Early Resuscitation of Children With
Moderate-to-Severe Traumatic Brain Injury
WHAT’S KNOWN ON THIS SUBJECT: Traumatic brain injury is the leading cause of death and disability in children. Postinjury hypotension and hypoxia are believed to induce secondary brain injury and are associated with increased morbidity and mortality.
WHAT THIS STUDY ADDS: Hypotension and hypoxia are common events in pediatric traumatic brain injury. One third of children are not properly monitored for hypotension or hypoxia, especially if they are small. Attempts to treat hypotension and hypoxia significantly improve outcomes.
abstract
OBJECTIVES:Traumatic brain injury is a leading cause of death and disability in children. Guidelines have been established to prevent sec-ondary brain injury caused by hypotension or hypoxia. The purpose of this study was to identify the prevalence, monitoring, and treatment of hypotension and hypoxia during “early” (prehospital and emergency department) care and to evaluate their relationship to vital status and neurologic outcomes at hospital discharge.
METHODS:This was a retrospective study of 299 children with moder-ate-to-severe traumatic brain injury presenting to a level 1 pediatric trauma center. We recorded vital signs and medical provider response to hypotension and/or hypoxia during all portions of early care.
RESULTS:Blood pressure (31%) and oxygenation (34%) were not re-corded during some portion of “early care.” Documented hypotension occurred in 118 children (39%). An attempt to treat documented hypo-tension was made in 48% (57 of 118 children). After adjusting for severity of illness, children who did not receive an attempt to treat hypotension had an increased odds of death of 3.4 and were 3.7 times more likely to suffer disability compared with treated hypotensive chil-dren. Documented hypoxia occurred in 131 children (44%). An attempt to treat hypoxia was made in 92% (121 of 131 children). Untreated hypoxia was not significantly associated with death or disability, except in the setting of hypotension.
CONCLUSIONS:Hypotension and hypoxia are common events in pedi-atric traumatic brain injury. Approximately one third of children are not properly monitored in the early phases of their management. At-tempts to treat hypotension and hypoxia significantly improved out-comes.Pediatrics2009;124:56–64
CONTRIBUTORS:Michelle Zebrack, MD,aChristopher Dandoy,
MD,bKristine Hansen, RN, BS,cEric Scaife, MD,dN. Clay Mann,
PhD, MS,eand Susan L. Bratton, MD, MPHa
aDivisions of Pediatric Critical Care anddPediatric Surgery and eIntermountain Injury Control Research Center, University of
Utah School of Medicine, Salt Lake City Utah;bDivision of
Pediatrics, Miami Children’s Hospital, Miami, Florida;cTrauma
Program, Primary Children’s Medical Center, Salt Lake City, Utah
KEY WORDS
traumatic brain injury, secondary brain injury, outcome, emergency department, emergency resuscitation, treatment, emergency medical services
ABBREVIATIONS
TBI—traumatic brain injury ED— emergency department
PCMC—Primary Children’s Medical Center GCS—Glasgow Coma Scale
EMS— emergency medical services ISS—Injury Severity Score CT— computed tomography GOS—Glasgow Outcome Scale RR—relative risk
CI— confidence interval aOR—adjusted odds ratio
www.pediatrics.org/cgi/doi/10.1542/peds.2008-1006 doi:10.1542/peds.2008-1006
Accepted for publication Nov 6, 2008
Address correspondence to Michelle Zebrack, MD, 295 Chipeta Way, PO Box 581289, Salt Lake City, UT 84158-1289. E-mail: michelle.zebrack@hsc.utah.edu
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275). Copyright © 2009 by the American Academy of Pediatrics
Traumatic brain injury (TBI) is the lead-ing cause of injury-related death and disability in children and is a major public health problem in the United States.1Approximately 475 000 TBIs
oc-cur annually in children⬍15 years of
age. Each year, ⬎2000 children die
from TBI, and 42 000 require hospital-ization,2yet pediatric TBI remains
un-derinvestigated.3
In addition to the primary injury occur-ring at the moment of impact, second-ary brain injury evolving over the ensu-ing minutes, hours, and days can occur, resulting in increased disability and mortality.4For this reason,
guide-lines have been published addressing the prehospital assessment, treat-ment, and transport of individuals with TBI.3,4Postinjury hypotension and
hyp-oxia are believed to induce secondary brain injury and are associated with increased morbidity and mortality.5–10
Few pediatric studies have identified the prevalence of hypotension and hyp-oxia during prehospital and emer-gency department (ED) care and linked these episodes to outcomes at hospital discharge. There are limited data re-garding how well children are moni-tored and treated for hypotension and hypoxia during their early care. We hypothesized that hypotension and hypoxia in moderately and severely brain-injured children are frequent oc-currences in the prehospital and ED settings and that failure to respond to early hypotension and/or hypoxia is associated with a worse outcome.
METHODS
Study Design
A retrospective cohort study of chil-dren with moderate-to-severe TBI was conducted from January 2002 to Sep-tember 2006. We identified all patients ⬍15 years of age with moderate or se-vere TBI presenting to Primary Chil-dren’s Medical Center (PCMC), a
free-standing Pediatric American College of Surgeons-accredited level 1 trauma center in Salt Lake City. PCMC serves 5 western states and had an annual ED volume of⬃40 000 patients during the study period. Moderate TBI was de-fined as postresuscitation Glasgow Coma Scale (GCS) score 9 to 12. Severe TBI was defined as postresuscitation GCS score 3 to 8. Postresuscitation GCS score was defined as the score ob-tained on arrival to the ED at PCMC. The University of Utah Institutional Review Board approved this study and waived the need for informed consent.
Data were abstracted from the trauma registry, hospital medical charts, and emergency medical services (EMS) “run sheets.” Early resuscitation was defined as all portions of care from the time of injury until departure from ED, including ground or air transport from the scene, community medical facility treatment, interfacility transfer, and ED care. Vital signs during all portions of early care were recorded. Hypoxia was defined as a saturation⬍90% or if
apnea⬎20 seconds was documented.
Hypotension was defined as systolic
blood pressure ⬍5th percentile for
the age appropriate norm.8,11Lack of
monitoring for blood pressure, oxime-try, and respiratory rate was also re-corded by location of care.
To evaluate medical provider response to hypotension or hypoxia/apnea, data were collected for all of the patient
in-terventions. Medical provider
re-sponse to hypotension or hypoxia/ap-nea was independently evaluated by 2 critical care physicians and rated as “treated” or “untreated.” Treatment for hypotension was defined as place-ment of an intraosseous or intrave-nous catheter and administration of fluid above hourly maintenance rates (Table 1). For patients with hypoxia, treatment for low oxygen saturation required administration of
supple-mental oxygen, and treatment for ap-nea required the use of bag mask ven-tilation or intubation. Correction of hy-potension or hypoxia was not required to achieve a rating of “treated.” In cases where a care provider did not document blood pressure, respiratory rate, or saturation, the patient was considered “not fully monitored” at that site of care. One set of vital signs was required during each site of care to be considered “fully monitored.” However, if hypotension or hypoxia was documented, a follow-up mea-surement was required to evaluate on-going treatment.
Patient injuries were enumerated and an Injury Severity Score12(ISS) was
cal-culated. Computed tomography (CT) scans obtained within the prehospi-tal/ED time period were reviewed and graded using the Marshall classifica-tion13(Table 2).
TABLE 1 Hourly Maintenance Rates Patient’s Weight
Range, kg
Hourly Maintenance Fluid Rate ⱕ10 4 mL/kg per h
11–20 40 mL/h plus 2 mL/kg per h for each kg⬎10 kg
⬎20 60 mL/h plus 1 mL/kg per h for each kg⬎20 kg
TABLE 2 Marshall Classification of CT Scans13
Level Classification I No visible intracranial pathology II Diffuse injury with cisterns present
and 0- to 5-mm shift III Diffuse injury with cisterns
compressed or absent and 0- to 5-mm shift
IV Diffuse injury with midline shift⬎5 mm but no lesion⬎25 mm EML Evacuated mass lesion, any
surgically evacuated mass lesion
NEML Nonevacuated mass lesion, any high- or mixed-density mass lesion⬎25 mm not surgically evacuated
Outcome Measures
The primary outcome measures were mortality and functional neurologic out-come at hospital discharge. Functional neurologic outcome was ranked as “poor” or “favorable” based on the Glas-gow Outcome Scale (GOS) score. The GOS ranges from 5 (good recovery) to 1 (death).14We defined a “poor” GOS score
as 1 (dead), 2 (vegetative), or 3 (severely disabled), whereas a “favorable” GOS score was defined as 4 (moderate dis-ability) or 5 (good recovery).
Statistical Analysis
Data were analyzed using SPSS 15.0 for Windows (SPSS Inc, Chicago, IL). Sum-mary results were expressed as medi-ans (with 25th and 75th quartiles) or
percentages. Bivariate analyses were conducted using2tests and the
Mann-Whitney U test or Wilcoxon signed rank test for continuous data. Unadjusted rel-ative risk (RR) ratios with 95% confi-dence intervals (CIs) were calculated. Statistical significance was defined as
P⬍.05.
As part of the multivariate analysis plan, logistic regression models were developed for evaluating potential as-sociations between lack of vital sign monitoring, as well as documented hy-potension and/or hypoxia, with poor neurologic outcome or death, control-ling for patient and injury characteris-tics. Four different models were con-structed and are summarized below. The first model evaluated whether
fail-ure to fully monitor patients was asso-ciated with patient demographics, clin-ical characteristics, or outcome. A second model evaluated potential as-sociations between lack of vital sign monitoring with poor neurologic out-come or death. Third, medical provider treatment of patients with docu-mented hypotension or hypoxia was evaluated by excluding the normoten-sive, nonhypoxic patients and desig-nating subjects with treated hypoten-sion or hypoxia as the comparison group. Finally, a related model in-cluded both exposure to hypotension and hypoxia/apnea graded by provider treatment response to evaluate the effects of both insults on clinical outcomes. Patients who did not have documented hypotension or hypoxia, including those not fully monitored,
were classified as normotensive
and/or nonhypoxic in our analyses.
Variables considered in the multivari-ate models included those that were significantly associated with either lack of monitoring or the presence of hypotension or hypoxia in the bivariate analysis. Several severity-of-illness markers, such as Marshall CT score, ISS, blood pH obtained in the ED at
PCMC, and postresuscitation GCS
score, were evaluated with the Spear-man’scorrelation coefficient for co-linearity. Measures of illness severity with autocorrelations in excess of 0.35 were considered potentially colinear and were not both included in the same model. ISS had the greatest cor-relation with failure to fully monitor patients, whereas Marshall CT scores had the greatest correlation with both mortality and poor outcome and were, thus, retained in the final models, which also included blood pH in the ED at PCMC. Variables were entered si-multaneously in the models. Blood pH (⬎7.34, 7.33–7.24, and⬍7.24) and ISS (⬎25, 18 –25, and 0 –17) were grouped as a tricategorical dummy variable
TABLE 3 Characteristics of Children Categorized by Adequacy of Blood Pressure Monitoring Characteristic Fully Monitored All Sites Not Fully Monitored
No Hypotension Documented
(N⫽129)
Hypotension Documented (N⫽77)
No Hypotension Documented
(N⫽52)
Hypotension Documented (N⫽41) Age, y
Median 8 9 3a 4b
IQR 4–12 3–12 1–7 2–9
Weight, kg
Median 25 28 16a 15b
IQR 15–40 13–40 10–22 11–30
GCS score
Median 5 3 3 3
IQR 3–9 3–4 3–7 3–3
Transport time, minc
Median 149 165 146 125
IQR 68–261 71–285 67–245 62–237
PCMC pHd
Median 7.34 7.31 7.33 7.20b
IQR 7.29–7.39 7.20–7.36 7.25–7.38 7.10–7.36 ISS
Median 25 29 25 29
IQR 16–26 19–38 17–34 25–44
Length of stay ICU, d
Median 2 2 3 2
IQR 1–6 1–6 1–7 0–6
GOS score
Median 5 4 5a 1b
IQR 5–5 1–5 3–5 1–5
Poor GOS score,n(%)e 10 (8) 33 (43) 13 (25)a 29 (71)b
Death,n(%) 5 (4) 28 (36) 9 (17)a 23 (56)b IQR indicates interquartile range.
aP⬍.05 compared with normotensive patients in the fully monitored group. bP⬍.05 compared with hypotensive patients in the fully monitored group. cData exclude 2 case subjects transported⬎24 hours after injury. dData are missing 22 values.
based on median and the lower 25th quartile. The reference groups for the tricategorical variables were the high-est pH and lowhigh-est ISS groups. A pseudomeasure of the variance ex-plained in the resulting logistic models was represented as a Nagelkerke’sR2.
Model fit was assessed with the Hos-mer and Lemeshow test statistic. Ad-justed odd ratios (aORs) with 95% CIs were calculated.
RESULTS
Of the 10 160 patients evaluated and treated for head injury during the study period, 311 patients met crite-ria for having moderate-to-severe head injury. Twelve patients were ex-cluded because of missing EMS/run
sheets. No significant difference in age, GCS score, or GOS score was found between the 12 excluded pa-tients and the 299 included papa-tients. Of the 299 patients, 245 (82%) had severe TBI, and 54 (18%) had moder-ate TBI. The median age was 6.5 years, and 60% were boys. Injuries mostly commonly occurred during the afternoon and evening hours. Median transport time was 148 min-utes. Care was provided by 187 dif-ferent agencies from 5 western states. The most common mecha-nisms of trauma were motor vehicle collisions (24%), falls (18%), and in-flicted injury (12%). The median post-resuscitation GCS score was 3, and
the median ISS was 25. The majority (94%) of children underwent CT im-aging during their prehospital/ED course. Children who did not un-dergo CT imaging died before it was possible to image or were taken di-rectly to the operating room for sur-gical intervention.
Hypotension and Monitoring
Hypotension was documented in 118 of 299 children (39%). Thirty-one percent of children were not monitored for blood pressure during a portion of their early care. Most often (89%) this occurred during the “scene” EMS time period. Documented hypotension was least likely to be treated at the scene (12%) and most likely to be treated in the ED at PCMC (86%).
Table 3 enumerates patient character-istics categorized by whether children were fully monitored for blood pres-sure at all of the sites. The 2 popula-tions were further subdivided by the absence or presence of documented hypotension. Children not fully
moni-tored for blood pressure were
younger and smaller than fully moni-tored children. In children without doc-umented hypotension, those who were not fully monitored had an increased risk of in-hospital death (RR: 4.5 [95% CI: 1.6 –12.7]) and an increased risk of poor GOS score at discharge (RR: 3.2 [95% CI: 1.5–7.9]) compared with chil-dren who were fully monitored. In the multivariate analysis, factors associ-ated with blood pressure monitoring included age (aOR: 1.2 [95% CI: 1.1– 1.2]) and severity of illness per the ISS, adjusted for blood pH. Compared with children with ISS 0 to 17, those with
scores ⬎25 were less likely to have
blood pressure documented (aOR: 0.4 [95% CI: 0.2– 0.8]). (Nagelkerke’sR2⫽
0.173) Thus, the absence of blood pres-sure monitoring was associated with young age, increased severity of ill-ness, and poor outcome.
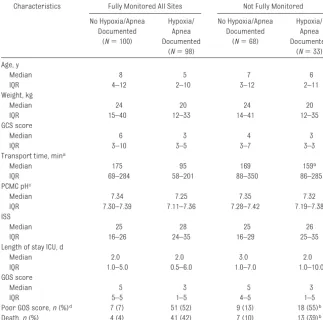
TABLE 4 Characteristics of Children Categorized by Adequacy of Saturation and Apnea Monitoring Characteristics Fully Monitored All Sites Not Fully Monitored
No Hypoxia/Apnea Documented
(N⫽100)
Hypoxia/ Apnea Documented
(N⫽98)
No Hypoxia/Apnea Documented
(N⫽68)
Hypoxia/ Apnea Documented
(N⫽33) Age, y
Median 8 5 7 6
IQR 4–12 2–10 3–12 2–11
Weight, kg
Median 24 20 24 20
IQR 15–40 12–33 14–41 12–35
GCS score
Median 6 3 4 3
IQR 3–10 3–5 3–7 3–3
Transport time, mina
Median 175 95 169 159b
IQR 69–284 58–201 88–350 86–285
PCMC pHc
Median 7.34 7.25 7.35 7.32
IQR 7.30–7.39 7.11–7.36 7.28–7.42 7.19–7.38 ISS
Median 25 28 25 26
IQR 16–26 24–35 16–29 25–35
Length of stay ICU, d
Median 2.0 2.0 3.0 2.0
IQR 1.0–5.0 0.5–6.0 1.0–7.0 1.0–10.0
GOS score
Median 5 3 5 3
IQR 5–5 1–5 4–5 1–5
Poor GOS score,n(%)d 7 (7) 51 (52) 9 (13) 18 (55)b
Death,n(%) 4 (4) 41 (42) 7 (10) 13 (39)b IQR indicates interquartile range.
aData exclude 2 case subjects transported⬎24 hours after injury. bP⬍.05 compared with hypotensive patients in the fully monitored group. cData are missing 22 values.
Hypoxia/Apnea and Monitoring
Hypoxia or apnea was documented in 131 (44%) of 299 children. Thirty-four percent of children were not moni-tored for oxygen saturation or apnea during a portion of their early care; most often this occurred at the scene
(69%). Documented hypoxia/apnea
also occurred most often at the scene. EMS personnel treated hypoxia/apnea 87% of the time. Air transport, commu-nity hospital, and PCMC ED personnel
treated hypoxia/apnea 100% of the time.
Table 4 addresses monitoring for hypoxia/apnea. Children with hypoxia
were significantly younger and
smaller than children without docu-mented hypoxia. Children with hypoxia and children who were not fully moni-tored had lower median GCS scores than children without documented hypoxia who were fully monitored. Fail-ure to monitor for hypoxia/apnea was
not associated with age, other mark-ers of injury severity, transport time, or poor outcome.
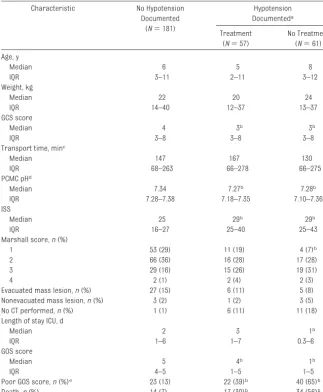
Response to Hypotension
Table 5 depicts provider response to hypotension as it relates to patient de-mographic and clinical characteris-tics. Patients were categorized by presence or absence of documented hypotension. Patients without docu-mented hypotension, including those who were not completely monitored, were classified as normotensive. If hy-potension was documented, patients were further subdivided by whether they were treated or untreated for hy-potension. Of the 118 children with documented hypotension, 57 (48%) were treated. Hypotensive children were more likely to die and had more severe Marshall CT scores, lower GCS scores, lower blood pH, and higher ISS compared with children without docu-mented hypotension. Children who were not treated for hypotension had significantly higher unadjusted risk of in-hospital death (RR: 1.9 [95% CI: 1.2– 3.0]) and poor GOS scores at discharge (RR: 1.7 [95% CI: 1.2–2.5]) compared with treated children. In a multi-variate model, adjusted for pH and Marshall CT scores, failure to treat documented hypotension was associ-ated with an increased odds of death (aOR: 3.400 [95% CI: 1.002–11.766]) and poor GOS score (aOR: 3.7 [95% CI: 1.1– 11.9]) compared with treated children (Nagelkerke’sR2⫽0.572 and 0.590,
re-spectively).
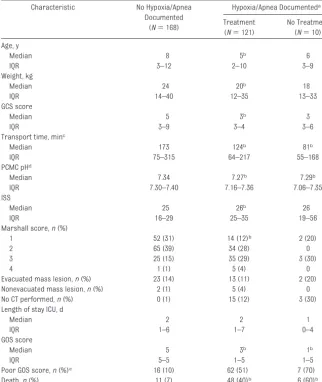
Response to Hypoxia/Apnea
Table 6 shows provider response to hypoxia or apnea as it relates to pa-tient demographic and clinical charac-teristics. The patients were catego-rized by presence or absence of documented hypoxia/apnea and were further subdivided by whether they were treated or untreated. Patients without documented hypoxia,
includ-TABLE 5 Characteristics of Children Categorized by Treated and Untreated Hypotension Characteristic No Hypotension
Documented (N⫽181)
Hypotension Documenteda
Treatment (N⫽57)
No Treatment (N⫽61) Age, y
Median 6 5 8
IQR 3–11 2–11 3–12
Weight, kg
Median 22 20 24
IQR 14–40 12–37 13–37
GCS score
Median 4 3b 3b
IQR 3–8 3–8 3–8
Transport time, minc
Median 147 167 130
IQR 68–263 66–278 66–275
PCMC pHd
Median 7.34 7.27b 7.28b
IQR 7.28–7.38 7.18–7.35 7.10–7.36
ISS
Median 25 29b 29b
IQR 16–27 25–40 25–43
Marshall score,n(%)
1 53 (29) 11 (19) 4 (7)b
2 66 (36) 16 (28) 17 (28)
3 29 (16) 15 (26) 19 (31)
4 2 (1) 2 (4) 2 (3)
Evacuated mass lesion,n(%) 27 (15) 6 (11) 5 (8) Nonevacuated mass lesion,n(%) 3 (2) 1 (2) 3 (5) No CT performed,n(%) 1 (1) 6 (11) 11 (18) Length of stay ICU, d
Median 2 3 1b
IQR 1–6 1–7 0.3–6
GOS score
Median 5 4b 1b
IQR 4–5 1–5 1–5
Poor GOS score,n(%)e 23 (13) 22 (39)b 40 (65)b
Death,n(%) 14 (7) 17 (30)b 34 (56)b
IQR indicates interquartile range.
aHypotension was documented: it includes both patients who were and were not fully monitored. bData are significantly different from the nonhypotension group.
cData exclude 2 case subjects transported⬎24 hours after injury. dTwenty-two values are missing.
ing those who were not completely monitored, were classified as nonhy-poxic. Of the 131 children with docu-mented hypoxia/apnea, 121 (92%) were treated. Children with hypoxia/ apnea were more likely to die and were younger with more severe Marshall CT scores, lower GCS scores, lower blood pH, and higher ISS compared with chil-dren without documented hypoxia/ apnea. The 10 children who were un-treated for hypoxia/apnea did not have a statistically higher unadjusted or ad-justed risk of death or poor GOS score compared with treated children.
Hypotension and Hypoxia Treatment and Association With Outcomes
To further explore the association among hypoxia, hypotension, and out-comes, models were developed (Table 7) that included both insults and whether the providers treated hypo-tension and/or hypoxia. Seventy-four children had both documented hypo-tension and hypoxia/apnea. Children with treated hypotension were no more likely to die or have a poor GOS score than children without
hypoten-sion, but children with untreated hypo-tension were significantly more likely to die (aOR: 8.9 [95% CI: 3.1–26.0]) or have a poor GOS score (aOR: 11.6 [95% CI: 4.0 –33.5]) compared with children without hypotension. Children with hypoxia/apnea had a worse GOS score whether they received treatment but were only more likely to die if they were untreated for hypoxia/apnea (aOR: 16.0 [95% CI: 1.6 –161.1]) com-pared with children without docu-mented hypoxia or apnea.
DISCUSSION
Our study demonstrates that early hy-potension and hypoxia/apnea are com-mon events in pediatric TBI and are strongly associated with worse out-comes. More than half of the children with documented hypotension and 8% of children with documented hypoxia/ apnea were untreated. Untreated chil-dren with hypotension were more likely to die or suffer severe disability. When both hypotension and hypoxia/ apnea were present, untreated hypoxia/ apnea was also associated with an in-creased risk of death and disability. At-tempted treatment was associated with improved outcome. Almost one third of children were not fully moni-tored in the prehospital and ED set-tings, especially those who were younger and sicker.
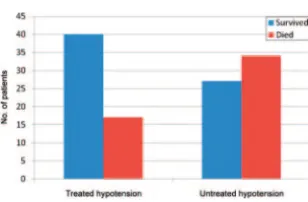
Hypotension was documented in 39% of our study population and was strongly associated with death (Fig 1). These findings are similar to existing
literature regarding brain-injured
adults5,7 and children.9,15,16 There is
some evidence that early hypotension (field or ED) predicts worse outcome than hypotension after admission to the PICU.8In our study, even in children
who received treatment for early hypo-tension, the risk of death was twice that of children without documented hypotension. However, left untreated, children with early hypotension were
TABLE 6 Characteristics of Children Categorized by Treated and Untreated Hypoxia/Apnea Characteristic No Hypoxia/Apnea
Documented (N⫽168)
Hypoxia/Apnea Documenteda
Treatment (N⫽121)
No Treatment (N⫽10) Age, y
Median 8 5b 6
IQR 3–12 2–10 3–9
Weight, kg
Median 24 20b 18
IQR 14–40 12–35 13–33
GCS score
Median 5 3b 3
IQR 3–9 3–4 3–6
Transport time, minc
Median 173 124b 81b
IQR 75–315 64–217 55–168
PCMC pHd
Median 7.34 7.27b 7.29b
IQR 7.30–7.40 7.16–7.36 7.06–7.35
ISS
Median 25 26b 26
IQR 16–29 25–35 19–56
Marshall score,n(%)
1 52 (31) 14 (12)b 2 (20)
2 65 (39) 34 (28) 0
3 25 (15) 35 (29) 3 (30)
4 1 (1) 5 (4) 0
Evacuated mass lesion,n(%) 23 (14) 13 (11) 2 (20) Nonevacuated mass lesion,n(%) 2 (1) 5 (4) 0 No CT performed,n(%) 0 (1) 15 (12) 3 (30) Length of stay ICU, d
Median 2 2 1
IQR 1–6 1–7 0–4
GOS score
Median 5 3b 1b
IQR 5–5 1–5 1–5
Poor GOS score,n(%)e 16 (10) 62 (51) 7 (70)
Death,n(%) 11 (7) 48 (40)b 6 (60)b
IQR indicates interquartile range.
aHypoxia/apnea was documented: this includes both patients who were and were not fully monitored. bData are significantly different from the nonhypoxic/apneic group.
cData exclude 2 case subjects transported⬎24 hours after injury. dTwenty-two values are missing.
⬃9 times as likely to die compared with children without documented hy-potension. If both hypoxia and sion were present, treated hypoten-sive patients did not have worse outcomes than patients without hypo-tension, illustrating the importance of treatment for hypotension.
Hypoxia occurred in 44% of our pa-tients, a finding similar to that re-ported in other TBI studies.5,7,9Children
with hypoxia had significantly worse outcomes than children without hyp-oxia, but we were unable to detect a significant difference in death for the treated versus untreated groups, ex-cept in the analysis that adjusted for the presence of both hypotension and hypoxia, suggesting that perhaps the neurologic insult from hypoxia is addi-tive to the insult from hypotension (Fig 2).
Although children were most likely to be hypotensive or hypoxic/apneic at the scene, they were least likely to
re-ceive corrective interventions for
these insults at the scene. Medical pro-viders at all sites of care were more likely to respond to hypoxia than hypo-tension. This finding does not seem to be because of restrictions on obtain-ing intravenous or intraosseous ac-cess. Resuscitation of critically injured children is a daunting task, and based on published reports, children
repre-sent ⬍10% of life-threatening EMS
prehospital calls.17,18 Su et al18
re-ported that children with hypotension and tachycardia were much less likely to receive an intravenous catheter during prehospital management when compared with adults with similar ab-normal physiologic parameters. Lack
of ongoing pediatric training for EMS personnel may be a contributing fac-tor, and although pediatric skills have been shown to deteriorate quickly without practice, continuing education in pediatric care is often not re-quired.19,20
A particularly worrisome finding in our study was the lack of consistent vital sign monitoring, especially in younger and sicker children. Worse outcomes and higher likelihoods of death were noted in the unmonitored population. Children were least likely to be fully monitored at the scene, where pre-sumably limited personnel were avail-able to resuscitate, monitor, and transport a critically injured child.
There were limitations in our study. Lack of complete documentation of vital signs by medical providers may have influenced our findings. Because vital signs were not consistently documented at all of the sites of care, hypotension or hypoxia/apnea may have occurred more frequently than we report. Increased risk of poor out-comes in the unmonitored patients without documented vital sign insta-bility suggests that unmonitored chil-dren were sicker, and if vital sign re-cording were more thorough, a greater proportion of children would have been classified as hypotensive and/or hypoxic. Thus, the associations be-tween hypotension and hypoxia and poor outcome or death were likely un-derestimated. Medical provider re-sponse to hypotension and hypoxia/ apnea was evaluated by consensus of 2 critical care physicians. We at-tempted to minimize subjective error by clearly defining the requirements for treatment. Medical providers were not expected to correct hypotension or hypoxia/apnea but rather to respond to and treat hypotension or hypoxia/ apnea. Although we controlled for in-jury severity on the multivariate mod-els, this may not sufficiently control for
FIGURE 1
Death and attempt to treat documented hypotension.
FIGURE 2
Death and attempt to treat documented hypoxia. TABLE 7 Presence of Hypoxia/Apnea and Hypotension: Treatment and Outcomes
Variable aOR CI
In-hospital deatha
No hypotension documented 1 Reference group
Hypotension treated 2.4 0.7–7.5
Hypotension untreated 8.9 3.1–26.0
No hypoxia/apnea documented 1 Reference group
Hypoxia/apnea treated 1.5 0.5–4.1
Hypoxia/apnea untreated 16.0 1.6–161.0
Nagelkerke’sR2⫽0.582
Poor GOS scorea
No hypotension documented 1 Reference group
Hypotension treated 2.9 1.0–8.4
Hypotension untreated 11.6 4.0–33.5
No hypoxia/apnea documented 1 Reference group
Hypoxia/apnea treated 2.9 1.2–7.4
Hypoxia/apnea untreated 26.2 2.5–280.2
Nagelkerke’sR2⫽0.637
risk of death or poor outcome. We did not collect ED length of stay data.
CONCLUSIONS
TBI is one of the most common disabling injuries in children. Adequate resuscita-tion is critical. All medical providers in-volved in the care of brain-injured chil-dren must be prepared to recognize and respond to hypotension and hypoxia/ apnea. The initial interventions are straightforward. For hypotension, place
an intravenous or intraosseous catheter and administer bolus isotonic fluid.21–23
For hypoxia, provide oxygen. For apnea, ventilate via bag mask or endotracheal intubation. As Chesnut et al7eloquently
pointed out more than a decade ago, “we see before us a clinical problem where any improvement has the poten-tial of making a significant impact on the outcome from head injury.” Fifteen years later, we are faced with the same challenge.
ACKNOWLEDGMENTS
This work was supported by a cooper-ative agreement (U07MC05036) from the Emergency Medical Services for Children Program in the Health Re-sources and Services Administration (Rockville, MD).
We acknowledge and extend our grati-tude to the trauma service nurse prac-titioners at Primary Children’s Medical Center who worked diligently to help complete this project.
REFERENCES
1. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview.J Head Trauma Rehabil.2006;21(5):375–378
2. Rutland-Brown W, Langlois JA, Thomas KE, Xi XL. Incidence of traumatic brain injury in the United States, 2003.J Head Trauma Rehabil.2006;21(6):544 –548
3. Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents: chapter 1–introduction.Pediatr Crit Care Med.2003;4(3 suppl):S2–S4
4. Gabriel EJ, Ghajar J, Jagoda A, Pons PT, Scalea T, Walters BC. Guidelines for prehospital manage-ment of traumatic brain injury.J Neurotrauma.2002;19(1):111–174
5. Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences.Arch Surg.2001;136(10):1118 –1123
6. Chi JH, Knudson MM, Vassar MJ, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study.J Trauma.2006;61(5):1134 –1141 7. Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining
outcome from severe head injury.J Trauma.1993;34(2):216 –222
8. Coates BM, Vavilala MS, Mack CD, et al. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury.Crit Care Med.2005;33(11):2645–2650 9. Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with
severe head injuries.J Pediatr Surg.1993;28(3):310 –316
10. Kokoska ER, Smith GS, Pittman T, Weber TR. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma.J Pediatr Surg.1998;33(2):333–338 11. 2005 American Heart Association for Cardiopulmonary Resuscitation and Emergency
Cardiovas-cular Care. Part 12: pediatric advanced life support.Circulation.2005;112(24 suppl):IV-167–IV-187 12. Baker SP, O’Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing
patients with multiple injuries and evaluating emergency care.J Trauma.1974;14(3):187–196 13. Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification
based on computed axial tomography.J Neurotrauma.1992;9(suppl 1):S287–S292
14. King JT Jr, Carlier PM, Marion DW. Early Glasgow Outcome Scale scores predict long-term func-tional outcome in patients with severe traumatic brain injury.J Neurotrauma.2005;22(9):947–954 15. Vavilala MS, Bowen A, Lam AM, et al. Blood pressure and outcome after severe pediatric traumatic
brain injury.J Trauma.2003;55(6):1039 –1044
16. Chiaretti A, Piastra M, Pulitano S, et al. Prognostic factors and outcome of children with severe head injury: an 8-year experience.Childs Nerv Syst.2002;18(3– 4):129 –136
17. Tsai A, Kallsen G. Epidemiology of pediatric prehospital care.Ann Emerg Med.1987;16(3):284 –292 18. Su E, Mann NC, McCall M, Hedges JR. Use of resuscitation skills by paramedics caring for critically
injured children in Oregon.Prehosp Emerg Care.1997;1(3):123–127
19. Su E, Schmidt TA, Mann NC, Zechnich AD. A randomized controlled trial to assess decay in acquired knowledge among paramedics completing a pediatric resuscitation course.Acad Emerg Med.
2000;7(7):779 –786
System.Emergency Care for Children: Growing Pains. Future of Emergency Care. Washington, DC: National Academies Press; 2007:xxii, 338
21. Banerjee S, Singhi SC, Singh S, Singh M. The intraosseous route is a suitable alternative to intravenous route for fluid resuscitation in severely dehydrated children.Indian Pediatr.1994; 31(12):1511–1520
22. Glaeser PW, Hellmich TR, Szewczuga D, Losek JD, Smith DS. Five-year experience in prehospital intraosseous infusions in children and adults.Ann Emerg Med.1993;22(7):1119 –1124
DOI: 10.1542/peds.2008-1006
2009;124;56
Pediatrics
and Susan L. Bratton
Michelle Zebrack, Christopher Dandoy, Kristine Hansen, Eric Scaife, N. Clay Mann
Services
Updated Information &
http://pediatrics.aappublications.org/content/124/1/56
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/124/1/56#BIBL
This article cites 22 articles, 1 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/trauma_sub
Trauma
sub
http://www.aappublications.org/cgi/collection/emergency_medicine_
Emergency Medicine
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2008-1006
2009;124;56
Pediatrics
and Susan L. Bratton
Michelle Zebrack, Christopher Dandoy, Kristine Hansen, Eric Scaife, N. Clay Mann
Injury
Early Resuscitation of Children With Moderate-to-Severe Traumatic Brain
http://pediatrics.aappublications.org/content/124/1/56
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.