Surgical Site Infection Reduction by the
Solutions for Patient Safety Hospital
Engagement Network
Joshua K. Schaffzin, MD, PhDa, Lory Harte, PharmD, CPHQb, Scott Marquette, MHSAc, Karen Zieker, MSd, Sharyl Wooton, MSd, Kathleen Walsh, MD, MScd, Jason G. Newland, MD, Medb
abstract
OBJECTIVE:Surgical site infections (SSIs) negatively affect patients and the healthcare system. National standards for SSI prevention do not exist in pediatric settings. We sought to reduce SSI-related harm by implementing a prevention bundle through the Solutions for Patient Safety (SPS) national hospital engagement network.
METHODS:Our study period was January 2011 to December 2013. We formed a national workgroup of content and quality improvement experts. We focused on 3 procedure types at high risk for SSIs: cardiothoracic, neurosurgical shunt, and spinal fusion surgeries. We used the Model for Improvement methodology and the Centers for Disease Control and Prevention SSI definition. After literature review and consultation with experts, we distributed a recommended bundle among network partners. Institutions were permitted to adopt all or part of the bundle and reported local bundle adherence and SSI rates monthly. Our learning network used webinars, discussion boards, targeted leader messaging, and in-person learning sessions.
RESULTS:Recommended bundle elements encompassed proper preoperative bathing, intraoperative skin antisepsis, and antibiotic delivery. Within 6 months, the network achieved 96.7% reliability among institutions reporting adherence data. A 21% reduction in SSI rate was reported across network hospitals, from a mean baseline rate of 2.5 SSIs per 100 procedures to a mean rate of 1.8 SSIs per 100 procedures. The reduced rate was sustained for 15 months.
CONCLUSIONS:Adoption of a SSI prevention bundle with concomitant reliability
measurement reduced the network SSI rate. Linking reliability measurement to standardization at an institutional level may lead to safer care.
Surgical site infections (SSIs) are common, accounting for nearly one-third of all health care–associated infections among hospitalized adults.1,2These infections increase patient morbidity and mortality and pose a high cost burden to the US health care system.3–5 In 1 study, the national SSI rate in children was reported to be 1.8%.6 Procedures that have been associated with higher SSI rates in children include
cardiothoracic, neurosurgical ventricular shunt, and spinal fusion surgeries. Reported rates of infection have large institutional variability: 2.3% to 5% for cardiothoracic,7–95.7% to 10.4% for neurosurgical ventricular shunt,10–12 and 4.4% to 10.2% for spinal fusion surgeries.13–16For this reason, these 3 types of procedures are commonly monitored for SSIs and targeted for SSI reduction.
a
Division of Hospital Medicine, anddChildren’s Hospitals’ Solutions for Patient Safety National Network, James M Anderson Center for Healthcare Systems Excellence, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio;bDepartment of Pediatrics, Children’s Mercy Hospital, Kansas City, Missouri; andcCS Mott Hospital Administration, University of Michigan Hospital and Health Systems, Ann Arbor, Michigan
Drs Schaffzin and Newland, Ms Harte, Mr Marquette, and Ms Wooton designed and executed
interventions; Ms Harte, Mr Marquette, Ms Zieker, Ms Wooton, and Drs Walsh and Newland reviewed and interpreted data and contributed to initial drafting and revision of the manuscript; Ms Zieker designed and executed data analysis; Dr Schaffzin drafted the initial manuscript and subsequent revisions; and all authors approved thefinal manuscript as submitted.
www.pediatrics.org/cgi/doi/10.1542/peds.2015-0580
DOI:10.1542/peds.2015-0580 Accepted for publication Jun 8, 2015
Address correspondence to Joshua K. Schaffzin, MD, PhD, Division of Infectious Diseases, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave MLC 7017, Cincinnati, OH 45229-3039. E-mail: joshua. schaffzin@cchmc.org
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2015 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have nofinancial relationships relevant to this article to disclose.
FUNDING:This improvement work was supported in part by the Centers for Medicare and Medicaid Services (grant HHSM-500-2012-0026C).
In 2011, a national hospital engagement network (HEN),
Solutions for Patient Safety (SPS), was established to eliminate harm in hospitalized children.17One goal of the HEN was to eliminate SSIs among cardiothoracic, neurosurgical
ventricular shunt, and spinal fusion surgeries. Previous work among a collaborative network of 8 children’s hospitals in Ohio demonstrated an increase in the number of months in which no SSI occurred when a common prevention bundle was adopted.18We describe the formation of a national pediatric SSI prevention network and its impact on reducing SSI rates among high-risk surgeries.
METHODS
Setting
Children’s Hospitals SPS is a national network of children’s hospitals working together to eliminate serious harm among children hospitalized in the United States.17At the time of the study, January 2011 through
December 2013, the network comprised 33 children’s hospitals nationwide. SPS was initiated as part of the federal Partnership for Patients initiative, a nationwide public-private collaboration to improve the quality, safety, and affordability of health care for all Americans.19SPS is the only HEN focused on pediatric patient safety and prevention of common hospital-acquired conditions.
Planning the Intervention
A network leadership team of subject matter and quality improvement experts was recruited. The Model for Improvement was used, including root cause and failure mode analysis and key driver identification.20 Analyses of process and outcome data were used to design interventions. Interventions were tested through a series of plan/do/study/act cycles.20The key driver diagram was updated periodically to incorporate
learning from observations, feedback, and testing and to guide future work. This work was determined not to be human subjects research and therefore was exempt from institutional review.
Operational Definitions
A detailed operational definition document was generated and shared with all network hospitals (see Supplemental Information). An included procedure was defined as a spinal fusion, neurosurgical ventricular shunt, or cardiothoracic surgery in which the chest was fully closed in the operating room, in concert with National Health Safety Network (NHSN) definitions at the time.21,22Procedures during which the patient had an active infection, growing construct adjustments, and refusion procedures were excluded. For neurosurgical ventricular shunts, facilities were given 2 options for reporting. The preferred method was dividing shunt procedures into 3 separate groups: primary shunts, secondary shunts, and revision shunts (Supplemental Appendix A). The second option was reporting all neurosurgical ventricular shunt procedures as 1 according to NHSN definitions.22
An SSI was defined according to definitions published by the Centers for Disease Control and Prevention and NHSN.21,22Changes made to the national definition in January 2013, which shortened the postoperative surveillance period for certain surgical procedures, were incorporated into the network’s outcome definition.22The incidence of SSI was measured as events per 100 included procedures.
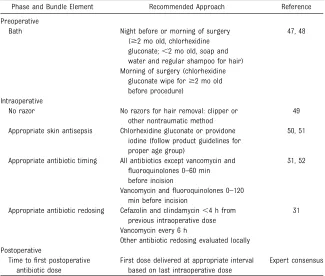
Based on published evidence and team consensus, 6 elements believed to reduce the risk of SSIs were included in a recommended bundle for all participating hospitals
(Table 1). The bundle was distributed to all network hospitals. Hospitals were given the option to implement
all or a set of individual bundle components. Hospitals were expected to measure the reliability of the bundle elements implemented. Reliability was measured as the number of audited opportunities per month in which all components of the locally implemented bundle were completed divided by the number of audited opportunities multiplied by 100.
Improvement Activities
Bundle implementation began in January 2012. The network SMART (specific, measurable, attainable, relevant, and time-based) aim was to reduce the mean network SSI rate from 2.5 to 1.5 SSIs per 100 procedures by December 31, 2013. This goal was determined by consensus among network partners that a 40% rate reduction would be considered a significant improvement. Achievement of this aim would rely on the following key drivers: reliable implementation of SSI prevention bundle, focus on 3 key surgical procedure types (cardiothoracic, neurosurgical ventricular shunt, and spinal fusion surgeries), transparency of data to drive continual learning and improvements, and effective use of high-reliability methods (Fig 1).
Training in Quality Improvement Methodology
We encouraged all institutions to use high-reliability methods, including regular reporting of process and outcomes measures with senior leaders, daily huddles for process failure review, systematic analysis of infections as they occurred, and promotion of a safety culture.23,24 Quality improvement science and application webinars were delivered monthly to network participants. The webinar curriculum followed the
Model for Improvement20and included use of hospital case studies to demonstrate methodology.
Network Dissemination Methods
SPS developed a learning network, based on the Institute for Healthcare Improvement Breakthrough Series methodology,25among member hospitals to improve several hospital-acquired conditions, including SSIs. SPS conducted regularly scheduled webinars and an annual in-person learning session. Webinars reviewed
the current process and outcome data and discussed successes and barriers with the bundle. The All Teach, All Learn philosophy of open sharing of successes, failures, and lessons learned was discussed in all
webinars. An SPS Sharepoint site was established for posting of relevant materials, as well as a discussion board where network members could post comments and questions. Network leaders were responsible for responding to questions and were available for informal coaching sessions for institutions as needed.
Data Collection and Analysis
Data on process and outcomes were collected locally and submitted on a monthly basis to SPS using a web-based form. Data submissions were due within 2 months, on the 10th of the month (eg, January data were due March 10). SSIs were counted according to the month when the procedure took place; hospitals could update the numbers of SSIs as they were identified. Network-level process control charts were maintained to track the bundle reliability (U-charts) and outcome measures (P-charts). Data were analyzed by using statistical process control methods. Initial centerline values were calculated by using the
first 12 months of measurement (January through December 2011). Special cause, a pattern of
performance that was not part of the existing system as a result of a change in the system, was defined as either
$8 consecutive points above or below the mean or any single point outside of the upper or lower control limits.26
RESULTS
Demographics of Hospital Network
The 33 HEN hospitals were located in 20 states and the District of Columbia. Regionally, 15% were located in the Northeast, 15% in the Southeast, 45% in the Midwest, 9% in the Southwest, and 15% in the Northwest. The
TABLE 1 Recommended Bundles
Phase and Bundle Element Recommended Approach Reference
Preoperative
Bath Night before or morning of surgery ($2 mo old, chlorhexidine gluconate;,2 mo old, soap and water and regular shampoo for hair)
47, 48
Morning of surgery (chlorhexidine gluconate wipe for$2 mo old before procedure)
Intraoperative
No razor No razors for hair removal: clipper or other nontraumatic method
49
Appropriate skin antisepsis Chlorhexidine gluconate or providone iodine (follow product guidelines for proper age group)
50, 51
Appropriate antibiotic timing All antibiotics except vancomycin and
fluoroquinolones 0–60 min before incision
31, 52
Vancomycin andfluoroquinolones 0–120 min before incision
Appropriate antibiotic redosing Cefazolin and clindamycin,4 h from previous intraoperative dose
31
Vancomycin every 6 h
Other antibiotic redosing evaluated locally Postoperative
Time tofirst postoperative antibiotic dose
First dose delivered at appropriate interval based on last intraoperative dose
Expert consensus
FIGURE 1
average number of licensed beds was 317 (range 72 to 595).
Bundle Adherence and SSI Rates
Of the 30 hospitals reporting baseline SSI rates, approximately one-third (8 to 10 hospitals per month) submitted data from January to December 2011. These data were used to calculate mean baseline reliability, which was 89.9%. The number of hospitals submitting reliability data increased steadily, with close to 90% of hospitals submitting reliability data by June 2013. Within 6 months, mean adherence increased to 93.7%, then reverted to a sustained rate of 91.4% (Fig 2).
The baseline network SSI rate for all 3 procedure types combined was 2.5 per 100 procedures. Within 10 months of network formation, the SSI rate decreased to 1.8 per 100 procedures and was sustained for 15 months (Fig 3). During the same time period, rate reduction after bundle implementation was also seen among cardiothoracic surgeries (1.7 to 1.3 per 100 procedures) and
neurosurgical ventricular shunt procedures (3.2 to 2.3 per 100 procedures). For spinal fusion surgeries, a reduction in rate (3.7 to 2.1 per 100 procedures) was observed, although the shift began before network formation (data not shown). Among individual hospitals, 12 (36%) of the 33 had a significant reduction (measured by a centerline shift) in SSI rates. Additionally, 27 (90%) of the 30 facilities reporting baseline data demonstrated
a significant reduction in SSI rates in at least 1 of the 3 procedure types followed.
DISCUSSION
The collaborative SPS HEN successfully reduced the SSI rate of cardiothoracic, neurosurgical ventricular shunt, and spinal fusion surgeries by 21%. This study is the
first in pediatrics to demonstrate on a national scale that increasing
reliability of part or all of a recommended SSI prevention bundle for high-risk surgeries can result in significant improvement.
Our choice of recommended bundle components was driven by high-quality, rigorous evidence. Because such evidence is limited for prevention of pediatric SSIs in the included high-risk surgeries,27–29the relevant pediatric literature was used along with adult literature. Surgical Care Improvement Project measures were evaluated because they represent a national standard and have been shown to reduce SSI rates among adult surgical patients.30We were careful to choose only those measures we believed would be most appropriate for pediatric populations. Specifically, we chose to not include proper choice of intraoperative antibiotic, despite its inclusion in adult guidance.31This decision was in part due to a lack at the time of comprehensive national guidance for antibiotic choice in pediatric populations. The most recent guidelines do include pediatric recommendations based on expert opinion.32
The network operational definitions for surgical procedures and SSIs were aligned with national standards for consistency across network hospitals. During the study period, the NHSN surveillance definition for SSI changed, shortening the surveillance period for tracked procedures from 365 to 90 days after the procedure.22 This change would be expected to reduce SSI rates, and therefore to affect our outcome measure. When we applied the new definition to data collected before the change, 3% of the reduction observed was due to the definition change (data not shown). The remainder of change, 21%, is how much we reduced the network SSI rate.
An important aspect of the improvement observed was the willingness of a diverse group of hospitals to work as a collaborative.
This strategy has been beneficial in improving other health care–acquired conditions, including central
line–associated bloodstream infections and ventilator-associated pneumonia.33–35As in similar cases, this allowed us to learn both as institutions and as a network. The Model for Improvement served as an excellent basis to enhance or initiate quality improvement work among network partners and ultimately to change outcomes. Network learning, rather than intense individual institutional training, seems to have been sufficient to enable groups to implement and measure successfully.
Institutional context is believed to affect organizational and practice change.36,37In our study, the SPS network partners represented a heterogeneous group of children’s hospitals with respect to geography, catchment area population, size, and quality improvement experience at the onset of our project. Furthermore, we permitted heterogeneity in the interventions implemented among institutions. Despite this heterogeneity, success was achieved at a network level in process reliability and outcome, and 27 of 30 hospitals reported a significant rate improvement in at least 1 area measured. Our project was based on the belief that standardization of processes with high reliability leads to safer care.38,39It is possible that the standardized approach played a significant role in the outcome improvement, perhaps to an extent equal to or greater than the bundle components themselves.
achieve our goal rate, we did achieve a∼21% improvement in overall SSI rate across the network (Fig 3). The establishment of clear goals is believed to be an important factor in quality improvement efforts,20which may have influenced our efforts as a network. In practice, many partners faced data-related challenges
that caused delays in SSI rate determination and prevented timely
SSI rate feedback to system participants. We instead focused on achieving high reliability with our processes, for which timely feedback was easily available. We used our rate chart as a long-term indicator of system change, in which movement toward our goal, if not beyond the goal, was considered a success.
High reliability is believed to be essential for a process to affect an
outcome.38,40The reliability of our system remained moderate throughout the study period. Therefore, it is unclear whether the increase in reliability observed during the study influenced the SSI rate directly. Specifically, system reliability improved by 1.5% (89.9% to 91.4%) and remained less than the.95% reliability standard established for other bundles.41,42However,
improvement in SSI rates may not be directly linked to bundle reliability. SSI prevention does notfit the original bundle model developed for catheter-associated infection or ventilator-associated pneumonia, mainly owing to a lack of a defined team in 1 geographic location. Additionally, a recently published SSI prevention bundle for colorectal surgery demonstrated significant improvement despite not demonstrating high reliability.43 Furthermore, significant
improvement in outcomes has been reported with lower reliability rates than observed in our study, as low as 51% in 1 instance.44–46Thus, it is likely that other factors contributed to the reduction in SSI rate, such as system learning and the development of a culture of safety, to which all SPS network partners commit.17,40
Interpretation of ourfindings is subject to limitations common to quality improvement methodology. Specifically, facilities selected which bundle components to implement, so interventions are likely not the same among all hospitals. Therefore, we cannot determine which bundle components most affected the SSI rate, nor can we determine to what degree, if any, adherence to a portion of the components influenced the outcome. Future studies are necessary to understand the interaction of practice standardization, adherence to bundle components, and the specific bundle components that result in the lowest SSI rates. Additionally, the change in SSI rates could have been due to a secular trend. Identification of special cause
FIGURE 2
P-chart depicting network bundle reliability. Baseline centerline was 89.9%.n, number of hospitals submitting data.
FIGURE 3
as well as sustainment of the lower SSI rate suggests it is indeed a result of our improvement work. Finally, our study was not designed to assess the effect on reliability and outcome of team composition, attendance at network sessions, or implementation strategy. Further study of these effects could inform future large-scale multicenter quality transformation efforts.
CONCLUSIONS
Using quality improvement methods to standardize care reduced SSI rates among high-risk pediatric surgeries. Despite the likely heterogeneity in actual interventions implemented across the network, we successfully reduced harm to patients and cost to the health care system. Future study is necessary to evaluate the effect of individual bundle elements on SSI prevention.
ACKNOWLEDGMENTS
We thank Kathy Ball for her
collaboration in the early part of this project, Dr Lloyd Provost for
statistical guidance, and Drs Steve Muething and Anne Lyren for their leadership and guidance. We also thank all of the network facility staff whose diligence and dedication ensured the success of this project.
ABBREVIATIONS
HEN: hospital engagement network
NHSN: National Healthcare Safety Network
SPS: Solutions for Patient Safety SSI: surgical site infection
REFERENCES
1. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002.Public Health Rep. 2007;122(2): 160–166
2. Magill SS, Hellinger W, Cohen J, et al. Prevalence of healthcare-associated
infections in acute care hospitals in Jacksonville, Florida.Infect Control Hosp Epidemiol. 2012;33(3):283–291
3. Scott R. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Division of Healthcare Quality Promotion National Center for Prevention, Detection, and Control of Infectious Diseases, Coordinating Center for Infectious Diseases, Centers for Disease Control and Prevention; 2009. Available at: www.cdc.gov/HAI/pdfs/hai/Scott_ CostPaper.pdf. Accessed June 2015
4. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs andfinancial impact on the US health care system.
JAMA Intern Med. 2013;173(22): 2039–2046
5. Lewis SS, Moehring RW, Chen LF, Sexton DJ, Anderson DJ. Assessing the relative burden of hospital-acquired infections in a network of community hospitals.Infect Control Hosp Epidemiol. 2013;34(11): 1229–1230
6. Bruny JL, Hall BL, Barnhart DC, et al. American College of Surgeons National Surgical Quality Improvement Program Pediatric: a beta phase report.J Pediatr Surg. 2013;48(1):74–80
7. Allpress AL, Rosenthal GL, Goodrich KM, Lupinetti FM, Zerr DM. Risk factors for surgical site infections after pediatric cardiovascular surgery.Pediatr Infect Dis J. 2004;23(3):231–234
8. Ben-Ami E, Levy I, Katz J, Dagan O, Shalit I. Risk factors for sternal wound infection in children undergoing cardiac surgery: a case-control study.J Hosp Infect. 2008; 70(4):335–340
9. Mehta PA, Cunningham CK, Colella CB, Alferis G, Weiner LB. Risk factors for sternal wound and other infections in pediatric cardiac surgery patients.
Pediatr Infect Dis J. 2000;19(10): 1000–1004
10. Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinalfluid shunt infection: a prospective study of risk factors.
J Neurosurg. 2001;94(2):195–201
11. Simon TD, Hall M, Riva-Cambrin J, et al; Hydrocephalus Clinical Research Network. Infection rates following initial cerebrospinalfluid shunt placement across pediatric hospitals in the United
States. Clinical article.J Neurosurg Pediatr. 2009;4(2):156–165
12. Kestle JR, Riva-Cambrin J, Wellons JC III, et al; Hydrocephalus Clinical Research Network. A standardized protocol to reduce cerebrospinalfluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative.J Neurosurg Pediatr. 2011;8(1): 22–29
13. Linam WM, Margolis PA, Staat MA, et al. Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure.Infect Control Hosp Epidemiol. 2009;30(2):109–116
14. Cahill PJ, Warnick DE, Lee MJ, et al. Infection after spinal fusion for pediatric spinal deformity: thirty years of experience at a single institution.Spine. 2010;35(12):1211–1217
15. Sponseller PD, Shah SA, Abel MF, Newton PO, Letko L, Marks M. Infection rate after spine surgery in cerebral palsy is high and impairs results: multicenter analysis of risk factors and treatment.Clin Orthop Relat Res. 2010;468(3):711–716
16. Mackenzie WG, Matsumoto H, Williams BA, et al. Surgical site infection following spinal instrumentation for scoliosis: a multicenter analysis of rates, risk factors, and pathogens.J Bone Joint Surg Am. 2013;95(9):800–806, S1–S2
17. Children’s Hospitals Solutions for Patient Safety. solutionsforpatientsafety.org/. Accessed January 23, 2015
18. Toltzis P, O’Riordan M, Cunningham DJ, et al. A statewide collaborative to reduce pediatric surgical site infections.
Pediatrics. 2014;134(4). Available at: www.pediatrics.org/cgi/content/full/134/ 4/e1174
19. Centers for Medicare and Medicaid Services. Partnerships for Patients. Available at: http:// partnershipforpatients.cms.gov/. Accessed January 23, 2015
20. Langley GJ, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP.The Improvement Guide: A Practical Approach to
Enhancing Organizational Performance, 2nd ed. San Francisco, CA: Jossey Bass; 2009
safety network.Infect Control Hosp Epidemiol. 2011;32(10):970–986
22. Centers for Disease Control and Prevention. Surgical Site Infection (SSI) Event. National Healthcare Safety Network Patient Safety Component Manual, 2013:9-1–9-14. www.cdc.gov/ nhsn/PDFs/pscManual/9pscSSIcurrent. pdf. Accessed June 2015
23. Sutcliffe KM. High reliability
organizations (HROs).Best Pract Res Clin Anaesthesiol. 2011;25(2):133–144
24. Halligan M, Zecevic A. Safety culture in healthcare: a review of concepts, dimensions, measures and progress.
BMJ Qual Saf. 2011;20(4):338–343
25. Institute for Healthcare Improvement. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series White Paper. Boston: 2003. Available at: www.IHI.org. Accessed June 2015
26. Provost LP, Murray SK.The health care data guide: learning from data for improvement. San Francisco, CA: Jossey-Bass; 2011
27. Glotzbecker MP, Riedel MD, Vitale MG, et al. What’s the evidence? Systematic literature review of risk factors and preventive strategies for surgical site infection following pediatric spine surgery.J Pediatr Orthop. 2013;33(5): 479–487
28. Prusseit J, Simon M, von der Brelie C, et al. Epidemiology, prevention and management of ventriculoperitoneal shunt infections in children.Pediatr Neurosurg. 2009;45(5):325–336
29. Murray MT, Corda R, Turcotte R, Bacha E, Saiman L, Krishnamurthy G.
Implementing a standardized perioperative antibiotic prophylaxis protocol for neonates undergoing cardiac surgery.Ann Thorac Surg. 2014; 98(3):927–933
30. Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections.JAMA. 2010; 303(24):2479–2485
31. Bratzler DW, Houck PM; Surgical Infection Prevention Guidelines Writers
Workgroup; American Academy of Orthopaedic Surgeons; American
Association of Critical Care Nurses; American Association of Nurse Anesthetists; American College of Surgeons; American College of Osteopathic Surgeons; American Geriatrics Society; American Society of Anesthesiologists; American Society of Colon and Rectal Surgeons; American Society of Health-System Pharmacists; American Society of PeriAnesthesia Nurses; Ascension Health; Association of periOperative Registered Nurses; Association for Professionals in Infection Control and Epidemiology; Infectious Diseases Society of America; Medical Letter; Premier; Society for Healthcare Epidemiology of America; Society of Thoracic Surgeons; Surgical Infection Society. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project.Clin Infect Dis. 2004;38(12): 1706–1715
32. Bratzler DW, Dellinger EP, Olsen KM, et al; American Society of Health-System Pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery.Am J Health Syst Pharm. 2013;70(3):195–283
33. Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia.Jt Comm J Qual Patient Saf. 2005;31(5):243–248
34. Miller MR, Griswold M, Harris JM II, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts.Pediatrics. 2010; 125(2). Available at: www.pediatrics.org/ cgi/content/full/125/2/e206
35. Berenholtz SM, Lubomski LH, Weeks K, et al; On the CUSP: Stop BSI program. Eliminating central line-associated bloodstream infections: a national patient safety imperative.Infect Control Hosp Epidemiol. 2014;35(1):56–62
36. Kaplan HC, Brady PW, Dritz MC, et al. The influence of context on quality
improvement success in health care: a systematic review of the literature.
Milbank Q. 2010;88(4):500–559
37. Tomoaia-Cotisel A, Scammon DL, Waitzman NJ, et al. Context matters: the experience of 14 research teams in systematically reporting contextual
factors important for practice change.
Ann Fam Med. 2013;11(suppl 1): S115–S123
38. Nolan T, Resar R, Haraden C, Griffin FA. Improving the Reliability of Health Care. IHI Innovation Series White Paper. Boston: 2004. Available at: www.IHI.org. Accessed June 2015
39. Luria JW, Muething SE, Schoettker PJ, Kotagal UR. Reliability science and patient safety.Pediatr Clin North Am. 2006;53(6):1121–1133
40. Resar RGF, Haraden C, Nolan TW. Using Care Bundles to Improve Health Care Quality. IHI Innovation Series White Paper. Boston: 2012. Available at: www.IHI.org. Accessed June 2015
41. Pogorzelska M, Stone PW, Furuya EY, et al. Impact of the ventilator bundle on ventilator-associated pneumonia in intensive care unit.Int J Qual Health Care. 2011;23(5):538–544
42. Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. Central line bundle implementation in US intensive care units and impact on bloodstream infections.PLoS ONE. 2011; 6(1):e15452
43. Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings.
JAMA Surg. 2014;149(10):1045–1052
44. Hawe CS, Ellis KS, Cairns CJ, Longmate A. Reduction of ventilator-associated pneumonia: active versus passive guideline implementation.Intensive Care Med. 2009;35(7):1180–1186
45. Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study.Crit Care. 2005;9(6):R764–R770
46. Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality.Crit Care Med. 2007;35(4):1105–1112
infection, 1999.Infect Control Hosp Epidemiol. 1999;20(4):250–278, quiz 279–280
48. National Institute for Health and Care Excellence. Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. 2008. Available at: www.nice.org.uk/guidance/cg74/ evidence/cg74-surgical-site-infection-full-guideline2. Accessed January 23, 2015
49. Tanner J, Norrie P, Melen K. Preoperative hair removal to reduce surgical site infection.Cochrane Database Syst Rev. 2011;(11):CD004122
50. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis.N Engl J Med. 2010;362(1):18–26
51. Swenson BR, Hedrick TL, Metzger R, Bonatti H, Pruett TL, Sawyer RG. Effects of
preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols.Infect Control Hosp Epidemiol. 2009;30(10):964–971
DOI: 10.1542/peds.2015-0580 originally published online October 5, 2015;
2015;136;e1353
Pediatrics
Kathleen Walsh and Jason G. Newland
Joshua K. Schaffzin, Lory Harte, Scott Marquette, Karen Zieker, Sharyl Wooton,
Engagement Network
Surgical Site Infection Reduction by the Solutions for Patient Safety Hospital
Services
Updated Information &
http://pediatrics.aappublications.org/content/136/5/e1353 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/136/5/e1353#BIBL This article cites 41 articles, 5 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/surgery_sub Surgery
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su Infectious Disease
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2015-0580 originally published online October 5, 2015;
2015;136;e1353
Pediatrics
Kathleen Walsh and Jason G. Newland
Joshua K. Schaffzin, Lory Harte, Scott Marquette, Karen Zieker, Sharyl Wooton,
Engagement Network
Surgical Site Infection Reduction by the Solutions for Patient Safety Hospital
http://pediatrics.aappublications.org/content/136/5/e1353
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
http://pediatrics.aappublications.org/content/suppl/2015/09/29/peds.2015-0580.DCSupplemental Data Supplement at:
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.