Mosaic Tetrasomy 9p: A Mendelian
Condition Associated With
Pediatric-Onset Overlap Myositis
Marie-Louise Frémond, MDa,b, Cyril Gitiaux, MDc, Damien Bonnet, MD, PhDd, Tamazoust Guiddir, MDa, Yanick J. Crow, MD, PhDe,f, Loïc de Pontual, MD, PhDa, Brigitte Bader-Meunier, MDb,f
abstract
Pediatric-onset inflammatory myositis (IM) and systemic lupus erythematosus(SLE) are rare inflammatory diseases. Both result from the complex
interaction of genetic and environmental factors. An increasing number of Mendelian conditions predisposing to the development of SLE have been recently identified. These include monogenic conditions, referred to as the type I interferonopathies, associated with a primary upregulation of type I interferon (IFN), a key cytokine in the pathogenesis of SLE and some cases of
IM. Here, we report on a pediatric-onset inflammatory overlap phenotype in
a 6-year-old girl who was shown to carry mosaic tetrasomy 9p. The patient presented with myositis overlapping with lupuslike features. Myositis was characterized by a proximal muscular weakness and HLA class I antigen
myofiber overexpression on muscle biopsy. Lupus-like manifestations
consisted of pericarditis, pleuritis, and positive antinuclear and anti-SSA (Sjögren-syndrome A) antibodies. Complete remission was achieved with corticosteroids and mycophenolate mofetyl. Analysis of tetrasomy 9p showed mosaic tetrasomy in the 9p24.3q12 region, including the type I IFN cluster, and increased expression of IFN-stimulated genes. These data suggest that mosaic tetrasomy 9p can be associated with an upregulation of type I IFN signaling, predisposing to inflammatory myositis and lupus-like features. Thus, unexplained muscle or other organ involvement in patients carrying mosaic tetrasomy of the type IFN cluster of chromosome 9p should lead to the search for IM and/or lupuslike disease, and karyotype should be performed in patients with SLE or IM with mental retardation.
Pediatric-onset inflammatory myositis (IM) and systemic lupus erythematosus (SLE) are rare inflammatory
phenotypes. These disorders result from the complex interaction between genetic and environmental factors. An increasing number of Mendelian conditions predisposing to the development of pediatric SLE have been identified.1,2 These include
a number of so-called type I interferonopathies,3which are
Mendelian diseases associated with a primary upregulation of type I interferon (IFN), a key cytokine in SLE and some IM pathophysiology.4Herein,
we report for thefirst time that mosaic forms of tetrasomy 9p5can predispose
to the development of overlap myositis associated with type I IFN
upregulation.
PATIENT PRESENTATION
A 6-year-old girl was born at 35 weeks to unrelated parents. There was no family history of autoimmunity. She had moderate mental retardation, pervasive developmental disorder, hypertelorism, strabismus, and severe myopia. She had normal weight, height (+1 SD), and cranial perimeter growth. aService de Pédiatrie, Université Paris 13, Sorbonne Paris
Cité, Hôpital Jean Verdier, Assistance Publique–Hôpitaux de Paris (AP-HP), Bondy Cedex, France;bUnité d’Immunologie, Hématologie et Rhumatologie Pédiatriques;cService de Neurologie Pédiatrique, Centre de Référence des Maladies Neuromusculaires, Hôpital Necker Enfants Malades, AP-HP, Paris, France;dM3C-Necker, Congenital and Pediatric Cardiology, Hôpital Necker-Enfants Malades, AP-HP, Paris, France;eManchester Centre for Genomic Medicine, Institute of Human Development Faculty of Medical and Human Sciences, Manchester Academic Health Sciences Centre, University of Manchester, Manchester, United Kingdom; and f
Université Paris Descartes-Sorbonne Paris Cité, Institut Imagine, Laboratory of Neurogenetics and
Neuroinflammation, Paris, France
Drs Frémond and Gitiaux conceptualized and designed the study and drafted the initial manuscript; Drs Bonnet and Guiddir carried out the initial analyses and reviewed and revised the manuscript; Drs Crow, Bader-Meunier and de Pontual coordinated and supervised the study and critically reviewed the manuscript; and all authors approved thefinal manuscript as submitted. www.pediatrics.org/cgi/doi/10.1542/peds.2015-0724
DOI:10.1542/peds.2015-0724 Accepted for publication May 6, 2015
Address correspondence to Marie-Louise Frémond, MD, Service de Pédiatrie, Université Paris 13, Sorbonne Paris Cité, Hôpital Jean Verdier, AP-HP, F-93143 Bondy Cedex, France. E-mail: marie-louise. fremond@jvr.aphp.fr
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2015 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have nofinancial relationships relevant to this article to disclose.
FUNDING:No external funding.
Her standard karyotype, performed after her parents’informed consent, was mos47,XX,+i(9)(p10)[33]/46,XX [17]. Confirmation of isochromosome 9p was performed by interphase
fluorescence in situ hybridization (FISH) by using commercial chromosome 9 centromeric probes. Parental conventional karyotypes were normal. The patient was referred to the pediatric cardiology department because of respiratory and cardiac failure. Physical examination revealed facial edema and rash, erythema of the soles of the feet, livedo reticularis of the legs, proximal muscular weakness, and an enlargement of liver and spleen in addition to polypnea and
tachycardia. Chest radiograph revealed bilateral pleural effusions and echocardiography showed a large circumferential pericardial effusion. Laboratory investigations revealed an elevation of erythrocyte sedimentation rate of 33 mm/h
(normal values:,25 mm/h),
C-reactive protein of 52 mg/L (normal values:,5 mg/L), and platelet count of 5903109/L (normal values:,4003109/L). Serum creatine kinase, glutamine oxaloacetic transaminases, lactate dehydrogenase, and aldolase levels were normal. Positivity for
antinuclear antibodies (ANAs; 1:800) and anti-Ro/SSA was found. Anti-DNA and antiphospholipid antibodies were absent, and serum complement levels were within the normal range. Deltoid muscle biopsy revealed some myofibers with ischemic, punched-out vacuoles and a heterogeneous major histocompatibility complex (MHC) class I antigen (human leukocyte antigen [HLA]-ABC) myofiber overexpression (Fig 1). A cerebral computed tomodensitometry scan was normal. Three pericardiocenteses were required because of recurrences of her pericardial effusion within thefirst 3 weeks, in addition to 1 pleural thoracentesis. Pleural and pericardialfluid analysis found an
exudate containing a majority of lymphocytes and an increased number of macrophages and mesothelial cells, respectively. Pericardial biopsy revealed
lymphocytic infiltration. There was no evidence of infection. Three pulses of methylprednisolone, followed by an oral course of steroids in combination with mycophenolate mofetyl, resulted in a complete clinical remission.
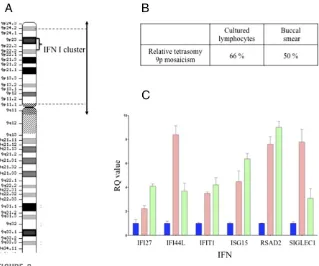
Comparative genomic hybridization arrays were used to better specify the breakpoints of the tetrasomy. The comparative genomic hybridization platform used was a single-nucleotide polymorphism microarray analysis performed by using the Illumina HumanHap300 BeadChips platform (Illumina, Inc, San Diego, CA). Data were analyzed with Genome Studio software (Illumina, Inc, San Diego, CA). Probe positions were established for all cases by using the Genome Browser Assembly hg19 (National Center for Biotechnology Information Build 37; http://genome.ucsc.edu/ cgi-bin/hgGateway). Mosaic tetrasomy concerns a 42-Mb region in 9p24.3q12 including 495 genes, among them 26 encoding for IFN (Fig 2A). Relative mosaicism in cultured lymphocytes and buccal smear was seen (Fig 2B). Single-nucleotide polymorphism array favored maternal origin of i(9p) with partial uniparental disomy of chromosome 9. An increased
expression of IFN-stimulated genes, a so-called IFN signature, was observed on 2 occasions (Fig 2C); specifically, the expression of 6 genes known to be IFN-stimulated was assessed from total RNA extracted from whole blood by using a PAXgene RNA isolation kit (PreAnalytix GmbH, Switzerland).
DISCUSSION
We report on a patient carrying a mosaic tetrasomy of 9p who presented with an overlap myositis. Myositis was characterized by a proximal muscular weakness and HLA class I antigen myofiber overexpression on muscle biopsy. Overlap features consisted of lupus manifestations comprising
pericarditis, pleuritis, and positive ANAs and anti-SSA antibodies.
Tetrasomy 9p has a highly variable phenotype due to the position of the breakpoints and number of cell lineages affected, resulting in differential levels and distribution of
mosaicism.5Tetrasomy 9p
mosaicism commonly leads to facial dysmorphy associated with
moderate to severe mental disability, mild growth retardation, renal and skeletal abnormalities, and
congenital heart disease. However, normal phenotypes are also
possible.6–8This report identifies, for
thefirst time to our knowledge, mosaic tetrasomy of 9p as
predisposing to the development of IM, which is associated with an upregulation of type I IFN activity.
Type I IFN plays a key role in the pathogenesis of SLE and some forms of IM.4In both diseases, type I IFN
genes are upregulated, and serum IFN-ais a biomarker of disease activity.4,9–11The genes encoding for
the 13 different IFN-aprotein isoforms are found together in a cluster on chromosome 9p22. In our patient, mosaic tetrasomy 9p resulted in a triplication of the 9p24.3q12 region, which includes a cluster of 17 genes encoding for type I IFN.12
FIGURE 1
Immunochemistry for anti-human MHC class I: overexpression of MHC class 1 antigens by myofibers in deltoid muscle biopsy. (Original magnification340.) MHC, major histocompati-bility complex.
As shown by the observation of an IFN signature, it appears that mosaic tetrasomy 9p results in an increased expression of genes induced by IFN, which is in accordance with the
findings of Zhuang et al13who
reported SLE-like disease associated with elevated levels of IFN-a/bin 2 patients with a trisomy of
9p–containing type I IFN cluster. Our report supports the hypothesis that several copies of the type I IFN cluster result in high levels of type I IFN due to a gene-dosage effect, and hence an increased susceptibility to some autoimmune diseases. Thus, we hypothesize that the presence of overlap myositis in our patient with tetrasomy of 9p reported here was related to the presence of 4 copies of the type I IFN gene cluster, and was not coincidental. However, the observation of a single patient is a limitation of our study, and additional reports are required to confirm the relationship between the
chromosomal aneuploidy and autoimmunity.
Pediatric-onset overlap myositis is characterized by the association of juvenile DM (dermatomyositis) or polymyositis with another autoimmune disease, such as SLE, scleroderma, juvenile idiopathic arthritis, inflammatory bowel disease, type 1 diabetes mellitus, or celiac disease.14An increasing number of
Mendelian conditions predisposing to the development of pediatric SLE have been identified.1,2To date,
3 major molecular pathways have been involved in the development of monogenic SLE: (1) a disturbed clearance of apoptotic material in complement deficiencies, leading to lupus in association with
a predisposition to infections15; (2)
an upregulated production of type I IFN in type I interferonopathies, including Aicardi-Goutières
syndrome,ACP5gene mutations
causing SLE associated with a skeletal
dysplasia, mutations inDNASE1L3
associated with lupus nephritis, and STING (Stimulator of interferon genes)-associated vasculopathy with onset in infancy (SAVI)3,16; and
(3) defective T-cell apoptosis coupled with dysregulated B-cell proliferation, which results from protein kinase Cddeficiency, leading to Mendelian lupus in association with
lymphoproliferative syndrome due to increased B-cell proliferation.17Until
now,ACP5gene mutation was the
only known monogenic condition associated with the development of IM.18The present report suggests
that mosaic tetrasomy of 9p might also predispose to this disease.
In conclusion, our report suggests that mosaic tetrasomy 9p predisposes to IM and lupuslike features. Our data implicate a dysregulation of type I IFN signaling due to the chromosomal aneuploidy. These observations emphasize that lupuslike disease and/or IM should be considered in patients with tetrasomy of 9p presenting with an acquired muscle or other organ involvement and that genetic studies should be performed in patients with mental retardation who develop IM and/or lupuslike features.
ACKNOWLEDGMENTS
We thank Dr Gillian I. Rice for her help and contribution, in particular for performing expression analyses of type I IFN–stimulated genes.
ABBREVIATIONS
ANA: antinuclear antibody IFN: interferon
IM: inflammatory myositis SLE: systemic lupus
erythematosus SSA: Sjögren-syndrome A
REFERENCES
1. Bader-Meunier B, Jeremiah N, Rieux-Laucat F. Childhood-onset systemic lupus erythematosus: polygenic or monogenic FIGURE 2
disorder? [in French].Rev Med Interne. 2013;34(4):230–233
2. Belot A, Cimaz R. Monogenic forms of systemic lupus erythematosus: new insights into SLE pathogenesis.Pediatr Rheumatol Online J. 2012;10(1):21
3. Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. 2011;1238;1:91–98
4. Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons.Curr Opin Immunol. 2006;18(6):676–682
5. Grass FS, Parke JC Jr, Kirkman HN, et al. Tetrasomy 9p: tissue-limited idic(9p) in a child with mild manifestations and a normal CVS result: report and review. Am J Med Genet. 1993;47(6):812–816
6. Baronchelli S, Conconi D, Panzeri E, et al. Cytogenetics of premature ovarian failure: an investigation on 269 affected women. J Biomed Biotechnol. 2011;2011:370195
7. McAuliffe F, Winsor EJT, Chitayat D. Tetrasomy 9p mosaicism associated with a normal phenotype.Fetal Diagn Ther. 2005;20(3):219–222
8. Papoulidis I, Kontodiou M, Tzimina M, et al. Tetrasomy 9p mosaicism associated with a normal phenotype in
two cases.Cytogenet Genome Res. 2012; 136(4):237–241
9. Kirou KA, Lee C, George S, Louca K, Peterson MGE, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease.Arthritis Rheum. 2005;52(5): 1491–1503
10. Becker-Merok A, Østli-Eilersten G, Lester S, Nossent J. Circulating interferon-a2 levels are increased in the majority of patients with systemic lupus erythematosus and are associated with disease activity and multiple cytokine activation.Lupus. 2013;22(2):155–163
11. Ernste FC, Reed AM. Recent advances in juvenile idiopathic inflammatory myopathies.Curr Opin Rheumatol. 2014; 26(6):671–678
12. Pestka S. The interferons: 50 years after their discovery, there is much more to learn.J Biol Chem. 2007;282(28): 20047–20051
13. Zhuang H, Kosboth M, Lee P, et al. Lupus-like disease and high interferon levels corresponding to trisomy of the type I
interferon cluster on chromosome 9p. Arthritis Rheum. 2006;54(5):1573–1579
14. Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood.Lancet. 2008; 371(9631):2201–2212
15. Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update.Ann Rheum Dis. 2014;73(9):1601–1606
16. Jeremiah N, Neven B, Gentili M, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like
manifestations.J Clin Invest. 2014; 124(12):5516–5520
17. Belot A, Kasher PR, Trotter EW, et al. Protein kinase cddeficiency causes Mendelian systemic lupus
erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 2013;65(8):2161–2171
18. Navarro V, Scott C, Briggs TA, et al. Two further cases of spondyloenchondrodysplasia
(SPENCD) with immune dysregulation. Am J Med Genet A. 2008;146A(21 146A): 2810–2815
DOI: 10.1542/peds.2015-0724 originally published online July 27, 2015;
2015;136;e544
Pediatrics
Crow, Loïc de Pontual and Brigitte Bader-Meunier
Marie-Louise Frémond, Cyril Gitiaux, Damien Bonnet, Tamazoust Guiddir, Yanick J.
Overlap Myositis
Mosaic Tetrasomy 9p: A Mendelian Condition Associated With Pediatric-Onset
Services
Updated Information &
http://pediatrics.aappublications.org/content/136/2/e544 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/136/2/e544#BIBL This article cites 18 articles, 2 of which you can access for free at:
Subspecialty Collections
ther_multisystem_disorders_sub
http://www.aappublications.org/cgi/collection/collagen_vascular_-_o Collagen Vascular & Other Multisystem Disorders
oskeletal_disorders_sub
http://www.aappublications.org/cgi/collection/rheumatology:muscul Rheumatology/Musculoskeletal Disorders
http://www.aappublications.org/cgi/collection/genetics_sub Genetics
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2015-0724 originally published online July 27, 2015;
2015;136;e544
Pediatrics
Crow, Loïc de Pontual and Brigitte Bader-Meunier
Marie-Louise Frémond, Cyril Gitiaux, Damien Bonnet, Tamazoust Guiddir, Yanick J.
Overlap Myositis
Mosaic Tetrasomy 9p: A Mendelian Condition Associated With Pediatric-Onset
http://pediatrics.aappublications.org/content/136/2/e544
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.
the American Academy of Pediatrics, 345 Park Avenue, Itasca, Illinois, 60143. Copyright © 2015 has been published continuously since 1948. Pediatrics is owned, published, and trademarked by Pediatrics is the official journal of the American Academy of Pediatrics. A monthly publication, it
at Viet Nam:AAP Sponsored on August 28, 2020
www.aappublications.org/news