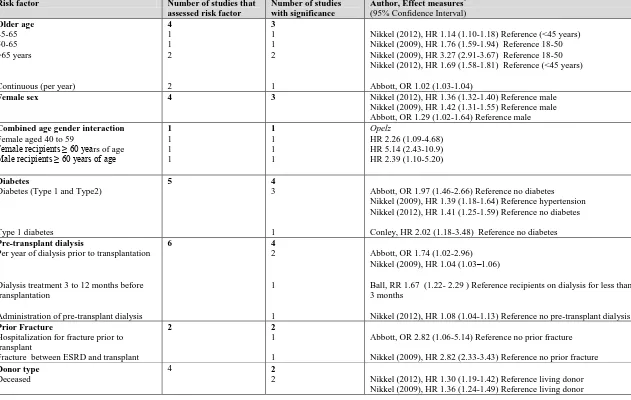
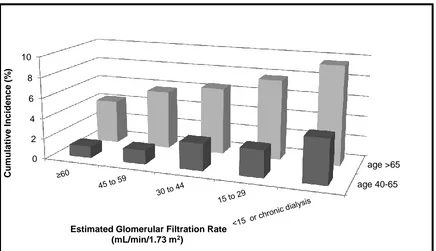
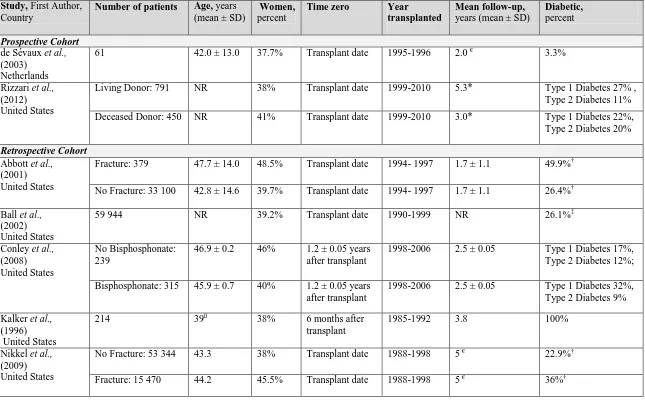
![Figure 3. 2. Mean predicted 5-year fracture risk from the Canadian FRAX tool (with and without bone mineral density [BMD]) and observed 5-year major osteoporotic fracture risk (Kaplan-Meier) according to estimated glomerular filtration rate](https://thumb-us.123doks.com/thumbv2/123dok_us/7765896.1276758/85.612.110.544.298.584/predicted-canadian-observed-osteoporotic-according-estimated-glomerular-filtration.webp)