Letters to the Editor
Statements appearing here are those of the writers and do not represent the official position of the American Academy of Pediatrics, Inc. or its Committees. Comments on any topic, including the contents ofPediatrics,are invited from all members of the profession: those accepted for publication will not be subject to major editorial revision but generally must be no more than 400 words in length. The editors reserve the right to publish replies and may solicit responses from authors and others.
• • •
Letters should be submitted in duplicate in double-spaced typing on plain white paper with name and address of sender(s) on the letter. Send them to Jerold F. Lucey, MD, Editor, Pediatrics Editorial Office, Fletcher Allen Health Care, Burlington, VT 05401.
Fungemia in Neonates: Report of 80 Cases From
Seven University Hospitals
To the Editor.—
Candidemia is an emerging infection in neonatology, due to multiple risk factors for fungal infections in neonates, eg, central venous catheter (CVC) insertion, total parenteral nutrition (TPN), corticosteroid and antibiotic therapy, very low birth weight (VLBW) and arteriovenous support (AV).1–3
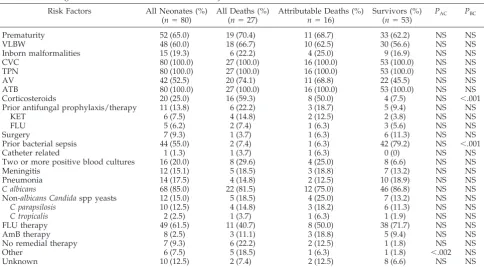
Within 10 years, from 1989 to 1998, a prospective nation-wide survey of fungemia in 7 university children’s clinics in Slovakia was performed. A total of 310 fungemias developed; 145 appeared in children (48.5%) and 80 (55.3% of all children and 23.3% of all cases) appeared in neonates. The most common risk factors (Table 1) for fungemia in neonates were prior antibiotic therapy, CVC insertion, and TPN, which appeared in all cases. Prematurity was diagnosed in 70.4% and very low birth weight in 66.7%.
The majority of fungemias were caused by Candida albicans, 85%, and non-albicans Candidaspp, 15% (C parapsilosis, 12.5%;C tropicalis, 2.5%). Table 1 presents a univariate analysis (Mantel-Haenzsel2test) of risk factors for mortality comparing 16 attrib-utable deaths (20% infection-associated mortality) to survivors (53
cases). Overall mortality (including deaths due to underlying dis-ease) was 32.5%.
There were no significant predictors of fungal infection-associ-ated deaths except previous use of corticosteroids (50% among attributable deaths vs 7.5% among survivors, P⬍.001).
As shown in Table 2, specific risk factors were documented to be statistically significant for neonatal fungemia, such as concomitant bacterial sepsis (P ⬍ .001), CVC, AV, TPN, and antibiotic therapy (all P ⬍ .001). Comparing neonates with other children, CVC, AV, TPN, and prior antibiotic therapy (Table 2; P ⬍ .01–.001) were significantly related to neonatal fungemia.C albicanscaused fungemia in neonates significantly more frequently than in other children (85.0 vs 52.0%,P⬍.001). Conversely, non-albicans Candida spp and non-Candida spp yeasts caused fungemia significantly more frequently in other children than in neonates (P⬍.035 and .015). However, mor-tality in both groups was similar.
Two risk factors always have been described: multiple pos-itive blood cultures were described by Viscoli et al1 to be associated with inferior outcome; Spanik et al4documented that inappropriate antifungal therapy is related to higher mortality. Our study did not describe higher attributable mortality in neonates having more than two positive blood cultures and, surprisingly, in those not appropriately treated. Only one risk factor (receipt of corticosteroids) was significantly related to
TABLE 1. Fungemia in Neonates—Predictors of Mortality
Risk Factors All Neonates (%) (n⫽80)
All Deaths (%) (n⫽27)
Attributable Deaths (%)
n⫽16)
Survivors (%) (n⫽53)
PAC PBC
Prematurity 52 (65.0) 19 (70.4) 11 (68.7) 33 (62.2) NS NS
VLBW 48 (60.0) 18 (66.7) 10 (62.5) 30 (56.6) NS NS
Inborn malformalities 15 (19.3) 6 (22.2) 4 (25.0) 9 (16.9) NS NS
CVC 80 (100.0) 27 (100.0) 16 (100.0) 53 (100.0) NS NS
TPN 80 (100.0) 27 (100.0) 16 (100.0) 53 (100.0) NS NS
AV 42 (52.5) 20 (74.1) 11 (68.8) 22 (45.5) NS NS
ATB 80 (100.0) 27 (100.0) 16 (100.0) 53 (100.0) NS NS
Corticosteroids 20 (25.0) 16 (59.3) 8 (50.0) 4 (7.5) NS ⬍.001
Prior antifungal prophylaxis/therapy 11 (13.8) 6 (22.2) 3 (18.7) 5 (9.4) NS NS
KET 6 (7.5) 4 (14.8) 2 (12.5) 2 (3.8) NS NS
FLU 5 (6.2) 2 (7.4) 1 (6.3) 3 (5.6) NS NS
Surgery 7 (9.3) 1 (3.7) 1 (6.3) 6 (11.3) NS NS
Prior bacterial sepsis 44 (55.0) 2 (7.4) 1 (6.3) 42 (79.2) NS ⬍.001
Catheter related 1 (1.3) 1 (3.7) 1 (6.3) 0 (0) NS NS
Two or more positive blood cultures 16 (20.0) 8 (29.6) 4 (25.0) 8 (6.6) NS NS
Meningitis 12 (15.1) 5 (18.5) 3 (18.8) 7 (13.2) NS NS
Pneumonia 14 (17.5) 4 (14.8) 2 (12.5) 10 (18.9) NS NS
C albicans 68 (85.0) 22 (81.5) 12 (75.0) 46 (86.8) NS NS
Non-albicans Candidaspp yeasts 12 (15.0) 5 (18.5) 4 (25.0) 7 (13.2) NS NS
C parapsilosis 10 (12.5) 4 (14.8) 3 (18.2) 6 (11.3) NS NS
C tropicalis 2 (2.5) 1 (3.7) 1 (6.3) 1 (1.9) NS NS
FLU therapy 49 (61.5) 11 (40.7) 8 (50.0) 38 (71.7) NS NS
AmB therapy 8 (2.5) 3 (11.1) 3 (18.8) 5 (9.4) NS NS
No remedial therapy 7 (9.3) 6 (22.2) 2 (12.5) 1 (1.8) NS NS
Other 6 (7.5) 5 (18.5) 1 (6.3) 1 (1.8) ⬍.002 NS
attributable deaths, probably because corticosteroids were used in high-risk neonates with higher APACHE score (sepsis, men-ingitis, etc). Several small series of neonatal fungemia were published.2– 6The majority of them were due toC albicans. Other species in combination with other groups of children (e.g. chil-dren with cancer, where non-albicans Candida spp and
non-Candidaspp reported 50 – 60%7were rare.C. parapsilosis, a can-cer pathogen related to use of vascular catheters in children5 caused 10 episodes (12.5%) in our group of neonates. Attribut-able mortality in our group of 80 neonates was 20%. Other small studies on neonates reporting 12 to 40 cases showed 19 to 54% mortality. However, none of them distinguished between over-all and fungemia-associated mortality; therefore, we assume that attributable mortality probably would be lower in those studies. In conclusion, this is, according to our knowledge, the largest series of fungemia in neonates published.
V. Krcˇme´ry M. Fricˇ
M. Pisarcˇikova´ M. Huttova´ J. Filka K. Kralinsky´ H. Hupkova´ J. Hanzen J. Trupl M. Lisˇkova´
University of Trnava School of Public Health Departments of Paediatrics
Bratislava, Petrzˇalka, Kosˇice, Banska´ Bystrica
Department of Microbiology Bratislava, Nitra, Kosˇice,
Banska´ Bystrica Heydukova 10 812 50 Bratislava Slovak Republic
REFERENCES
1. Viscoli C, Girmenia C, Marinus A, et al. Candidemia in cancer patients: results of a prospective, multicenter surveillance study in Europe.Clin Infect Dis.In press
2. Stamos JK, Rowley AH. Candidemia in pediatric population.Clin Infect Dis.1995;20:571–575
3. Roillides E, Kadiltsoglou I, Zahides D, Bibaski E. Invasive candidiasis in pediatric patients.Clin Microbiol Infect.1997;4:192–195
4. Huttova´ M, Filka J, Kurak J, Kralinsky K, Krcmery V Jr.Candida funge-mia in neonates treated with fluconazole.Pediatr Infect Dis J.1998;17: 1112–1116
5. McDonald L, Baker C, Chenoweth C. Risk factors for candidemia in a children’s hospital.Clin Infect Dis.1998;26:642– 645
6. Kaiserova E, Krcmery V Jr. Fungemia in children with cancer.Pediatr Infect Dis J.1998;19:844 – 845
7. Walsh JT, Gonzalez C, Roilides E, et al. Fungemia in children infected with the human immunodeficiency virus: new epidemiologic patterns, emerging pathogens, and improved outcome with antifungal therapy.
Clin Infect Dis.1995;20:900 –906
TABLE 2. Neonatal Versus Other Pediatric (Non-Neonatal) Fungemia: Risk Factors, Therapy, and Outcome
Neonates (%) (n⫽80)
Other Children (%) (n⫽75)
P
Underlying disease
Two or more positive blood cultures 16 (20.0) 15 (20.0) NS
Trauma 1 (1.3) 7 (9.5) ⬍.03
Bacterial sepsis 44 (55.0) 15 (20.0) NS
Prior surgery 7 (8.8) 13 (17.2) NS
Diabetes mellitus 1 (1.3) 1 (1.4) NS
CVC 80 (100.0) 43 (65.4) ⬍.001
AV 42 (52.5) 17 (17.7) ⬍.001
TPN 80 (100.0) 21 (27.3) ⬍.001
Prior antibiotic therapy 80 (100.0) 57 (76.0) ⬍.001
Fungemia during antifungal prophylaxis/therapy (breakthrough) 11 (13.7) 22 (29.2) ⬍.03
During therapy with FLU 6 (7.5) 8 (10.8) NS
During therapy with KET 5 (6.3) 8 (10.8) NS
During therapy with AmB 0 (0) 5 (6.8) ⬍.025
Catheter-related fungemia 1 (1.3) 8 (10.8) ⬍.035
Etiology
C albicans 68 (85.0) 39 (52.0) ⬍.0014
Non-albicans Candidaspp 12 (12.5) 23 (30.6) ⬍.035
C parapsilosis 10 (12.5) 11 (14.6) NS
C tropicalis 2 (2.5) 3 (4.0) NS
C glabrata 0 (0) 3 (4.0) NS
C krusei 0 (0) 3 (4.0) NS
C guillermondii 0 (0) 3 (4.0) NS
Non-Candidaspp yeasts 0 (0) 6 (8.0) ⬍.015
Number of positive blood cultures 1.6 1.8 NS*
Fungemia due to in vitro resistant yeasts 1 (1.3) 3 (4.0) NS
Therapy
FLU 49 (61.5) 40 (53.) NS
AmB 8 (12.5) 11 (14.6) ⬍.035
FLU⫹AmB 6 (7.5) 2 (2.7) NS
No remedial therapy 7 (8.8) 11 (14.7) NS
Outcome
Cure 53 (66.3) 39 (52.0) NS
Death due to underlying disease with fungemia 11 (13.8) 7 (9.5) NS
Death due to fungemia (attributable mortality) 16 (20.0) 19 (25.3) NS
Overall mortality 27 (33.8) 36 (48.0) NS
Insulin Infusions in Extremely Low Birth Weight
Infants
To the Editor.—
We read with interest the article by Fuloria et al on insulin infusions in extremely low birth weight (ELBW) infants.1 The authors concluded that priming the tubing with a higher concen-tration of insulin (5 U/mL) before the initiation of a standard insulin infusion therapy should accelerate the achievement of steady-state insulin delivery and correction of hyperglycemia in ELBW.1This approach would require two insulin concentrations for the routine preparation of these infusions. In our view, this increases the likelihood of errors, especially during shifts when less experienced personnel are present in the NICU. To combat this problem and minimize the loss of insulin in the infusion system, we have, until recently, added serum albumin to the insulin infusion solutions. However, shortages and costs of albu-min have led us to retrospectively compare the effectiveness of insulin infusions in ELBW infants with and without albumin. The albumin insulin solutions had 10 mL of the 5% albumin added to the solution, and the nonalbumin insulin infusions were prepared by flushing the microbore tubing with 10 mL of the insulin ad-mixture. The tubing and syringe were allowed to stand for at least 30 minutes prior to use. The demographics of the patients are presented in Table 1. The insulin infusion rates and blood sugars are shown in Table 2. In comparing the two groups on day 1, the patients whose insulin admixture did not contain albumin dem-onstrated a slower decline in glucose. However, by 12 hours, the two groups appeared to have very similar insulin requirements and the blood glucose levels were consistently⬍200 mg/dL (11.1 mmol/L). Although the time difference between the two groups in achieving serum glucose levels of⬍200 mg/dL is about 12 hours, clinically and physiologically it is not clear whether this would compromise the care of the patient.2,3
We also analyzed the cost of using albumin in the preparation of the insulin admixtures, and the estimated annual cost was $47 328. Therefore, not adding albumin to the insulin admixture results in a considerable cost savings and avoids the exposure of the patient to human sera.
In conclusion, it appears that adequate control of hyperglyce-mia is achieved by flushing the intravenous tubing with 10 mL of insulin admixture and allowing the insulin syringe and tubing to sit for 30 minutes.
Minyon Avent, PharmD* Jonathan Whitfield, MBChB‡
*Department of Pharmacy and ‡Neonatal Intensive Care Unit, Department of Pediatrics
Baylor University Medical Center Dallas, TX 75246
REFERENCES
1. Fuloria M, Friedberg MA, DuRant RH, Aschner JL. Effect of flow rate and insulin priming on the recovery of insulin from microbore infusion tubing.Pediatrics.1998;102:1401–1406
2. Pildes RS. Neonatal hyperglycemia.J Pediatr.1986;109:905–907 3. Lilien LD, Rosenfield RL, Baccaro MM, Pildes RS. Hyperglycemia in
stressed small premature neonates.J Pediatr.1979;94:454 – 459
In Reply.—
We thank Drs. Avent and Whitfield for their comments on our article “Effect of Flow Rate and Insulin Priming on the Recovery of Insulin from Microbore Infusion Tubing,” in which we compared
insulin recovery from unprimed infusion tubing and tubing primed with a higher concentration of insulin. Similar to our own data, Drs. Avent and Whitfield found a 12-hour delay in correction of hyperglycemia when the insulin infusion was administered through tubing flushed for 30 minutes with 10 mL of the “insulin admixture” (presumably 0.2 U/mL) compared with administra-tion through tubing flushed with 5% albumin. Their informaadministra-tion on the cost associated with the use of 5% albumin, in addition to the risk of exposure to human sera, provides a strong disincentive to this practice. They also point out that use of two different insulin concentrations may increase the likelihood of errors in the nursery. We believe this risk can be circumvented with the proper safeguards. It requires less than 1 mL of insulin (5 U/mL) to prime the infusion tubing. This can be dispensed by the pharmacy in a small, properly labeled 1-mL syringe. The standard insulin infu-sion solution (0.2 U/mL) used to flush the tubing after priming, and for continuous insulin infusion to patients, should be dis-pensed in a large (20- or 50-mL) syringe. This approach should make the possibility of errors remote.
Drs Avent and Whitfield imply that a delay of 12 hours before correction of hyperglycemia is unlikely to compromise patient care. However, there is little evidence to support or refute that supposition. Persistent hyperglycemia can be detrimental to in-fants, resulting in osmotic diuresis and alterations in blood osmo-lality, which can affect the integrity of the blood-brain barrier and increase the risk of intraventricular hemorrhage.1,2Ideally, when a continuous infusion of insulin is deemed clinically indicated, the delivery method should result in rapid correction of hyperglyce-mia and utilize the lowest possible insulin infusion rate. Apropos, we found it interesting that the insulin infusion rates used by Drs. Avent and Whitfield in the no-albumin group were higher than those infused through tubing with albumin. We believe that the higher infusion rates required to correct hyperglycemia in neo-nates receiving infusions through unprimed tubing increases the risk of hypoglycemia once saturation of the binding sites on the tubing has occurred. We are currently conducting a randomized clinical trial comparing safety and efficacy of insulin delivery to extremely low birth weight infants through unprimed and insulin-primed tubing, which we hope will address some of these impor-tant issues.
Mamta Fuloria, MD Judy L. Aschner, MD
Department of Pediatrics
Wake Forest University School of Medicine Winston-Salem, NC 27157-1081
REFERENCES
1. Finberg L. Dangers to infants caused by changes in osmolar concentra-tion.Pediatrics.1967;40:1031–1034
2. Dweck HS, Cassady G. Glucose intolerance in infants of very low birth weight. I. Incidence of hyperglycemia in infants of birth weight 1100 grams or less.Pediatrics.1974;52:189
Sleep Location and Suffocation: How Good Is the
Evidence?
To the Editor.—
In their article “Infant Mechanical Suffocation Deaths in the United States, 1980 –1997,” Drago and Dannenberg analyze a case series of 2178 infant deaths that they classified as “suffocation” based on the Consumer Product Safety Commission’s (CPSC) Death Certificate File (DCF), providing a sobering reminder of TABLE 1. Demographics of Infants
Infant Characteristics Insulin No-Albumin (Mean⫾SD)
Insulin No-Albumin (Range)
Insulin With Albumin (Mean⫾SD)
Insulin With Albumin (Range)
Gestational age (wk) 24.8⫾2.6 23–31 23⫾0.84 23–25
Birth weight (g) 702.4⫾320.78 560–1650 566.6⫾86.79 475–704
Days of life at start of insulin
5.3⫾3.23 1–10 8.4⫾5.94 2–18
environmental hazards to infants, such as wedge spaces around mattresses/cushions and strangulation risks from cords or widely spaced crib rails.1However, the data do not necessarily support their recommendations that “bed sharing and the use of adult beds for infants should be discouraged” or that regulations should “forbid the use of a bed for an infant or toddler.” Such recom-mendations require knowledge of the relative risk of different sleepinglocations(eg, cribs vs playpens vs beds, etc), information which is not discernable from the CSPC-DCF for two reasons. First, the denominator is unknown (the proportion of time infants in the population spent in each location). We will focus on the second and more complicated issue of numerator reliability.
Concerns about the reliability of case definition stem first from variability in the gathering, interpretation, and reporting of infor-mation to the CPSC.2,3Variation arises from 1) inconsistent data collection procedures, 2) disparate qualifications of those who investigate and certify death, with certifiers ranging from coroners with no medical training to forensic pathologists, and 3) lack of objective criteria to distinguish suffocation from sudden infant death syndrome (SIDS).4 As an example of possible resulting classification bias, O’Hara noted a geographic clustering of the suffocation subcategory “overlaying” on review of the CPSC-DCF data since 1995. Certifiers from areas where the term was used most frequently related by phone interview that they would not diagnose SIDS in the context of co-sleeping, assuming sleep loca-tion to be a possible contributory factor. In contrast, some other certifiers classify an infant death in any location as SIDS unless evidence of a specific etiology is found. Only the former cases would likely be classified as a type of “suffocation” in the authors’ analysis, increasing both the number of deaths ascribed to suffo-cation and the apparent percentage of suffosuffo-cation deaths involv-ing beds and co-sleepinvolv-ing.
Classification is also limited by the scant amount of information provided to the CPSC, consisting only of a code for the cause of death (E-code), a one-line narrative, and demographic informa-tion. Based on such sparse data, the authors note that their clas-sifications proved to be inaccurate for half of the subset (18/38) of crib-related deaths that were compared with in-depth investiga-tions by the CPSC.1Unfortunately, no validation of accuracy was presented for the cases involving beds and/or more speculative mechanisms of death (eg, case 9734052045: “baby found unrespon-sive, father slept in same bed: asphyxiation by overlay [sic]”5). Reasons to question classifications related to beds and co-sleeping include 1) the inability of E-codes to distinguish beds from cribs, 2) possible use of the general term “bed” in the narrative to refer to a variety of specific sleeping locations, 3) lack of any informa-tion about co-existing environmental risks (eg, caregiver intoxica-tion), and 4) the potential for cultural beliefs about proper sleep location to influence the interpretation of death circumstances.
History has humbled us with the hazards of making strong recommendations about infant sleep without an adequate empiric basis. One example is the widespread recommendation in the 1950s to place infants prone, followed decades later by the recog-nition that prone sleeping is a major risk factor for SIDS.6 Simi-larly, premature recommendations about optimal infant sleep lo-cationmight 1) unnecessarily limit cultural choices about infant care, 2) subject parents to unfounded guilt/blame, 3) obscure the need for further research, and 4) inadvertently compromise child health. This is particularly important in light of evidence7–10 sug-gesting that co-sleeping may be protective against SIDS. In addi-tion, co-sleeping facilitates breastfeeding,11which in turn has sig-nificant benefits for maternal and child health.12Until better data are available to determine the impact of infant sleepinglocationon overall infant health, we should focus our recommendations on evidence-based information about infant sleeppositionand envi-ronment.
Maryann O’Hara, MD, MSt
Robert Wood Johnson Clinical Scholars Program University of Washington
Seattle, WA 98104
Richard Harruff, MD, PhD
Acting Chief Medical Examiner Public Health Department Seattle, WA 98104
President-elect of the Washington SIDS Foundation
TABLE 2. Blood Glucose Levels and Insulin Requirements Time (h) Insulin No-Albumin Glucose (mg/dL) Day 1, Mean ⫾ SD Insulin With Albumin Glucose (mg/dL) Day 1, Mean ⫾ SD Insulin
No-Albumin Glucose (mg/dL)
John E. Smialek, MD*
David R. Fowler, MB, ChBM‡
*Chief and ‡Deputy Chief Medical Examiners Office of the Chief Medical Examiner for the State
of Maryland Baltimore, MD 21201
REFERENCES
1. Drago DA, Dannenberg AL. Infant mechanical suffocation deaths in the United States, 1980 –1997. Pediatrics. 1999;103(5). URL: http:// www.pediatrics.org/cgi/content/full/103/5/e59
2. US Consumer Product Safety Commission. A description of the Death Certificate Project and its data files. Washington, DC, 1998
3. Sturner WQ. Common errors in forensic pediatric pathology. Am J Forensic Med Pathol.1998;19:317–320
4. Sheers NJ. Infant Suffocation Project: fiscal year 1993 status report. Washington, DC: US Consumer Product Safety Commission, 1993 5. US Consumer Product Safety Commission. Death Certificate File.
Washington, DC, 1995–1998
6. Mitchell EA, Ford RP, Taylor BJ, et al. Further evidence supporting a causal relationship between prone sleeping position and SIDS.J Paediatr Child Health.1992;28:S9 –12
7. Farooqi S. Ethnic differences in infant care practices and in the incidence of sudden infant death syndrome in Birmingham.Early Hum Dev.
1994;38:209 –213
8. Mosko S, Richard C, McKenna J, Drummond S, Mukai D. Maternal proximity and infant CO2 environment during bedsharing and possible implications for SIDS research.Am J Phys Anthropol1997;103:315–328 9. Mosko S, Richard C, McKenna J. Infant arousals during mother-infant
bed-sharing: implications for infant sleep and sudden infant death syndrome research.Pediatrics.1997;100:841– 849
10. McKenna JJ, Thoman EB, Anders TF, Sadeh A, Schechtman VL, Glotzbach SF. Infant-parent co-sleeping in an evolutionary perspective: implications for understanding infant sleep development and the sud-den infant death syndrome.Sleep.1993;16:263– 82
11. McKenna J, Moso S, Richard C. Bedsharing promotes breastfeeding.
Pediatrics.1997;100:214 –219
12. American Academy of Pediatrics. Working Group on Breastfeeding. Breastfeeding and the use of human milk.Pediatrics.1998;100:1035–1039
In Reply.—
O’Hara et al have raised concerns about the validity of the data to support conclusions by Drago and Dannenberg, stating that knowledge of relative risk of different sleeping locations is neces-sary before we can discourage the use of adult beds or bed-sharing for infants, and before we can recommend regulations to forbid the use of a bed for an infant or toddler. They also question the reliability of the data in view of possible variations in procedures for gathering, reporting, and interpreting the data.
First, it is important to clarify that the recommendation for a regulation to forbid the use of a bed for an infant or toddler was offered only for daycare settings. We believe this is an appropriate recommendation because daycare providers may not always be aware of hazards associated with sleeping, and because their motivation for using a bed instead of a crib is probably for con-venience rather than for bonding, feeding, or reducing the risk of SIDS.
We do not necessarily agree that one always needs relative-risk data to recommend injury intervention strategies. Intervention strategies address ways to reduce or eliminate hazards and con-tributing factors, based on what is known about how the injuries occur. Because mechanical suffocation is the leading cause of unintentional death among infants, and because 30% of the infant suffocation deaths reported to CPSC involved beds, it would be irresponsible to not look to solutions which address the bed. Relying on behavioral interventions to make a bed safe (such as changing attitudes and behavior about soft mattresses, pillows, fluffy bedding, and the location of a bed against the wall) reduces the likelihood that the intervention will succeed. Most products for infants, including cribs, playpens, high chairs, and toys, meet either a mandatory federal safety requirement or a voluntary industry safety standard. Such regulations and standards typically incorporate design or performance specifications, and although they may include to some complementary degree a behavioral intervention component, that is certainly not their primary focus.
We do agree that there probably is variation in the way in which coroners, medical examiners, etc, investigate and certify deaths and also that there may be inconsistencies in coding causes of death and/or products involved. Nonetheless, we believe the number (2178) of cases in our study reasonably reflects infant suffocation deaths associated with consumer products. The Con-sumer Product Safety Commission (CPSC) has always collected data on deaths with the E-Code 913; therefore, we do not agree that data collection procedures varied during the period our study covers. More than 90% of the cases in our study had been assigned this E-Code.
Although we agree that the narrative in the CPSC database is scant, there is a product code, which O’Hara et al fail to mention, that was used by the authors in conjunction with the narrative text to arrive at a product code for the study. Because only one person (Drago) assigned product codes for the study, any errors would have at least been systematic.
The attempt to use the reported revision of codes for 18 of 38 cases in a subset to support the contention of data inaccuracy is flawed. The subset was in no way representative of the data set. Further, it should be noted that 8 of the 18 cases in question had been coded as “mechanism unknown” and that the additional information only served to allow a more accurate classification; the remaining 10 of the 18 had been coded as “wedgings,” whereas 9 were found to be a special circumstance of wedging, namely, “entrapment with suspension.” Only one case would have to be reversed from “wedging” to “unknown.”
As for misclassification of SIDS deaths as “overlaying,” that may be true to some degree; however, because there are no ob-jective criteria for distinguishing SIDS from mechanical suffoca-tion, that distinction relies on evidence from the death scene. The number of “overlain” cases in our study was 180, less than 10% of the data set, with 102 of 180 (56%) occurring in a bed. Whereas O’Hara et al noted a geographic clustering of “overlaying” in their data review, our study showed that one state (Michigan) was the source of 15 cases; 3 states (Indiana, Texas, and Wisconsin) were the sources of 10 cases each; five states (California, Georgia, Illi-nois, New Jersey, and Pennsylvania) were the sources of 7 to 9 cases each. We cannot draw any conclusions about clustering.
It is important to appreciate that nearly one third of the cases in our study involved beds, and that except for the small number of cases coded as “overlain,” the authors drew no conclusions about bed-sharing. The point is that whether an infant is placed in a bed alone or with another person, there are immediate hazards presented to the infant which would not be presented by the use of a compliant crib. If the positive aspects of bed-sharing are publicized, the hazards associated with bed-sharing also need to be made known.
Dorothy A. Drago, MA, MPH
Product Safety Consultant Gaithersburg, MD
To the Editor.—
We are writing to express our concern with several of the points raised in the recent article by Drago and Dannenberg (Drago DA, Dannenberg AL. Infant mechanical suffocation deaths in the United States, 1980 –1997. Pediatrics. 1999;103:(5). URL: http:// www.pediatrics.org/cgi/content/full/103/5/e59).1 The authors suggest that the reported increase in infant deaths by suffocation and “overlying” could be the result of “an increase in the rate of infant-parent co-sleeping related to reported benefits, including increased breastfeeding and reduction in the rate of SIDS. . . .” They acknowledge that rates of co-sleeping are unknown and that this association is theoretical.
ap-neas in deep sleep, longer infant sleep, and more positive evalu-ations by bedsharing mothers of their nighttime experiences.3– 8
It has been estimated that more than half of the families in the United States practice co-sleeping with infants for some period of time. Drago and Dannenberg casually dismiss the biologically important role of co-sleeping when they state: “New parents may take their infants to bed with them. . .for feeding convenience.” Catastrophic accidents in the co-sleeping environment are tragic exceptions to the act of co-sleeping itself and are almost always attributable to avoidable, unsafe conditions, most frequently found in high-risk populations where most such tragedies occur. In recent years in Cook County, Illinois (Chicago), the medical examiner has found thatalloverlying deaths were in situations in which the adult was intoxicated with either alcohol or illegal drugs. We agree with the authors and others that special precau-tions need to be taken to minimize catastrophic accidents, but the need for such precautions is no more an argument against all co-sleeping and, specifically, bedsharing, than is the reality of infants accidentally strangling, suffocating or dying from SIDS alone in cribs a reason to recommend against all solitary, unsu-pervised infant sleep (cribs). The goal is to avoid dangerous adult beds, and dangerous bedsharing conditions, while preserving the proven benefits of co-sleeping in safe beds involving safety-conscious adults, if that is the parents’ choice.
Breastfeeding (at an all-time recorded high in the United States) and co-sleeping in the form of bedsharing mutually reinforce each other.9,10That is, studies show that bedsharing increases the fre-quency and duration of nightly breastfeeding, while breastfeeding makes bedsharing convenient for mothers, thereby increasing the chances of its adoption as a routine practice.4 Maintenance of breastfeeding is a proven preventive action against increased in-fant illness and death, even in developed countries, and a signif-icant factor in reducing maternal illness. It is unfortunate that Drago and Dannenberg were unable to report the specific condi-tions and/or circumstances in which alleged overlaying and other bedsharing infant deaths occurred. It is those specific conditions that transform co-sleeping (in the form of bedsharing) into some-thing potentially dangerous. Of the total bedsharing deaths they report, it is important to know, for example, how many infants were found lying prone, or were sleeping on sagging mattresses, on waterbeds, or sofas—all highly risky forms of co-sleeping. Of the bedsharing deaths, how many mothers smoked during their pregnancies, or smoked at the time of the infant’s death, laid their babies prone for sleep, were intoxicated, used drugs, or were perhaps unaware that the baby was sleeping alongside? Was there a previous infant or child death in the family, suggesting possible infanticide or a Munchausen-by-proxy syndrome? Of even greater importance is the question: how many of these overlays involved nonsmoking, non-intoxicated, breastfeeding mothers? These data are critical to assess the actual causes of death. Mere location of infant sleep is insufficient for assessing the actual cause of the tragedy.
Furthermore, it is important to recognize that an infant can die from SIDS while bedsharing without any contributory role from co-sleeping. Most adults die in bed, but we do not indict the bed as a factor in causing the death. Cultural biases against mother-infant co-sleeping in our society make it very difficult to think of a bedsharing death simply as yet another tragic SIDS. All too frequently the assumption is that the adult in the bed probably overlaid the baby either accidentally or purposefully. Unfortu-nately, autopsy examination is unable to differentiate between “SIDS” and suffocation in the absence of physical signs of injury. A priori assumptions make it less likely that an accurate assess-ment will be achieved.
The distinction between co-sleeping and particular forms of it, likebedsharing,was introduced several years ago as a way to make more precise the discourse surrounding co-sleeping and SIDS11,12 and to help clarify and potentially reconcile the legitimate diverse positions argued by researchers in this controversial area. The authors erroneously use these terms interchangeably. Co-sleeping takes hundreds of different forms worldwide, and no single out-come necessarily can be associated with it. Differential outout-comes for different types of co-sleeping, including different types of bedsharing, can be predicted only by considering both the nature of relationships involved while co-sleeping (what happens be-tween the caregiver and infant once in bed, or outside of the bed) and the qualities of the physical environment and social circum-stances within which particular types of infant care (as, for
exam-ple, breastfeeding) are integrated with, or are absent from, the act of co-sleeping.
That a proactive, involved, and nurturing caregiver changes the outcomes in the co-sleeping environment are suggested by the New Zealand epidemiological study showing that when infants sleep in the same room with their mothers, but not when sleeping in a room with siblings, they are four times less likely to die from SIDS.13Similarly, the CESDI (Great Britain) epide-miological study shows that infants who sleep in a separate room alone are more likely to die from SIDS than are infants of nonsmoking mothers who are brought in and out of their mother’s bed throughout the night for breastfeeding, and who are kept in the room, close to the mother all night long.14 Moreover, Japan exhibits the lowest SIDS rates in the world and, there, mother-infant co-sleeping (on floor-positioned fu-tons) is the cultural norm! By distinguishing between co-sleep-ing in a generic sense and particular forms of co-sleepco-sleep-ing (such as safe bedsharing, exhibited by breastfeeding, nonsmoking mothers sleeping on firm mattresses vs unsafe bedsharing, exhibited by non-breastfeeding, smoking mothers sleeping on soft, over-blanketed beds) health professionals can preserve and acknowledge the importance of parents and infants sleep-ing within arms reach—within proximity (co-sleepsleep-ing).
Drago and Dannenberg speculate that the increase in overlay deaths in the last decade might be attributed to the promulgation and acceptance of McKenna’s documented benefits of co-sleeping in the form of safe bedsharing. At the very least, information on why the parents or caregivers of overlain infants elected to bed-share as well as data on whether or not they did sosafelyon the night the infant died, would be required before such an assertion could be proven. Sound scientific methods and procedures were used in all of McKenna’s studies, and all work was peer-reviewed on multiple occasions. It is true that the AAP Committee on Infant Sleep Position sees no reason to recommend bedsharing as a way to reduce SIDS (and, at this point, neither does McKenna). It is also true that AAP committee warns appropriately (as does McKenna and colleagues) that under special unsafe circumstances bedshar-ing can increase SIDS risk; nevertheless, there remain valid, peer-reviewed data which justify scientific speculation that under safe bedsharing/co-sleeping circumstances (especially where breast-feeding is involved), infants may have an increased chance to avoid a SIDS death. This speculation emerged initially from a detailed peer-reviewed monograph, which proposed a theoretical model and a series of testable hypotheses, all of which integrated cross-cultural SIDS epidemiology, and developmental, experi-mental, and evolutionary data.15It led to two pilot studies of mother-infant co-sleeping16,17and a carefully controlled NICHD-funded scientific study which documented significant physiolog-ical and behavioral changes in sleep, arousal, and feeding patterns induced by the presence of a breastfeeding, co-sleeping mother.18 At least 10 peer-reviewed articles have been published, two of which appeared inPediatrics.
James J. McKenna, PhD
Director, Mother-Baby Behavioral Sleep Laboratory University of Notre Dame
Lawrence M. Gartner, MD
University of Chicago Chicago, IL
REFERENCES
1. Drago DA, Dannenberg AL. Infant mechanical suffocation deaths in the United States, 1980 –1997.Pediatrics.1999;103:e59
2. Konner MJ. Evolution of human behavior development. In: Munroe RH, Munroe RL, Whiting JM eds.Handbook of Cross-Cultural Human Devel-opment.New York, NY: Garland STPM Press, 1981:3–52
3. McKenna JJ, Mosko S, Richard C, et al. Mutual behavioral and physio-logical influences among solitary and co-sleeping mother-infant pairs: implications for SIDS.Early Hum Dev.1994;38:182–201
4. McKenna J, Mosko S, Richard C. Bedsharing promotes breast feeding.
Pediatrics.1997;100:214 –219
5. Richard C, Mosko S, McKenna J. Sleeping position, orientation, and proximity in bedsharing infants and mothers.Sleep.1996;19:667– 684 6. Mosko S, Richard C, McKenna J. Infant arousals during mother-infant
bedsharing: implications for infant sleep and SIDS research.Pediatrics.
1997;100:841– 849
7. Richard C, Mosko S, McKenna J. Apnea and periodic breathing in the bedsharing infant.Am J Applied Phys.1998;84:1374 –1380
8. Mosko S, Richard C, McKenna J, Drummond S. Infant sleep architecture during bedsharing and possible implications for SIDS.Sleep.1996;19: 677– 684
9. Ross Mothers Survey (1997). Published and available through Ross Laboratories, Ross Products Division of Abbott Laboratories 10. Mitchell EA, Scragg L, Clements M. Factors related to infant bedsharing.
NZ Med J.1994;107:466 – 467
11. McKenna JJ, Thoman E, Anders T, Sadeh A, Schechtman V, Glotzbach S. Infant-parent co-sleeping in evolutionary perspective: implications for understanding infant sleep development and the Sudden Infant Death Syndrome (SIDS).Sleep.1993;16:263–282
12. McKenna JJ. The potential benefits of infant-parent co-sleeping in rela-tion to SIDS prevenrela-tion: overview and critique of epidemiological bed sharing studies. In: Rognum TO, ed,Sudden Infant Death Syndrome: New Trends in the Nineties. Oslo: Scandinavian University Press, 1995: 256 –265
13. Mitchell EA, Thompson JMD. Cosleeping increases the risks of the sudden infant death syndrome, but sleeping in the parent’s bedroom lowers it. In: Rognum TO, ed.Sudden Infant Death Syndrome: New Trends in the Nineties.Oslo: Scandinavian University Press, 1995:266 –269 14. Fleming P, Blair P. Safe environments for infant sleep: community and
laboratory investigations or folk wisdom? Symposium on Breast Feed-ing, Parental Proximity and Contact in Promoting Infant Health. Paper delivered at University of Notre Dame, South Bend, September 1998. 15. McKenna JJ. An anthropological perspective on the sudden infant death
syndrome (SIDS): the role of parental breathing cues and speech breath-ing adaptations.Med Anthropol.1986;10:9 –53
16. McKenna JJ, Mosko S, Dungy C, McAninch P. Sleep and arousal pat-terns of co-sleeping human mothers/infant pairs: a preliminary physi-ological study with implications for the study of Sudden Infant Death Syndrome (SIDS).Am J Phys Anthropol.1990;83:331–347
17. Mosko S, McKenna JJ, Dicker M, Hunt L. Parent-infant co-sleeping: the appropriate context for the study of infant sleep and implications for SIDS research.J Behav Med.1993;16:589 – 610
18. McKenna JJ, Mosko S, Richard C. Breast feeding and mother-infant cosleeping in relation to SIDS prevention. In: Trevathan W, Smith N, McKenna J, eds. Oxford, UK:Evolutionary Medicine.Oxford, UK: Oxford Universty Press: Oxford 1999:53–74
19. McKenna J. Cultural influences on infant and childhood sleep biology and the science that studies it: toward a more inclusive paradigm. In: Loughlin J, Carroll J, Marcus C, eds.Sleep in Development and Pediatrics.
New York, NY: Marcel Dekker; In press
In Reply.—
It appears that Dr. McKenna’s work in the field of co-sleeping/ bed-sharing causes him to focus on a subset of less than 10% of the 2178 deaths we described. According to Dr. McKenna, bed-shar-ing, a particular form of co-sleepbed-shar-ing, describes the co-occupancy of a bed. Co-sleeping describes sleeping in the same environment, perhaps the same room, usually nearby, and may take a variety of
forms. We admit our mistaken use of these terms interchangeably and thank Dr. McKenna for clarifying the issue. We concluded that increased rates of co-sleeping (instead of bed-sharing) could have been a factor for the 102 cases of overlaying in a bed. We drew no conclusions about bed-sharing or co-sleeping for the more than 500 other cases involving beds. In fact, we focused on the location of the bed against the wall as the major hazard.
It was never our intent to weigh the risks and benefits of bed-sharing. We did not address the benefits of co-sleeping be-cause it was never our intent to do anything but better understand the circumstances surrounding infant suffocation deaths.
Drs. McKenna and Gartner believe we casually dismissed the biological importance of co-sleeping by stating, “New parents may take their infants to bed with them. . .for feeding conve-nience.” How convenient for their argument that they omitted a phrase from our sentence. The full sentence reads, “New parents may take their infants to bed with them to enhance infant-parent bonding or for feeding convenience.”
It is outrageous and absurd for Drs McKenna and Gartner to compare adult deaths taking place in a bed with infant deaths taking place in a bed. Most adults may die in bed; however, that is a spurious association. Adults do not die stuck between the bed and wall or trapped between the mattress and bed frame, as infants do.
It is also erroneous for them to compare Japanese customs with American customs, when they readily admit that Japanese sleep on floor-positioned futons. If Drs. McKenna and Gartner had provided a description of the typical American bed-sharing scene, I believe it would be quite different from the Japanese.
We agree with Drs. McKenna and Gartner that the goal is to avoid dangerous adult beds for infants, but our study suggests that adults do not recognize what makes a bed safe vs dangerous. We had an obligation to conclude and report that bed location, presence of pillows or soft bedding, and bed-sharing made beds hazardous for infants.
Dorothy A. Drago, MA, MPH
Product Safety Consultant Gaithersburg, MD
To the Editor.—
I am concerned about the unqualified recommendation against co-sleeping published in your journal by Drago and Dannenberg.1 The authors argue that the rates of suffocation death by “overly-ing” have risen in this country over the last 20 years and that co-sleeping should therefore be considered “unsafe.” They go as far as suggesting a prohibition against “the use of a bed for an infant or toddler.”
There are several flaws in their reasoning. First, “overlying” is a notoriously unreliable autopsy diagnosis. It is frequently impos-sible to distinguish it from SIDS, which is a much more common condition. Yet, a death from SIDS of a newborn who was sleeping next to his or her parents is much more likely to be mistakenly attributed to “overlying” than a SIDS death occurring in a crib. Clinical experience suggests that true overlying is a rather rare cause of infant death under usual circumstances. Detailed inves-tigations show that confirmed accidents occur primarily when the adults are severely intoxicated by alcohol or drugs or when they are markedly obese.2This is not surprising given that a normal adult would be easily awakened by the discomfort caused by lying over a child (as anyone who has ever accidentally lain over a cat can attest).
Clearly, some sleeping arrangements are unsafe for infants. These include soft and deep pillows or comforters, as well as waterbeds. However, this is as true for unsupervised solitary sleeping of infants as it is for co-sleeping arrangements. Instead of a sweeping recommendation against co-sleeping, Drago and Dan-nenberg would have done a greater service to infant safety if they had emphasized such unsafe bedding.
David Servan-Schreiber, MD, PhD
Chief, Division of Psychiatry
University of Pittsburgh Medical Center, Shadyside Pittsburgh, PA
REFERENCES
1. Drago DA, Dannenberg AL. Infant mechanical suffocation deaths in the United States, 1980 –1997.Pediatrics.1999;103:e59
2. Bass M, Bravath RE, Glass L. Sudden infant death scene investigation.
N Engl J Med.1986;315:100 –105
3. Harlow HF, Harlow MK. Effects of various mother-infant relationships on rhesus monkey behaviors. In: Foss BM, ed.Determinants of Infant Behaviour,Vol 4. London: Methuen, 1969:15–36
4. Meyer J, Novak MA, Bowman RE, Harlow HF. Behavioral and hor-monal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Dev Psychobiol. 1975;8: 425– 435
5. Cummins MS, Suomi SJ. Behavioural stability of rhesus monkeys fol-lowing various rearing.Primates.1976;17:42–51
6. Ainsworth MDS. I. Patterns of infant-mother attachment: antecedents and effects on development. Bulletin of New York Academy of Medi-cine. 61:771–791
7. American Academy of Pediatrics, Task Force on Infant Positioning and SIDS. Does Bed Sharing Affect the Risk of SIDS? (RE9725)Pediatrics.
1997;100:272
In Reply.—
Dr. Servan-Schreiber chooses to focus on one suffocation pat-tern, overlaying, as the basis for our recommendations, and selec-tively focuses on “discouraging bed-sharing,” as if it were our only conclusion.
In fact, it was “wedging between the bed and a wall” as the leading circumstance of death that led us to discourage the use of an adult bed for an infant, regardless of whether the infant is alone or with another individual. Our conclusions state that “. . .beds involved were more likely to become hazardous because of their location near a wall, the presence of pillows or soft bedding or because of bed-sharing.”
Furthermore, we recommend a regulation to forbid the use of a bed for an infant or toddler only for daycare settings. We believe this is an appropriate recommendation because daycare providers may not always be aware of hazards associated with sleeping, and because their motivation for using a bed instead of a crib is probably for convenience rather than for bonding, feeding, or reducing the risk of SIDS.
We did not address the benefits of co-sleeping because it was never our intent to do anything but better understand the circum-stances surrounding infant suffocation deaths.
Dorothy A. Drago, MA, MPH
Product Safety Consultant Gaithersburg, MD
Theophylline-Induced Convulsions in Children
With Epilepsy
To the Editor.—
In Japan, theophylline is widely used as a basic bronchodilator in asthmatic children and other patients with severe cough. How-ever, many recent papers have reported that theophylline induces convulsions.1–3 Therefore, we retrospectively investigated theo-phylline-induced convulsions in epileptic children.
In our outpatient clinic, 143 epileptic patients are receiving anti-epileptic drugs. Among them, 43 had previously received theophylline and 16 of these patients had a history of convulsions during theophylline treatment. We defined theophylline-induced convulsions as follows: 1) no convulsion occurred for at least 6 months before administration of theophylline; 2) convulsions oc-curred while receiving theophylline; 3) no convulsion ococ-curred for at least 6 months after discontinuing theophylline; and 4) no other factors were present that could cause convulsions, such as de-creased serum concentrations of anti-epileptic drugs, noncompli-ance with such treatment, lack of sleep, etc. Six patients with
convulsions were excluded from the study, because their convul-sions were not considered theophylline-induced.
We divided the remaining 37 children into two groups: a con-vulsion group (10/37, 27.0%) and a non-concon-vulsion group (27/37, 73.0%). We compared the two groups with respect to age, sex, epilepsy type, anti-epileptic drugs, and duration and dosage of theophylline treatment. The only factor that correlated closely with convulsions was age. In infants under 1 year of age, we found theophylline-induced convulsions more often than in children over 1 year of age (P⬍.05). We assayed theophylline concentra-tions in three children with convulsions; all levels were below 10 g/mL.
Kato et al4reported that the ratio of free theophylline to total serum concentration of the drug is highest in neonates and de-creases with age. Furthermore, free theophylline in serum is equal to that in spinal fluid.5 These observations suggest that infants may be predisposed to developing theophylline-induced convul-sion.
In conclusion, infants under 1 year of age with epilepsy have a higher risk of theophylline-induced convulsions, so the use of theophylline should be avoided in this group.
Takuma Miura, MD Kyoko Kimura, MD
Department of Pediatrics Haga Red Cross Hospital Tochigi, Japan
REFERENCES
1. Weinberger M, Hendeles L. Drug therapy: theophylline in asthma.
N Engl J Med.1996;334:1380 –1388
2. Tsiu SJ, Self TH, Burns R. Theophylline toxicity: update.Ann Allergy.
1990;64:241–257
3. Bahls FH, Ma KK, Bird TD. Theophylline-associated seizures with “therapeutic” or low toxic serum concentrations: risk factors for serious outcome in adults.Neurology.1991;41:1309 –1312
4. Kato Z, Fukutomi O, Kondo N. Developmental changes of unbound theophylline.Ann Allergy Asthma Immunol.1998;80:517
5. Suzuka T. Theophylline therapy for premature infants and its effect on central nervous systems.Acta Neonatol Japonica.[In Japanese] 1981;17: 412– 418
Multiethnic Families: An Underrecognized
Influence on Health Statistics
To the Editor.—
In Southern California, Agran et al1found that Hispanic (main-ly Mexican) children had much higher rates of serious injury requiring hospitalization than did non-Hispanic white children. To investigate possible reasons for this disparity, we designed an ethnographic study involving in-depth interviews and observa-tions in homes in geographical areas where large numbers of such injuries had occurred. Our subjects consisted of Mexican mothers (born and educated in Mexico), Mexican American mothers (of Mexican ancestry but born and educated in the United States), and non-Hispanic US-born white mothers. We assumed that the chil-dren in these families would have the same racial and ethnic identity as their mothers and that in this way we could study possible effects of cultural factors on injury rates.
We found that the children of the Mexican and Mexican Amer-ican mothers did indeed share their mothers’ ethnicities, but of the 30 white mothers interviewed, 10 had children who had Hispanic surnames because their biological fathers were Hispanic. These children, many of whom spoke Spanish as well as English, would have been classified as “Hispanic” in most studies. Furthermore, in 5 of the 10 cases the biological father was not living with the family and, thus, whatever attitudes and behaviors were present in the home were much more a function of the non-Hispanic white mother than of the absent Hispanic father.
condi-tion or behavior is purely the result of ethnicity rather than, for example, a function of social class. Indeed, Krieger and Fee4and Krieger et al5have called for the reintroduction of social class variables into health statistics—variables now used in all devel-oped nations with the exception of the United States.
Recent articles have pointed out two major problems besetting attempts to study ethnicity in relation to health status. One is the fact that in virtually all published research, including that on Hispanics,6the ethnic classifications used are not clearly defined.7 Another is the marked lack of reliability in ethnic identifications regardless of whether the identifications are provided by subjects themselves or by other people, especially where classifications other than “white” and “black” are in question.8,9Misclassification may cause erroneous conclusions to be drawn, leading to inap-propriate health interventions.10
Our research reveals yet another complication in studies of ethnicity focusing on children: the adult controlling the physical and emotional environment in the household may be of a different ethnicity from the child whose health status is being investigated. As stated in a recent task force report on the health of immigrant children sponsored by the National Research Council and the Institute of Medicine,11in-depth ethnographic studies of house-hold factors influencing health are essential if statistics from broader surveys are to be fully understood.
Dorothy S. Mull, PhD Phyllis F. Agran, MD, MPH Diane G. Winn, RN, MPH Craig L. Anderson, DHSc, PhD
Pediatric Injury Prevention Research Group Center for Health Policy and Research University of California, Irvine Irvine, CA 92697-5800
REFERENCES
1. Agran PF, Winn DG, Anderson CL, Del Valle CP. Pediatric injury hospitalization in Hispanic children and non-Hispanic white children in Southern California.Arch Pediatr Adolesc Med.1996;150:400 – 406 2. Qian Z. Breaking the racial barriers: variations in interracial marriage
between 1980 and 1990.Demography.1997;34:263–276
3. Chew KSY. The rise of multiracial households in California: how many, how fast, how different? Paper presented at the annual meeting of the American Sociological Association, Toronto, Canada, August 9 –13, 1997 4. Krieger N, Fee E. Social class: the missing link in US health data.Int
J Health Serv.1994;24:25– 44
5. Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines.Annu Rev Public Health.1997;18:341–378
6. Hayes-Bautista DE. Identifying “Hispanic” populations: the influence of research methodology upon public policy.Am J Public Health.1980; 70:353–356
7. Anderson MR, Moscou S. Race and ethnicity in research on infant mortality.Fam Med.1998;30:224 –227
8. Hahn RA, Mulinare J, Teutsch SM. Inconsistencies in coding of race and ethnicity between birth and death in US infants. JAMA. 1992;267: 259 –263
9. Hahn RA, Truman BI, Barker ND. Identifying ancestry: the reliability of ancestral identification in the United States by self, proxy, interviewer, and funeral director.Epidemiology.1996;7:75– 80
10. Dakis P, Rubin L. Obstruction of valid race/ethnicity data acquisition by current data collection instruments. Methods Inf Med. 1998;37: 188 –191
11. Hernandez DJ, Charney E, eds.From Generation to Generation: The Health and Well-Being of Children in Immigrant Families.Washington, DC: Na-tional Academy Press, 1998
Ultrasonography Detects Appendicular Mucocele
in Cystic Fibrosis Patients Suffering Recurrent
Abdominal Pain
To the Editor.—
Cystic fibrosis (CF) patients frequently suffer from recurrent ab-dominal pain1because of various mechanisms such as gut motility
disorders and uncontrolled pancreatic insufficiency, but also surgical events. This makes diagnosis difficult.2–5We report on 8 children (5 boys, 3 girls) with chronic abdominal pain due to appendicular mucocele (AM) detected by colonic transabdominal ultrasonography (CUS). Abdominal pain symptoms appeared between ages 3 and 11. All patients have pancreatic insufficiency controlled with an effective pancreatic enzyme preparation (6000⫾2000 U lipase/kg/d); 4 had a neonatal meconium ileus. Genotype is homozygous␦F 508 for 4 children. Shwachman scores are 70 to 95.
All but one suffered from constipation or distal intestinal obstruc-tion syndrome (DIOS); 6 had mild abdominal pain and 2 had severe pain with colicky abdominal cramps. Pain was initially intermittent and then became continuous in 2 children. Otherwise, the children appeared well and had no weight loss, no bloody stools, or vomiting. One child had a fever for a few days just before diagnosis.
Physical examination revealed tenderness of the right lower quad-rant in 5 children, with a mobile mass in this area in 3. X-rays of the abdomen showed only a large amount of fecal material in the right colon. Their failure to respond to the usual medical management of symptoms of DIOS led to further radiological investigation.
CUS (Hitachi EU B 415 CFM, Tokyo, Japan; 0.6 mm lateral resolution, 7.5 MHz linear transducer) was used to scan the entire abdomen in both longitudinal and transverse directions focusing on the right iliac fossa. Patients were not required to fast and the bowel was not prepared. One patient had a typical ileocecal intussusception (ICI) with a target pattern.2Another had a large, multilayered mass in the right lower quadrant (4⫻ 3 cm). The remaining cases had appendices which were en-larged (7–20 mm, normal ⬍6 mm), with a lumen obstructed with echogenic material, and a clearly stratified wall. A barium enema confirmed both intussusceptions, with a partial reduc-tion for 1 child; patients had an impression of the internal side of the cecum. The appendix was not visible in any patients, and the ileum was not opacified in 6 of them. A barium enema was not required for the most recent case because ultrasonography provided sufficient information.
Surgery was performed: large ileocecal junction resections were necessary for the two ileocecal intussusceptions. One periappendicu-lar abcess was identified. The 6 other children underwent appendec-tomy with resection of the cecal tip to prevent any recurrence. Patho-logical studies disclosed appendicular mucocele in all patients.
All the patients are doing well, with follow-ups of 20 to 42 months. Transabdominal ultrasonography focusing on the right iliac fossa is a rational approach to abdominal pain in patients with CF inasmuch as appendicular mucocele is readily diagnosed. Early surgery may prevent complications such as intussusception3–5 and/or appendicular abscess.
A. Munck* N. Belarbi‡ P. de Lagausie§ M. Peuchmaur㛳 J. Navarro*
Departments of *Pediatric Gastroenterology, ‡Radiology, §Surgery and㛳Anatomopathology Hospital Robert Debre´
75019 Paris, France
REFERENCES
1. Gregory PC. Gastrointestinal pH motility, transit and permeability in cystic fibrosis.J Pediatr Gastroenterol Nutr.1996;55:3–23
2. Martens M. A right lower quadrant mass in cystic fibrosis: a diagnostic challenge.Eur J Pediatr.1992;151:329 –331
3. Mulvihill DM. Ultrasound findings of chronic intussusception in a patient with cystic fibrosis.J Ultrasound Med.1998;7:353–355 4. Boyez M, Le Cudonnec B, Valette M. Pseudo tumeur caecale et
muco-cele appendiculaire au cours de la mucoviscidose. A propos d’un cas.J Radiol.1983;64:627– 630