ABSTRACT
ISLAM, MOHAMMED SHAHIDUL. Prevalence and Dissemination of Antimicrobial Resistance among Campylobacter jejuni and Campylobacter coli from Meat-Animals. (Under the direction of Dr. Sophia Kathariou.)
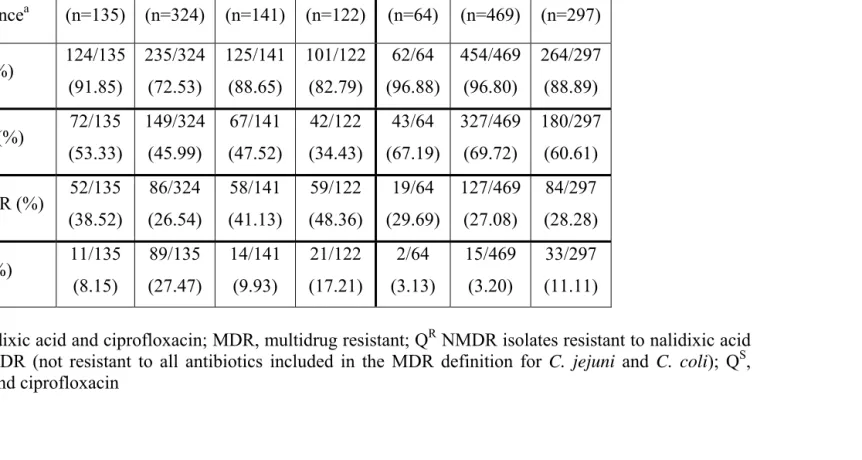
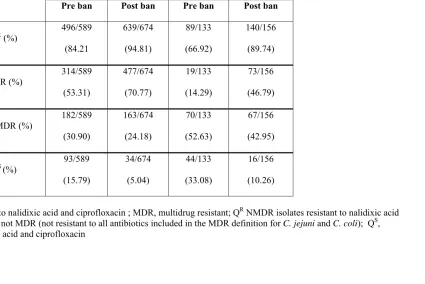
Ciprofloxacin MIC determinations suggested that there was no obvious difference in MIC distribution before and after the enrofloxacin ban. Our results suggest that there was no detectable decrease in prevalence of fluoroquinolone resistance among turkey-derived campylobacters within the surveyed three-year period following the enrofloxacin ban. Instead, and for reasons that remain unknown, prevalence values for fluoroquinolone resistance and for multidrug resistance were higher post-ban than pre-ban (p<0.0001). Continued surveys are needed to further evaluate the potential impact of the ban on resistance of campylobacters from turkeys to fluoroquinolones and other antibiotics.
turkey-derived as well as bovine C. jejuni to tetracycline resistance. Further studies are needed to evaluate the impact of transformation in dissemination of tetracycline resistance among C. jejuni and C. coli from animal production systems.
Prevalence and Dissemination of Antimicrobial Resistance among Campylobacter jejuni and Campylobacter coli from Meat-Animals
by
Mohammed Shahidul Islam
A thesis submitted to the Graduate Faculty of North Carolina State University
in partial fulfillment of the requirements for the degree of
Master of Science
Food Science
Raleigh, North Carolina 2009
APPROVED BY:
_______________________________ ______________________________ Dr. Jonathan Olson Dr. Jonathan C. Allen
________________________________ Dr. Sophia Kathariou
DEDICATION
BIOGRAPHY
ACKNOWLEDGEMENTS
I am in debt of Dr. Sophia Kathariou for giving me the opportunity and support to study microbiology in her laboratory. Her excellent guidance and teaching enabled me to carry out my research and complete my thesis.
I am grateful to Dr. Jonathan C. Allen who supported me every possible way. I would like to thank my committee member Dr. Jonathan Olson for his precious time, constructive suggestions and help to facilitate my research progress. I am grateful to Ms. Robin Siletzky for all her technical assistance throughout my research. I would like thank Dr. Donna Carver for statistical analysis of my research.
I would like to thank Dr. Weimin Gu for his help during the setup of my research. I also would like to thank past and current lab members: Dr. Ying Cheng, Dr. Jae-Won Kim, Dr. Reha Onur Azizoglu, Dr. Driss Elhanafi, Vikrant, Savitri, Sangmi, Sheea, Shakir, Harun, Jiajuan, and Danielle for their friendship.
TABLE OF CONTENTS
LIST OF TABLES ... ix
LIST OF FIGURES ... xii
CHAPTER 1: COMPREHENSIVE REVIEW OF LITERATURE ON CAMPYLOBACTER... 1
1.1 GENERAL DESCRIPTION OF CAMPYLOBACTER... 2
1.2 HISTORICAL EMERGENCE OF CAMPYLOBACTER... 4
1.3 EPIDEMIOLOGY ... 5
1.4 MODES OF TRANSMISSION... 9
1.4.1 Introduction... 9
1.4.2 Vertical transmissions... 10
1.4.3 Horizontal transmission ... 12
1.5 PATHOGENESIS AND VIRULENCE FACTORS ... 15
1.5.1 Introduction... 15
1.5.2 Flagella and motility ... 15
1.5.3 Chemotaxis ... 16
1.5.4 Adhesion and invasion... 19
1.5.5 Toxins ... 20
1.5.6 Iron acquisition ... 22
1.6 ANTIMICROBIAL RESISTANCE MECHANISMS... 25
1.6.1 Fluoroquinolone resistance ... 25
1.6.2 Tetracycline resistance... 28
1.6.3 Macrolide resistance ... 31
1.6.4 Aminoglycoside resistance ... 33
1.6.4.1 Kanamycin resistance ... 34
1.6.4.2 Streptomycin resistance ... 35
1.7 EFFECTS OF ANTIBIOTICS IN FOOD ANIMALS ON HUMAN HEALTH ... 36
1.7.1 Effects of fluoroquinolones (FQs) in food animals on human health... 37
1.7.2 Effects of tetracyclines in food animals on human health ... 41
1.7.3 Effects of marcolides in food animals on human health... 42
1.7.4 Effects of avoparcin and vancomycin in food animals on human health ... 43
1.7.5 Interventions aimed at reducing the occurrence of antimicrobial resistant Campylobacter... 44
1.8 NATURAL TRANSFORMATION... 45
1.8.1 Binding and uptake of DNA in Gram-positive bacteria ... 46
1.8.2 Binding and uptake of DNA in Gram-negative bacteria ... 47
1.8.3 Transport of DNA in Gram-positive and Gram-negative Bacteria... 48
1.8.4 Function of DNA uptake... 48
1.8.6 Transformation in Campylobacter... 53
1.9 REFERENCES ... 59
CHAPTER 2: PREVALENCE OF RESISTANCE TO FLUOROQUINOLONE AND PREVALENCE OF MULTIDRUG RESISTANCE AMONG CAMPYLOBACTER JEJUNI AND C. COLI FROM TURKEYS PRIOR TO AND FOLLOWING THE WITHDRAWAL OF ENROFLOXACIN... 90
2.1 ABSTRACT... 91
2.2 INTRODUCTION ... 92
2.3 MATERIALS AND METHODS... 95
2.3.1 Bacterial isolates and growth media ... 95
2.3.2 Antimicrobial susceptibility Testing... 96
2.3.3 Statistical analysis... 97
2.4 RESULTS ... 98
2.4.1 Prevalence of thermophilic campylobacters in the pre-and post-ban periods ... 98
2.4.2 Prevalence of fluoroquinolone resistance pre- and post-ban ... 99
2.4.3 Prevalence of multidrug resistance pre-and post-ban ... 100
2.4.4 Flock-associated changes in prevalence of fluoroquinolone resistance pre- and post-ban... 102
2.4.5 Ciprofloxacin MIC value distributions pre- and post-ban ... 103
2.5 DISCUSSION ... 104
2.6 REFERENCES ... 108
CHAPTER 3: TRANSFORMATION-MEDIATED TRANSFER OF TETRACYCLINE RESISTANCE FROM TURKEY AND SWINE-DERIVED CAMPYLOBACTER COLI TO TETRACYCLINE-SUSCEPTIBLE C. COLI AND C. JEJUNI... 124
3.1 ABSTRACT... 125
3.2 INTRODUCTION ... 126
3.3 MATERIALS AND METHODS... 128
3.3.1 Sample collection... 128
3.3.2 Campylobacter isolation and preservation... 128
3.3.3 DNA extraction... 129
3.3.4 Species determination ... 129
3.3.5 Susceptibility testing... 130
3.3.6 Mueller Hinton Broth transformation assay ... 130
3.3.7 Determination of strain competence using isogenic mutants ... 131
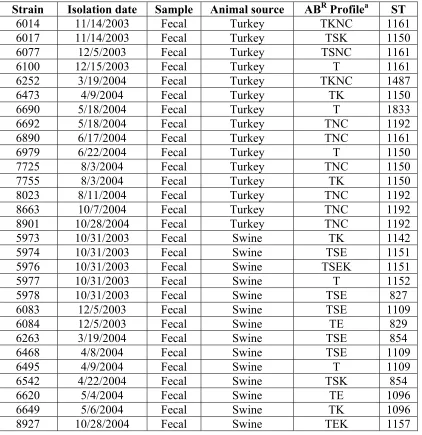
3.4 RESULTS ... 131
3.4.1 Ability of derived C. coli to serve as donors for transformation of turkey-derived C. coli to tetracycline resistance ... 131
3.4.3 Tetracycline-resistant, kanamycin-susceptible turkey-derived C. coli is unable to
transform swine-derived C. coli to tetracycline resistance ... 133
3.4.4 Tetracycline-resistant C. coli strains from swine are efficient in transforming turkey or swine-derived C. coli to tetracycline resistance ... 134
3.4.5. Limited ability of tetracycline resistance to be transferred via transformation from tetracycline-resistant C. coli to C. jejuni... 135
3.4.6 Determination of possible restriction barrier in C. coli 6461(TR) ... 136
3.5 DISCUSSION ... 137
3.6 REFERENCES ... 144
CHAPTER 4: TRANSFORMATION-MEDIATED TRANSFER OF TETRACYCLINE RESISTANCE FROM TETRACYCLINE- RESISTANT C. JEJUNI TO TETRACYCLINE-SUSCEPTIBLE C. JEJUNI AND C. COLI FROM MEAT ANIMALS... 159
4.1 ABSTRACT... 160
4.2 INTRODUCTION ... 161
4.3 MATERIALS AND METHODS... 162
4.3.1 Bacterial strains and culture conditions ... 162
4.3.2 DNA extractions, transformation assays, and determination of transformation frequency... 162
4.4 RESULTS ... 163
4.4.1 Limited ability of tetracycline-resistant, kanamycin-susceptible C. jejuni strains to serve as donors of DNA in transformation of C. coli 7474 to tetracycline resistance.. 163
4.4.2 DNA of the TRKS bovine strain C. jejuni 410E could transform several multisensitive C. jejuni strains to tetracycline resistance ... 163
4.4.3 Failure of C. jejuni to be transformed to tetracycline resistance by DNA from C. jejuni resistant both to tetracycline and to kanamycin... 164
4.4.4 TRKR C. jejuni from turkeys could not transform turkey-derived multisensitive C. coli 7474... 165
4.4.5 DNA from cattle-derived TRKR C. jejuni strains was unable to transform swine derived C. coli to tetracycline resistance ... 165
4.4.6 Comparison of the relative ability of C. jejuni and C. coli to transform multisensitive C. jejuni to tetracycline resistance... 166
4.5 DISCUSSION ... 166
4.6 REFERENCES ... 170
APPENDICES ... 181
APPENDIX 1 ANTIBIOTIC RESISTANCE PROFILE OF 1552 ISOLATES ... 182
APPENDIX 2 ANTIBIOTIC RESISTANCE PROFILE AND MICS OF CIPROFLOXACIN, TETRACYCLINE, AND KANAMYCIN OF 261 ISOLATES... 231
APPENDIX 3 PREVALENCE OF QR AND MDR OF CAMPYLOBACTER ISOLATES ... 241
LIST OF TABLES
Table 2.1 Sampling dates and integrator sources for samples used in this study ... 112 Table 2. 2 Prevalence of Campylobacter-positive samples pre-and post-ban ... 113 Table 2. 3 Yearly prevalence of total thermophilic campylobacters, C. coli and C. jejuni.. 114 Table 2. 4 Prevalence of fluoroquinolone-resistant (QR) thermophilic Campylobacter isolates pre- and post-ban... 115 Table 2. 5 Prevalence of fluoroquinolone-resistant (QR) C. jejuni and C. coli isolates pre - and post-ban... 116 Table 2. 6 Prevalence of QR change across integrator by year ... 117 Table 2. 7 Prevalence of multidrug resistant (MDR) C. jejuni and C. coli isolates pre-and post-ban... 118 Table 2. 8 Yearly prevalence of multidrug resistant (MDR) isolates from integrators A, B, and C ... 119 Table 2. 9 Pre-and post-ban prevalence of fluoroquinolone and multidrug resistance among isolates from integrators A, B, and C... 120 Table 2. 10 Pre- and post-ban prevalence of fluoroquinolone and multidrug resistance in C. jejuni and C. coli... 121 Table 2. 11 Flock-associated prevalence of fluoroquinolone resistance pre-and post-ban among flocks from integrators A, B, and C. ... 122 Table 2. 12 Pre- and post-ban prevalence of isolates with high ciprofloxacin MICs (16-64 μg/ml) among 261 isolates of C. coli and C. jejuni from integrators A, B, and C ... 123 Table 3. 1a C. coli strains used as donors in transformations done in this study………...148
Table 3. 3 Transformation of tetracycline resistance from turkey-derived TRKR C. coli donor to turkey-derived multisensitive C. coli 7474... 151 Table 3. 4 Transformation of swine-derived C. coli to tetracycline resistance and nalidixic acid resistance using TRKSNR C. coli 7725 as donor... 152 Table 3. 5 Transformation of multisensitive turkey-derived C. coli 7474 to tetracycline resistance using swine-derived TRKS C. coli as donors... 153 Table 3. 6 Transformation of multisensitive turkey C. coli 7474 to tetracycline resistance using swine derived TRKR C. coli as donors... 154 Table 3. 7 Transformation of multisensitive swine C. coli to tetracycline resistance using swine C. coli TRKS donor 6263... 155 Table 3. 8 Transformation of multisensitive turkey C. jejuni to tetracycline resistance using turkey TRKS C. coli donor 7725... 156 Table 3. 9 Transformation of multisensitive cattle C. jejuni to tetracycline resistance using turkey derived TRKS C. coli donor 7725... 157 Table 3. 10 Transformation of multisensitive human C. jejuni to tetracycline resistance using turkey derived TRKS C. coli donor 7725... 157 Table 3. 11 Transformation of C. jejuni recipient from turkey and cattle to tetracycline
Table 4. 6 Dissemination of tetracycline resistance using cattle-derived TRKR C. jejuni
LIST OF FIGURES
Figure A.6. 1: Prevalence of C. coli and C. jejuni year to year ... 266
Figure A.6. 2 Prevalence of QR Campylobacter isolates by year ... 266
Figure A.6. 3 Prevalence of total QR Campylobacter isolates... 267
Figure A.6. 4 Prevalence of QR C. coli... 267
Figure A.6. 5 Prevalence of QR C. jejuni... 268
Figure A.6. 6 Prevalence of MDR Campylobacter... 268
Figure A.6. 7 Prevalence of MDR Campylobacter by year... 269
Figure A.6. 8 Prevalence of MDR C. coli... 269
Figure A.6. 9 Prevalence of MDR C. jejuni... 270
Figure A.6. 10 Correlation of MDR, QR, and QRNMDR Campylobacter isolates... 270
Figure A.6. 11 Prevalence of different flocks year to year... 271
Figure A.6. 12 Prevalence of QR flock ... 271
Figure A.6. 13 Prevalence of QR+S flock ... 272
Figure A.6. 14 Prevalence of QS flock... 272
Figure A.6. 15 Prevalence of Pre-Ban flocks ... 273
Figure A.6. 16 Prevalence of Post-Ban flocks... 273
Figure A.6. 17 Prevalence of Pre-Ban flocks Integrator A... 274
Figure A.6. 18 Prevalence of Post-Ban flocks Integrator A ... 274
Figure A.6. 19 Prevalence of Pre-Ban flocks Integrator B... 275
CHAPTER 1
1.1 GENERAL DESCRIPTION OF CAMPYLOBACTER
Campylobacter is a gram negative, microaerophilic, non spore forming bacterium (Kelly, 2001). At present, the genus Campylobacter contains 15 species. Member of the genus cause a wide variety of diseases in humans and animals. Twelve species are associated with human diseases, even though some are found as commensals in animals (Park, 2002; On, 1996). Three species including C. jejuni, C. coli, and C. lari are known as “thermophilic campylobacters”. Among these three species C. jejuni is responsible for 80-90% of foodborne Campylobacter infections in humans, followed by C. coli, and to lesser extent by C. lari (Jacob-Reitsma, 2000; Skirrow and Blaser, 2000). Campylobacter cells are 0.2 to 0.8 µm wide and 0.5 to 5 µm long (Vandamme, 2000.). They are usually spiral in shape when actively growing (Nachamkin and Blaser, 2000), but the cells may transform to spherical or coccoid shapes upon prolonged exposure to air or when the cultures are aged (Buck et al., 1983; Nachamkin et al., 2000).
Thermophilic Campylobacter can be transmitted mainly by consumption of contaminated foods of animal origin, which includes undercooked poultry, beef or pork, unpasteurized milk, and dairy products. Untreated water can contribute to sporadic infection and outbreaks (Friedman et al., 2000). C. jejuni and C. coli have been recognized as one of the major causes of acute gastroenteritis in humans in the world (Adak et al., 2005). In most cases Campylobacter infections are characterized by a self limited watery diarrhea and do not require antibiotic treatment. However, antimicrobial treatment may be necessary for systemic infections and severe or long-lasting Campylobacter infections in immunosuppressed patients. Such serious infections can lead to reactive arthritis (Reiter’s syndrome), Guillain-Barre syndrome, osteomyelitis, nephritis, myocarditis, cystitis, pancreatitis, septic abortion, and bacteremia (Altekruse et al., 1999; Blaser 1997; Skirrow and Blaser, 2000).
1.2 HISTORICAL EMERGENCE OF CAMPYLOBACTER
In 1886, the German scientist Escherich, found spiral bacteria in the colons of children with diarrhea. He named the illness ‘cholera infantum’ and published his findings in a series of articles. This scientist might have possibly discovered Campylobacter spp. because his work in the German language remained largely unrecognized until 1985 (Moore et al., 2005). In 1913, for the first time McFadyean and Stockman isolated and described Campylobacter species as an important cause of bovine and ovine infertility and abortion. In 1918, Smith confirmed the results of McFadeyan and Stockman. He isolated similar organisms from aborted bovine fetuses and proposed the name ‘Vibrio fetus’ (Moore et al., 2005). In 1931, Jones et al. confirmed that Vibrio caused dysentery in cattle. They determined that the infection site for this organism is the jejunum (part of the intestinal tract), hence they designated these vibrios as ‘Vibrio jejuni’ (Jones et al., 1931). In 1944, Doyle isolated vibrios from the colonic mucosa of pigs with diarrhea. He determined that these microaerophilic vibrios were responsible for the disease and named them Vibrio coli (Doyle, 1948).
of organisms was designated as Vibrio fetus with optimal growth at 37ºC and the second group had a optimal growth at 42ºC. In 1972, Dekeyser et al. isolated a ‘related Vibrio’ (Campylobacter jejuni) from the blood and feces of a previously healthy woman with acute febrile hemorrhagic enteritis (Dekeyser et al., 1972). In 1973, Butzler reported the first cases of Campylobacter enteritis in Zaire, now the Democratic Republic of Congo (Butzler, 1982). In 1977, Skirrow confirmed the findings of Dekeyser et al. and described a simpler technique for culturing C. jejuni and C. coli from stool specimens, which allowed widespread isolation of these organisms. He used selective antibiotics in the media to select against competing flora and to promote the isolation of Campylobacter. This improved isolation method of Campylobacter has been used extensively in microbiological studies (Skirrow, 1977; Butzler et al., 1983; Bolton et al., 1984; Karmali et al., 1986; Goossen et al., 1989; Endlz et al., 1991).
1.3 EPIDEMIOLOGY
Campylobacter infections became reportable illness in the United States in the early 1980s (Aloos, 2001). In the early years of Campylobacter surveillance, stool samples were not tested for Campylobacter, they were tested for other enteric pathogens such as
Salmonella or Shigella (Allos, 2001). In later studies it was mentioned that diarrheal stool samples were cultured for Salmonella, Shigella as well as Campylobacter. Mead et al. (1999) reported that Campylobacter was implicated in diarrheal disease 2-7 times more frequently than Salmonella and Shigella (Mead et al., 1999).
year, Campylobacter was detected more frequently than other pathogens and even more frequently than Salmonella and Shigella combined (Allos, 2001).
According to the Centers for Disease Control and Prevention (CDC) approximately 2.4 million cases of Campylobacter infection occur in the United States each year, which involves almost 1% of the entire population (Friedman et al., 2000). In England and Wales, estimates showed that there were approximately 360,000 cases of food-borne Campylobacter infection in 2000, accounting for 27% of all food-borne disease (Adak et al., 2002). One study reported that in the Netherlands there are approximately 80,000 cases per year. This study also mentioned that unreported cases are significant and the true incidence could be five to 10 times higher (de Wit et al., 2001). The epidemiology of campylobacteriosis reveals two distinct patterns. One involves outbreaks in which a large number of people develop clinical symptoms; the other pattern is individual infections, or sporadic cases (White et al., 1997).
The vast majority of Campylobacter infections are sporadic; unlike with Salmonella and Escherichia coli (Kirk et al., 1997). The countries that have monitored prevalence of campylobacteriosis over time have observed that campylobacteriosis occurs much more frequently in the summer months than in the winter (O'Reilly et al, 2007).
early in life due to contaminated drinking water and close contact with animals and therefore have elevated Campylobacter- specific antibody levels in comparison to those children in the United States (Blaser et al., 1985; Blaser et al., 1986a; Martin et al., 1989). In contrast, in industrialized countries exposure is relative infrequent and protective immunity is only acquired by some groups with heavy occupational exposure (Cawthraw et al., 2000). Asymptomatic infections occur commonly in both children and adults in developing countries. On the other hand, asymptomatic Campylobacter infections are unusual in industrialized countries. However, in both developed and developing countries, Campylobacter remains one of the most common bacterial causes of diarrhea (Lee and Newell, 2006).
Contaminated water, raw milk, and farms are the common vehicles of transmission of Campylobacter in humans. Campylobacter is a zoonotic pathogen with many animal species serving as reservoirs for human disease. Possible animal sources of infection include poultry, cows, pigs, sheep, rabbits, rodents, wild birds, and domestic pets (Nachamkin, 1997; Saeed et al., 1993).
(Moore et al., 2005). The final slaughterhouse stages of washing and chilling the carcasses with chlorinated water reduce the contamination, but do not eliminate it (Sanchez et al., 2002). Stern and associates (1995) reported that slaughtering and processing can increase bacterial counts on carcasses up to 1000fold (Stern et al., 1995). C. jejuni has been found to survive on fresh, chilled or frozen meat for a long period of time (Butzler, 2004; Jorgensen et al., 2002). C. jejuni is a prevalent pathogen on poultry products found in retail stores and was recovered from approximately 70% of tested poultry from grocery stores in the United States (Zhao et al., 2001). Not only has C. jejuni been isolated from the external packaging of chicken, but also nearly 90% of chicken tested was found to be contaminated with C. coli as well as C. jejuni (Wong et al., 2007).
Other food animals: It was reported that the gastrointestinal tract of other food animal species such as cattle, sheep and pigs is frequently colonized with campylobacters, particularly C. jejuni and C. coli (Minihan et al., 2004). The digestive tract of clinically normal cattle has been demonstrated to be a significant reservoir for a number of
Campylobacter spp. (Atabay and Corry, 1998). Prevalence of the enteropathogen in cattle ranged from 0-80%. Prevalence of Campylobacter in sheep was reported to be generally lower, approximately 20% (Zweifel et al., 2004). Numerous studies have reported high prevalence of campylobacters in pigs. It is also reported that dressed pig carcasses are more frequently contaminated than either beef or sheep (Nesbakken et al., 2003).
Hopkins et al., 1984; Lehner et al., 2000; Peterson, 2003; Riordan et al., 1993; Sibbald and Sharp, 1985).
Water and Seafood: Campylobacter spp. contamination of surface water likely comes from fecal contamination by wild birds, domestic animals or sewage effluent (Engberg et al., 1998; Terzieva and McFeters, 1991; Waldenstrom et al., 2002). Surface water contamination is recognized as a source of campylobacteriosis outbreaks (Jones and Roworth, 1996). Campylobacter spp. have also been isolated from streams, seawater and recreational waters (Korhonen and Martikainen, 1991; Terzieva and Mcfeters, 1991). Accidental ingestion can account for exposure to the organism, and may cause some sporadic cases in the community. Drinking water supplies are generally chlorinated, thus are regarded as being free of Campylobacter spp., provided safety procedures are maintained to prevent contamination with untreated water (Blaser et al., 1986). An increased risk of infection has been associated with drinking water from non-urban supplies (Ikram et al., 1994). Shellfish contaminated with Campylobacter spp. have been reported after harvesting from Campylobacter-contaminated water (Abeyta et al., 1993; Engberg et al., 1998).
1.4 MODES OF TRANSMISSION 1.4.1 Introduction
broiler flocks, and it is unlikely that there is a single dominating source of Campylobacter transmission. Rather, diverse sources of infection may exist on different farms.
1.4.2 Vertical transmissions
Even though several observations from different studies support the theory that vertical transmission can be a source of Campylobacter infection, this type of transmission of campylobacters to flocks via contaminated eggs remains controversial for several reasons. First, young broiler chickens usually lack C. jejuni before 2 or 3 weeks of age, even when the chicks are hatched from eggs from infected parent flocks (Annan-Prah and Janc, 1988; Berndtson et al., 1996a). Secondly, broilers from the same parent flocks can be colonized by Campylobacter or they can be Campylobacter-free (Jacobs-Reitsma, 1995). Thirdly, broiler flocks are frequently infected with strains different from those infecting breeder flocks (Berndtson et al., 1996b; Shanker et al., 1983; Chuma et al., 1997; Sahin et al., 2003; van de Giessen et al, 1998). In addition, unlike Salmonella, Campylobacter survive poorly in egg contents and shell. It was reported that it is very difficult and rare to find C. jejuni from eggs from naturally or experimentally infected chickens (Doyle, 1984; Shanker et al., 1986). Live Campylobacter cells have not been detected in hatcheries or young hatchlings (Shanker et al., 1986; Annan-Prah and Janc, 1988; Kazwala et al., 1990; Chuma et al., 1994)
with C. jejuni, when a pool of whole eggs were mixed in a blender and subjected to selective enrichment for isolation (Sahin et al., 2001). Shane et al. (1986) isolated the organism both from the interior surface of the eggshell and the egg contents after swabbing feces containing Campylobacter onto the surface of the eggs (Shane et al., 1986).
Campylobacter isolates have been recovered from various segments of the reproductive tract including the oviduct (Camarda et al., 2000). This organism has also been recovered from rooster semen, suggesting that the genital tract of hens may also be infected venereally, providing a possibility for egg contamination (Cox et al., 2002).
1.4.3 Horizontal transmission
Horizontal transmission is the most probable route of Campylobacter transmission for infection of poultry (Sahin et al., 2002) and swine (Alter et al., 2005). Humans can become infected with Campylobacter either by direct contact with feces of infected animals or more likely through consumption of fecally contaminated animal products. Campylobacter does not gain entry into the poultry houses through feed. Because of its low moisture content, feed does not promote survival or growth of Campylobacter (Evans, 1992; Jacobs-Reitsma et al., 1995, van de Giessen et al., 1998) but it can be contaminated from other sources, such as feces in the chicken house (Gregory et al., 1997). Litter is a common component in poultry houses but fresh litter is not a likley source of infection (Berndtson et al., 1996a). Litter is relatively dry and Campylobacter may not grow under such arid conditions. However, used litter may become contaminated by Campylobacter and may play a role in maintaining
Campylobacter in the farm environment (Montrose et al., 1985). A study by Payne et al. (1999) did not support the role of litter in the transmission of the organism to successive flocks in the same poultry house. Usually broiler houses are cleaned and disinfected and the litter is changed between consecutive flocks. Therefore litter seems an unlikely source of infection in commercial broiler production (Payne et al., 1999).
Several studies reported that insects such as house flies, darkling beetles, cockroaches, and mealworms can act as mechanical vectors and may transmit Campylobacter from animal reservoirs to chicken flocks (Rosef and Kapperud, 1983; Shane et al., 1985; Jacob-Reitsma et al., 1995, 1997). Insects in poultry houses are usually not positive for Campylobacter until the organism is isolated from broilers (Brendtson et al., 1996a; Nesbit et al., 2001). Therefore, the possibility of insects as an original source of infection for broiler houses is small, but they may carry the organism from one location to another within or between flocks (Rosef and Kapperud, 1983; Shane et al., 1985; Berndtson et al., 1996a; Gregory et al., 1997). In several studies, the rodent (mice and rats) and small wild animals such as raccoons have been shown as carriers of Campylobacter (Annan-Prah and Janc, 1988; Kapperud et al., 1993; Berndtson et al., 1994; Nesbit et al., 2001). Wild birds have been indicated as a potential vector of Campylobacter (Yogasundram et al., 1989; Broman et al., 2000; Chuma et al., 2000; Fallacara et al., 2001; Jeffrey et al., 2001). Wild birds around the poultry production facilities are often found infected with Campylobacter, however, isolates from wild birds are typically different from those colonizing the chickens (Rosef et al., 1985; Annan-Parah and Janc, 1988; Gregory et al., 1997; Nesbit et al., 2001). The exact role of wild birds in the introduction of Campylobacter into broiler house is debatable.
Cardinale et al., 2004). Farm workers may carry Campylobacter from one flock to another if they move between different flocks without changing clothes and boots. Many studies have indicated that the application of hygiene barriers significantly reduced the prevalence of Campylobacter in broiler flocks (Kapperud et al., 1993; van de Giessen et al., 1996; Hald et al., 2000; Saleha, 2004).
Most studies have suggested that the best way to control Campylobacter transmission during production is through biosecurity measures. In particular, important methods appear to include treatment of drinking water and cleaning of the drinking water systems between flocks, hygiene measures for workers or visitors such as disinfection or change of boots and clothing before entering into the rearing environment; and finally control of rodents, wild birds, and flies on the farm (Corry and Atabay, 2001).
1.5 PATHOGENESIS AND VIRULENCE FACTORS 1.5.1 Introduction
C. jejuni and C. coli are leading causes of food-borne gastroenteritis. Through their virulence factors they are able to colonize the host, avoid the host’s immune response, enter into and exit out of the cells and obtain nutrition from the host. Several major virulence factors have been recognized to be important for the induction of human gastroenteritis due to Campylobacter infection.
1.5.2 Flagella and motility
demonstrated that motility plays an important role in pathogenesis because non-motile strains were unable to colonize experimental animals or volunteers (Pavlovskis et al., 1991).
Two genes, flaA and flaB, that are involved in production of the flagellar filament, was identified in C. jejuni and C. coli (Fischer and Nachamkin, 1991; Guerry et al., 1991; Wassenar et al., 1991). Wassenar and associates (1993) demonstrated that mutants that did not express subunit A but expressed subunits B produced truncated, stubby flagella, and were completely non-motile, whereas mutants that did not express the B subunit showed slightly decreased motility, but were still capable of movement (Wassenar et al., 1993).
When the pflA gene was mutated (deleted) it resulted in a non-motile bacterium with paralyzed flagella (Yao et al., 1994), suggesting that the gene was required for function of the flagellar motor. Immobilized flagella could mediate some adherence but in absence of motility adherence did not result in invasion (Yao et al., 1994). The importance of motility in colonization has been demonstrated in several animal models, including mice (Newell et al., 1985), rabbits and hamsters (Pavlovskis et al., 1991). A human volunteer experiment also suggested that motility was required for human intestinal infection (Black et al., 1988). 1.5.3 Chemotaxis
tract by bacteria and almost every motile bacterial species studied to date was found to possess this response system. Chemotactic responses allow bacteria to identify optimal growth conditions, avoid toxic substances, or target specific tissues for interaction or invasion of a host (Lux and Shi, 2004). It was mentioned as an important factor in C. jejuni and C. coli colonization of the avian intestinal tract (van Vliet and Ketley, 2001). In one study, chemotactic motility was described as central to the intestinal lifestyle of C. jejuni and as an essential prerequisite to pathogenesis in human disease (Marchant et al., 2002). By using a mouse model, it was found that non-chemotactic mutants were incapable of colonizing the intestine (Takata et al., 1992).
The importance of chemotaxis in colonization by C. jejuni has been mentioned in several studies. It was first demonstrated using chemically mutagenized motile but non-chemotactic strains. Even though the strains were actively motile, they failed to colonize the intestinal tract of suckling mice, indicating that chemotactic movement was important for colonization of the intestinal tract of the mice by C. jejuni (Taka et al., 1992).
A study was performed to compare the expression of chemotaxis in C. jejuni at two physiologically important temperatures, 37ºC and 42ºC. The chemotactic ability was greater at a temperature of 37ºC (human temperature) than at 42ºC (avian temperature), which suggest that the physiological temperature of humans might be more favorable for the expression of virulence in Campylobacter than that of birds (Khanna et al., 2006).
During periods of smooth swimming in viscous solution, C. jejuni traveled much farther and with greater velocity than during tumbling (Szymanski et al., 1995). Ferrero and Lee (1988) measured the path lengths of C. jejuni in two solutions of different viscosities and they found rapid increasea in the proportion of cells that displayed longer path lengths during smooth swimming in a high-velocity medium (Ferrero and Lee, 1988).
1.5.4 Adhesion and invasion
Thermophilic Campylobacter crosses through the mucus layer of the intestine, and adheres to the epithelial cells that lie underneath. Adherence is often an essential step in bacterial pathogenesis or infection, required for colonization of a new host (Coutte et al., 2003). To effectively adhere to host surfaces, many bacteria produce multiple adherence factors called adhesins. Possible reported adhesins include flagella, outer membrane proteins (OMPs), and lipopolysaccharides (LPS) (Fauchere et al., 1989; McSweegan et al., 1987).
In C. jejuni flagellin was identified as an adhesin. Fields and Swerdlow (1999) reported that the flagellum is necessary to invade intestinal epithelial cells. In the study, mutants of flaA, the primary structural gene for flagella, were unable to colonize 3-day-old chicks and could not invade human intestinal epithelial cells in vitro (Fields and Swerdlow 1999). Several studies indicated that adhesion and invasion are dependent on both motility and flagellar expression. In these studies mutant C. jejuni with paralyzed flagella showed reduced adhesion and no invasion. The studies suggested that flagella are involved in adherence, and adhesins are involved in subsequent internalization (van Vilet and Ketley 2001). Nachamkin et al. (1993) orally administered a wild type flagellated, a nonflagellated mutant and a reduced flagella mutant to 3-day-old chicks at levels of 6.6×108 CFU per chick. Only the fully motile, wild type strain colonized the chick ceca, indicating that flagella are essential for colonization (Nachamkin et al., 1993).
binding of C. jejuni to epithelial cells and fibronectin, respectively. PEB1 mutants showed decreased adherence and invasion of HeLa cells, and their ability to colonize mice was compromised (Pei et al., 1998).
Konkel et al. (1997) showed that CadF mediates the binding of C. jejuni to fibronectin. CadF mutants could not bind fibronectin, a cell surface glycoprotein, and were thus unable to colonize newly hatched chicks (Konkel et al., 1997). A surface-exposed lipoprotein, J1pA (42.3 kDa), was reported to play a role in adherence of C. jejuni to HEp-2 epithelial cells (Jin et al., 2001). Thus a number of Campylobacter OMPs have been associated with binding to various eukaryotic cells.
The major outer membrane constituents lipopolysaccharides (e.g. possessing an O-antigen) and lipooligosaccharides (LOS) are the major surface antigens of Gram-negative bacteria and play an important role in the interaction of these bacteria with their host and the environment. These surface polysaccharide molecules in C. jejuni are involved in serum resistance, endotoxicity and adhesion (Jin et al., 2001; van Vliet and Ketley, 2001). Bacon et al. (2001) demonstrated that the high molecular weight glycan capsule produced by some C. jejuni strains is required for cell invasion in vitro and for full virulence in the ferret animal model (Bacon et al., 2001).
1.5.5 Toxins
intracellular cyclic AMP (cAMP) levels, whereas cytotoxins are defined as proteins that kill target cells via intracellular activity or pore formation (Wassenaar, 1997).
Enterotoxin production by C. jejuni was first described by Ruiz-Palacios et al, (1983). They showed that C. jejuni can produce heat labile (HL) enterotoxins that share functional properties and have partial immunological homology with cholera toxin (CT) and E. coli heat labile (HL) enterotoxin (Ruiz-Palacios et al., 1983).
The Chinese Hamster Ovary (CHO) test is a common method to show production of Campylobacter HL enterotoxin. In this method enterotoxin raises the intracellular cyclic AMP level, which causes cytotoxic changes in the CHO cells (Klipstein and Engert, 1984). Some investigators use the rat ileal loop test for enterotoxin, and agreement between the rat ileal loop and the CHO cell test was found in comparative studies (Ruiz-Palacios et al., 1983).
Klipstein and associates (1985) documented avirulent C. jejuni strains. In this study avirulent isolates from asymptomatic carriers did not produce any enterotoxin or cytotoxin or cause fluid accumulation in rat ligated ileal loops. (Klipstein et al., 1985). This study also mentioned that isolates from individuals with invasive-bloody diarrhea failed to produce enterotoxin, but all of them produced cytotoxin (Klipstein et al., 1985).
in the suckling mouse assay, and it was not neutralized by anti-Shiga-like and cholera toxins (Guerrant et al., 1987).
The best characterized toxin attributed to Campylobacter spp. is the cytolethal distending toxin (CDT). Johnson and Lior (1988) first described CDT production by C. jejuni (Johnson and Lior, 1988). CDT causes eukaryotic cells to arrest in the G2/M phase of the cell cycle, preventing them from entering mitosis, leading to cell death (Newell, 2001). The C. jejuni cdt operon consists of three adjacent genes, cdtA, cdtB and cdtC that encode proteins with predicted molecular masses of 30, 28, and 21kDa, respectively. All three genes were required for toxic activity with HeLa cells (Pickett et al., 1996). Most C. jejuni and C. coli strains carry the cdt genes but there are differences in the amount of CDT produced (Pickett et al., 1996). Another study indicated that 100 of 101 isolates of C. jejuni contained the cdt gene and also showed cytotoxic effects in a Vero cell line (Bang et al., 2001). Some studies mentioned that cdt-negative strains are able to exhibit cytotoxic effects (Jain et al., 2008). 1.5.6 Iron acquisition
vivo growth (Bullen, 1981; Litwin et al., 1993). In order to survive they maintain iron homeostasis through the differential expression of iron uptake and storage systems.
In response to an iron-deficient environment many bacteria acquire iron by siderophores (Palyada et al., 2004). Campylobacters produce few or no siderophores, but they are able to use exogenous ferrichrome and enterochelin from other bacteria and heme compounds, which might be released in the site of inflammation (Baig et al., 1986; Field et al., 1986; Pickett et al., 1992). Thus Campylobacter may acquire iron in the gastrointestinal tract via siderophores (such as enterobactin) produced by the indigenous microflora, even if it does not synthesize its own siderophores (Field et al., 1986). Campylobacters posses a transport system, encoded by the ceu operon (ceuBCDE; campylobacter enterochelin uptake) that might scavenge siderophores in the intestinal tract (Richardson & Park, 1995). However, chick colonization studies with a ceuE mutant showed that this system is not necessary for chick colonization (Ketley, 1997). This study suggested that campylobacters possess additional iron uptake system(s) that complements for the loss of the Ceu uptake system. Campylobacters from this chick study showed colonization, but they were not capable of tissue invasion. This particular uptake system may play a role during tissue invasion (Ketley, 1997).
Like many Gram-negative bacteria Campylobacter achieve iron-responsive genetic regulation via the ferric uptake regulator (Fur) protein (Holmes et al., 2005). When intracellular ferrous iron is present in excess it is bound by the Fur protein. A functional C. jejuni fur has been shown to regulate a number of genes in response to iron levels (van Vliet et al., 1998). Holmes and associates (2005) demonstrated the role of Fur in iron acquisition in C. jejuni. They reported that iron limitation resulted in the expression of a siderophore-medited system at higher levels in the wild-type strain than in the fur mutant, whereas higher levels of expression were detected in the fur mutant under iron-rich conditions. Such findings suggested that the transport system and genes involved in iron acquisition were regulated by the Fur protein (Holmes et al., 2005). The growth rate of the C jejuni fur mutant was lower than the wild type under both iron-limited and iron-rich condition (van Vliet, 1998).
Palyada and co-workers (2004) mentioned that a total of 53 genes were regulated by Fur and showed a significantly reduced rate of colonization of the fur mutant in comparison to the wild-type strain in the gastrointestinal tract of chicks, thus indicating the importance of iron homeostasis in vivo (Palyada et al., 2004).
role in protection against oxidative stress when intracellular iron levels are high (Ketley, et al., 1997).
1.6 ANTIMICROBIAL RESISTANCE MECHANISMS 1.6.1 Fluoroquinolone resistance
The quinolones are a family of synthetic broad-spectrum antibiotics. Nalidixic acid is the parent group of quinolones, and it was the first commercialized quinolone antimicrobial. Fluoroquinolones (FQs) are derived from the quinolone structure of nalidixic acid and contain a fluorine at the C-6 position. This fluorine enhances gyrase inhibition and cell penetration (Appelbaum and Hunter, 2000). FQ antimicrobials include ciprofloxacin, enrofloxacin, levofloxacin and others.
In bacteria the enzymes DNA gyrase and topoisomerase IV are both involved in DNA replication and transcription. GyrA and GyrB catalyze the ATP-dependent negative supercoiling of double-stranded closed-circular DNA and relieve topological stress arising from the translocation of transcription and replication complexes along the DNA. Topoisomerase IV (ParC and ParE subunits) resolves interlinked daughter chromosomes following DNA replication (Drlica and Zhao, 1997; Hooper, 2001). After entering into the bacterial cells FQs interact with these two target enzymes (Hooper, 2001).
were also reported (Ge et al., 2005). Even though high-level FQ resistance was reported to be caused by a point mutation (Arg-139-Gln) in parC (Gibreel et al., 1998), several independent investigations later indicated that C. jejuni and C. coli lack parC (Parkhill et al., 2000; Bachoual et al., 2001; Piddock et al., 2003).
Pore-forming proteins ‘porins’ allow exchange of hydrophilic compounds across the outer membrane of Gram-negative bacteria. Porins are involved in adaptation of many bacteria to their environment. Two porins, a major outer membrane protein (MOMP) (de et al., 2000) and a minor one (Omp 50) (Bolla et al., 2000) were characterized in C. jejuni. Omp 50 is not found in C. coli (Dedieu et al., 2004). However, modification in expression or porin sequence was not associated with antibiotic resistance in Campylobacter (Payot et al., 2002; Luo et al., 2003; Pumbwe et al., 2004).
Active extrusion of FQs can take place via an efflux pump system in Campylobacter (Charvalos et al., 1995). CmeABC is the most commonly identified efflux pump that contributes to antibiotic resistance in C. jejuni and C. coli (Lin et al., 2002; Pumbwe and Piddock, 2002; Luo et al., 2003; Ge et al., 2005; Lin et al., 2005). This pump contributes significantly to both intrinsic and acquired resistance of C. jejuni to FQs and other antimicrobials (Ge et al., 2005; Lin et al., 2002; Luo et al., 2003; Pumbwe and Piddock, 2002).
FQ in Campylobacter cells and acted in conjunction with the gyrA mutations in conferring and maintaining high-level FQ resistance in clinical isolates (Ge et al., 2005; Luo et al., 2003). Lin et al. (2005) reported that inactivation of cmeABC increased the susceptibility of certain strains (Lin et al., 2005). Yan et al. (2006) reported that this efflux pump plays an important role in the emergence of FQ-resistant Campylobacter under selection pressure because many of the spontaneous gyrA mutants can not survive the selection by ciprofloxacin in the absence of CmeABC (Yan et al., 2006). This study also showed that over-expression of CmeABC reduced the aggregation of ciprofloxacin in C. jejuni and increased the resistance conferred by many types of gyrA mutations.
The most important characteristic of fluoroquinolone resistance in Campylobacter is that it does not require stepwise accumulation of point mutations in gyrA; rather, a single point mutation in gyrA can lead to clinically relevant levels of resistance to FQs (Ge et al., 2005; Zhang, 2003). Studies in chickens demonstrated that in vivo FQ-resistant Campylobacter mutants arose within 24 h of FQ treatment and reached up to 107 CFU/g feces (Luo et al., 2003; Griggs et al., 2005; Farnell et al., 2005).
1.6.2 Tetracycline resistance
In Gram-positive bacteria tetracyclines freely diffuse through the cell membrane and accumulate in the cytoplasm (Chopra and Roberts, 2001). But in Gram-negative bacteria tetracyclines bind with Mg2+ cations and pass through outer membrane porins into the periplasmic space, where the drug dissociates from magnesium and moves passively through the inner membrane (Chopra and Roberts, 2001). After entering into the cytoplasm tetracycline binds reversibly with the ribosome and inhibits translation by preventing the incoming aminoacyl-tRNA from binding to the A site of the ribosome (Connell et al., 2003). Four different bacterial strategies of resistance have been identified: (i) protection of the ribosomal binding site of tetracycline by a soluble protein, (ii) prevention of cytoplasmic accumulation, e.g., by an energy-dependent efflux of tetracycline from the cell, (iii) chemical modification of the drug, and (iv) a mutation in the 16S rRNA that affects the drug’s binding site (Schnappinger and Hillen 1996).
CmeABC and Tet(O) has been mentioned in several studies (Gibreel et al., 2007; Lin et al., 2002).
Tetracycline resistance in C. jejuni is primarily shown to be plasmid-mediated (Taylor et al., 1981; Tenover et al., 1983.). A study of 15 tetracycline-resistant C. jejuni isolates to analyze plasmid-mediated tetracycline resistance found that resistant isolates harbored one of two plasmids (pFKT1000 and pFKT20) (Tenover et al., 1983). Taylor et al. (1983) characterized several plasmids responsible for tetracycline resistance in C. coli and C. jejuni and found that tetracycline resistance plasmid from C. coli and C. jejuni from various sources showed pronounced DNA homology (Taylor et al., 1983). The study demonstrated that tetracycline resistance plasmids were able to transfer via conjugation within the same species and from one species to another (Batchelor et al., 2004; Gibreel et al., 2004; Pratt and Korolik, 2005). In addition, in vivo transfer studies showed a rapid transmission of tet(O) gene transfer of C. jejuni in the chicken digestive tract (Avrain et al., 2004).
The size of plasmids carrying tet(O) ranged from 45 to 58 kb and level of tetracycline resistance was extremely high (512µg/ml) in Campylobacter spp. recipients (Gibreel et al., 2004). Two large tetracycline-resistance plasmids, pTet 45.2(kb) from C. jejuni strain 81-176, and pCC31 (44.7kb) from C. coli strain CC31, have been sequenced (Batchelor et al., 2004). Even though tet(O) is mainly located on transferable plasmids, it can be located on the chromosome in some strains (Dasti et al., 2007; Manavathu et al., 1988; Ng et al., 1987; Pratt and Korolik, 2005; Taylor et al., 1988).
sequence identity with that of the tet(O) gene in Streptococcus mutans DL5. The guanine-plus-cytosine content of tet(O) gene was 40%, which is considerably higher than that typical for C. jejuni and C. coli plasmids or chromosomes (31 to 33%). Codon usage in tet(O) was more similar to that of Streptococcus tet(O) genes than to Campylobacter spp., and the ribosomal binding site of tet(O) is complementary to the 3' end of the 16S rRNA of Bacillus subtilis (Batchelor et al., 2004; Khachatryan et al., 2006; Sougakoff et al., 1987; Taylor et al., 1983). Also, Southern blot experiments showed the similarity between Campylobacter tet(O) and genomic DNA from tetracycline-resistant Streptococcus and Enterococcus spp. (Taylor et al., 1983; Zilhao et al., 1988). All these observations strongly suggest that Campylobacter acquired the tet(O) gene from Gram-positive bacteria by horizontal gene transfer.
1.6.3 Macrolide resistance
Macrolides are a group of drugs (typically antibiotics) that are characterized by the large lactone ring (12-16 members) and are broad-spectrum antibiotics. They bind to a site in 23S rRNA causing early release of peptidyl-tRNA and resulting in inhibition of translation (Payot et al., 2006). Yan and Taylor (1991) indicated that erythromycin resistance in C. jejuni and C. coli is quite possibly chromosomally mediated (Yan and Taylor, 1991).
(Leclercq, 2002). However, the other mechanisms were described in Campylobacter (Lin et al, 2005; Taylor et al., 2005).
Several point mutations in 23S rRNA have been associated with Campylobacter resistance to macrolides (Gibreel et al., 2005). These mutations occur at the base position 2074 (A2074C, A2074G, or A2074T) or 2075 (A2075G, A2075T, or A2075C) or both. Both the A2074G and A2075G changes can confer high-level resistance (MIC>128µg/mL) to macrolide antibiotics, but the A2075G mutation is more frequently observed than the A2074G mutation (Gibreel and Taylor, 2006). The point mutation probably weakens the binding between the ribosome and the lactone ring of macrolides (Franceschi et al., 2004).
Payot et al. (2006) identified that mutations in the ribosomal proteins L4 and L22 affected macrolide binding. This study also reported that even though mutation in ribosomal proteins L4 and L22 confer resistance in Streptococcus pneumoniae, Streptococcus pyogenes, and Haemophilus influenzae, mutations in either the L4 or L22 proteins in Campylobacter were not detected (Payot et al., 2006); Davydova et al. (2002) demonstrated that mutation in L22 changes its beta-hairpin structure, which affects the interactions between L22 and 23S rRNA. As a result, the erythromycin-binding pocket becomes destabilized, which allows the passage of the nascent peptide (Davydova et al., 2002). However, mutations in ribosomal proteins L4 and L22 conferred only low levels of macrolide resistance in Campylobacter (Cagliero et al., 2006; Corcoran et al., 2006).
(Mamelli et al., 2003; Payot et al., 2004). Even though the efflux pump system CmeABC itself confers low-level resistance to macrolides (Corcoran et al., 2006), it can act in conjunction with other mechanisms such as the A2075G mutation in the 23S rRNA in conferring the acquired resistance to macrolides in C. coli (Cagliero et al., 2005; Cagliero et al., 2006). Another way of macrolide resistance was described by erythromycin ribosome methylase (erm) in E. coli and Campylobacter rectus (Roe et al., 1995).
1.6.4 Aminoglycoside resistance
Aminoglycosides such as kanamycin and streptomycin are widely used in human and animal medicine and have broad-spectrum activity (Luangtongkum et al., 2006). In several studies, four general mechanisms of aminoglycoside resistance in bacteria have been described. The mechanisms include reduced accumulation of the drug in the intracellular environment, the methylation of 16S rRNA in sites that interfere with efficient binding of the drug, mutations of binding sites of the ribosomal RNA and enzymatic modification of the drug. Resistance due to the enzymatic modification of the drug is the most common mechanism (Jana and Deb, 2006; Magnet and Blanchard, 2005).
common form of resistance regarding kanamycin and structurally related antibiotics involves the synthesis of 3'-aminoglycoside phosphotranferases [APH (3')] (Davies and Smith 1978). 1.6.4.1 Kanamycin resistance
Resistance to kanamycin was first described in France in C. coli strain BM2509 and in another C. coli strain isolated in Spain (Rivera et al., 1986). Several studies reported that kanamycin resistance is plasmid-mediated and more often associated with C. coli than with C. jejuni (Tenover et al., 1992; Rivera et al., 1986; Diane and Patrice, 1988). In general, resistance to kanamycin is conferred by APH (3') enzymes. These enzymes are classified into eight sub-groups symbolized by roman number (I to VIII) (Smith and Baker, 2002). In Campylobacter types I, III, IV, and VII APH (3') enzymes have been described (Taylor et al., 1988).
Trieu et al. (1985) cloned aphA-3 from C. coli plasmid pIP1433 and showed that the gene conferred kanamycin resistance in E. coli. This aphA-3 gene encoded 3'aminoglycoside phosphotransferase of type III, that was previously found only in Gram-positive cocci. This gene was also shown to be transcribed in B. subtilis (Trieu-Cuot et al., 1985). Thus, resistance to kanamycin in C. coli is probably due to in vivo acquisition of a gene from a Gram-positive coccus (Lambert et al., 1985). Recently aphA-3 has been found in plasmids of C. jejuni strains (Gibreel et al., 2004). Tenover et al. (1989) mentioned another kanamycin-resistance phosphotransferase gene, apha-7, which was identified on a 14-kb C. jejuni plasmid, pS1178 (Tenover et al., 1989).
1.6.4.2 Streptomycin resistance
1.7 EFFECTS OF ANTIBIOTICS IN FOOD ANIMALS ON HUMAN HEALTH
In the last few years, human campylobacteriosis has dramatically increased in industrialized countries, thus representing one of the main causes of bacterial foodborne diseases. Zoonotic Campylobacters are routinely found in cattle, sheep, swine, and avian species. The avian species are the most common host for both C. jejuni and C. coli (Skirrow, 1977). Most infections in humans are caused by C. jejuni, and only a small fraction is caused by C. coli (Pezzotti et al, 2003).
Worldwide the concern for foodborne infections has increased because of the frequent isolation of antimicrobial resistant strains from humans and animals. Antibiotic resistance development in Campylobacter has been speculated to be a consequence of the excessive use of antibiotics in modern intensive animal production for therapeutic and subtherapeutic purposes. Campylobacters that are resistant to antibiotics and present in food-producing animals may transfer from food animals to humans by various means (Zhang et al., 2003). Therefore, food of animal origin may represent a vehicle of transmission of resistant Campylobacter to humans (Luangtongkum et al., 2009). Campylobacter has been reported to develop resistance against several antimicrobials including fluorquinolones, macrolides, aminoglycosides, and tetracycline (Padungton and Kaneene, 2003; Moore et al., 2006).
(Chopra and Roberts, 2001). The antimicrobials cause high and continuous selective pressure for the animal-colonizing bacteria, ultimately resulting in the acquisition of antimicrobial resistance genes (Butaye et al., 2003).
1.7.1 Effects of fluoroquinolones (FQs) in food animals on human health
FQs are generally used to treat colibacillosis and Mycoplasma infections in poultry but as a side effect, the Campylobacter spp. present in the intestines of the bird become resistant (Wagener, 2006). However, FQs do not completely eliminate Campylobacter from the bird’s intestinal tracts. They create selection pressure on the surviving campylobacters and rapidly select for mutations during the treatment, which ultimately causes emergence and dissemination of FQ-resistant campylobacters (Wagener, 2006).
Before 1989, FQs were mainly used in human medicine and resistance was rare (Endtz et al., 1991), but after the introduction of FQs in veterinary medicine in the early 1990s in Asia and Africa, in the United States and in European countries such as Sweden, the Netherlands, and Spain, the resistant Campylobacter infections in humans increased (Luangtongkum et al., 2009).
(Gupta et al., 2004; Nachamkin et al., 2002; Luangtongkum et al., 2009). In 1999, a Minnesota study reported significant increases in quinolone resistant Campylobacter infections acquired domestically. The study also indicated that increased prevalence was associated with the approval of FQs for use in poultry in the United States (Smith et al., 1999). According to the Food and Drug Administration (FDA), the use of FQs in chickens in the United States compromised the treatment with FQs of almost 10,000 people each year (Angulo et al., 2004). Several studies reported that FQ- resistant Campylobacter infections cause prolonged diarrhea (Nelson et al., 2004). In the Minnesota study, Smith et al. (1999) also reported that persons with quinolone-resistant infections had a median duration of diarrhea that was 3 days longer than that of persons with quinolone-susceptible infections (Smith et al., 1999). In Denmark, Engberg et al. (2004) also reported similar finding (Engberg et al., 2004).
Spain: In Spain, enrofloxacin was approved for veterinary use in 1990 and one year before the approval no FQ-resistant Campylobacter was found from human samples. In 1991, 30-50% campylobacters from human fecal samples were resistant. A few years later (1997-1998), the FQ-resistant Campylobacter isolates increased to 99% in broiler and 75% in humans, and to 100% in pigs (Saenz et al., 2000). The level of FQ-resistant Campylobacter observed in Spain was highest among the surveyed European countries (Luangtongkum et al., 2009).
enrofloxacin was introduced for veterinary use in the Netherlands in 1987. The FQ-resistant Campylobacter isolates were 0 percent in poultry products from 1982 to 1983 or in humans from 1982 to 1983 or 1985. After introducing enrofloxacin the percentage of FQ resistant isolates in poultry products increased to 8.4% in 1987/1988 and 14% in 1989 (Endtz et al., 1991). Another study in the Netherlands documented that in 1992 and 1993 the percentage of FQ-resistant isolates from broilers increased to 29% (Jacobs-Reitsman et al., 1994). The emergence and subsequent increase in resistance among isolates causing infections in humans has been observed after the emergence of resistance among poultry products and broilers. In 1982-1983, no FQ-resistant Campylobacter was detected in humans, but in 1989, the resistance prevalence in Campylobacter was 11% and by 1997 it increased to 29% (Endtz et al., 1991; Talsma et al., 1999). Similar trends have been observed in other countries where FQs were approved in veterinary medicine.
Belgium: In Belgium, flumequine (a quinolone) was licensed for use in poultry in 1982, enrofloxcin (FQ) in 1988, and difloxacin (FQ) in 1998. One study conducted in Belgium reported that 44% of broiler C. jejuni isolates and 35% of turkey C. jejuni isolates were resistant to ciprofloxacin (Zhang et al., 2003).
Germany: In 2001, in Germany, 41-46% of Campylobacter isolated from humans was resistant to ciprofloxacin, whereas 42% of C. jejuni and 71% of C. coli isolates from chickens were resistant (Luber et al., 2003).
Thailand: In 1995, the incidence of the FQ-resistant Campylobacter infection in Thailand was reported as 84% (Unicomb et al., 2003; Luangtongkum et al., 2009).
Hongkong. In Hongkong, the prevalence of quinolone resistance among C. jejuni from clinical isolates in 2002 was 85.9% (Chu et al., 2002; Luangtongkum et al., 2009).
Japan. In Japan, enrofloxacin was introduced for veterinary use in 1992. The Japanese Ministry of Agriculture, Forestry and Fisheries approved dosage (50 ppm for 3 days) of enrofloxacin in drinking water of chickens. However, studies showed that even minimal amount of FQ can increase the prevalence of FQ-resistant Campylobacter in chickens (Takahashi et al., 2005).
Australia and New Zealand. The rate of FQ-resistant Campylobacter for these two countries has been very low (Luangtongkum et al., 2009). In Australia, use of fluoroquinolones in food producing animals has been prohibited. Domestically acquired infections with fluoroquinolone-resistant Campylobacter spp. were rarely found in humans (Unicomb et al., 2003). Since the prevalence of FQ is very rare, the FQ remains an effective antibiotic for campylobacteriosis treatment.
1.7.2 Effects of tetracyclines in food animals on human health
The tetracyclines have been used for the treatment of infections in poultry, cattle, sheep, and swine for several decades. In some cases, the antibiotics are added directly to feed or water for therapeutic treatment of large numbers of poultry reared on commercial farms (Chopra and Roberts, 2001). The growth- promoting properties of tetracyclines were first reported in 1949. Initially this group of antibiotics was used in chickens. Because of its beneficial affect in the rate of growth and feed utilization by young chickens, afterward it was used with swine and cattle (Chopra and Roberts, 2001).
Tetracyclines were approved by FDA as feed additives in 1951 (chlortetracycline) and 1953 (oxytetracycline) (Chopra and Roberts, 2001). In 1969, Swann (from the United Kingdom) speculated that subtherapeutic use of tetracyclines and other antibiotics in farm animals might contribute to the development of resistant human isolates (Chopra and Roberts, 2001). This study influenced the ban of the use of tetracyclines for growth promotion in Europe in the early 1970s (Chopra and Roberts, 2001). Even though many countries restricted the use of this class of antimicrobials in food animals some countries are still using them, especially the United States and Australia (Chopra and Roberts, 2001). In countries where subtherapeutic uses of tetracyclines are still permitted, about 90% of tetracycline is used in cattle and swine, and only 10% in poultry (Chopra and Roberts, 2001).
contribute to tetracycline-resistant Campylobacter infections in humans (Chopra and Roberts, 2001).
Looveren et al. (2001) found higher frequency of tetracycline resistance in C. coli than C. jejuni. Particularly higher levels (62.3%) of tetracycline resistance were observed in C. coli strains from pigs (Looveren et al., 2001). A similar observation was described in Spain, where 94.4% of the C. coli strains isolated from pigs between 1997 and 1998 were resistant to tetracycline (Looveren et al., 2001). In Denmark, antibiotic growth promoter uses were banned in 1998. According to the Danish integrated antimicrobial resistance monitoring and research program after the ban almost no tetracycline resistance was found in Campylobacter strains from food animals (Looveren et al., 2001). Due to the increased prevalence of tetracycline-resistant infections in humans, many countries have reduced use of this antibiotic (Chopra and Roberts, 2001).
1.7.3 Effects of marcolides in food animals on human health
5% macrolide-resistant C. jejuni was found in humans, swine, and cattle (Luangtongkum et al., 2009). In the USA and Canada, 10% or less of Campylobacter from humans, broilers and cattle, was reported to be macrolide-resistant, while more than 40% of C. coli from turkeys and swine were found to be resistant to this antimicrobial in the USA. Increased macrolide resistance in C. coli isolated from swine was reported in Australia (Luangtongkum et al., 2009). The incidence of erythromycin resistance in human Campylobacter is still low and stable comparing to ciprofloxacin and tetracycline. Therefore, in many countries erythromycin has been considered as the drug of choice for Campylobacter enteritis (Gibreel et al., 2006).
1.7.4 Effects of avoparcin and vancomycin in food animals on human health
The Gram-positive glycopeptide antibiotics avoparcin and vancomycin are used in food animals as growth promoters. Avoparcin is generally used in broiler chickens, growing pigs, calves and beef cattle. It can also be used in the prevention of necrotic enteritis. Vancomycin is generally used in broilers and turkeys.
1.7.5 Interventions aimed at reducing the occurrence of antimicrobial resistant
Campylobacter
The incidence of Campylobacter infection is increasing worldwide and trends in antimicrobial resistance have shown a clear association between use of antibiotics in the veterinary industry and resistant isolates of Campylobacter in humans (Luangtongkum et al., 2009). It is hypothesized that antibiotic resistance will disappear if the selection pressure of antibiotic is removed. Many countries have discussed the issue of banning antibiotics as growth promoters but only few countries have taken specific measures.
1.8 NATURAL TRANSFORMATION
Most bacterial cells cannot take up DNA efficiently unless they are exposed to special chemical or electrical treatments to make them more permeable. However, some species of bacteria are able to take up DNA from their environment without requiring special treatment and they are called naturally competent bacteria. More than 40 species have been found to be naturally competent and transformable (Lorez and Wackernagel, 1994). Transformation was the first mechanism of bacterial gene exchange to be discovered (Griffith, 1928). In 1928, Fred Griffith found that one form of the pathogenic pneumococci (Streptococcus pneumoniae) could be transformed into another form. In later years, transformation was linked to uptake of DNA. Naturally competent transformable bacteria include Gram-positive organisms such as Bacillus subtilis and Streptococcus pneumoniae or Gram-negative species as Haemophilus inflenzae, Helibacter pylori and Neisseria gonorrhoeae (Dubnau, 1999).
1.8.1 Binding and uptake of DNA in Gram-positive bacteria
Two Gram-positive bacteria that have been well studied are Bacillus subtilis and Streptococcus pneumoniae. As an initial step in transformation double-stranded DNA binds to the binding sites of competent cell surface (Dubnau, 1999). Approximately 50 DNA binding sites have been estimated in each B. subtilis competent cell (Dubnau, 1999) and about 30-80 DNA binding site per competent cell have reported in S. pneumoniae (Lorenz and Wackernagel, 1994). During transformation in B. subtilis and S. pneumoniae double-stranded DNA associates rapidly with competent cells to form a complex and DNA can bind to the cell surface without base sequence preference (Lorenz and Wackernagel, 1994). B. subtilis does not have a preference between single-stranded and double-stranded DNA in the uptake (Vagner et al., 1990). Double-stranded transforming DNA has been detected intracellularly in one instance (Feitelson and Ganesan, 1982). Under acidic conditions, B. subtilis can bind and take up single-stranded DNA (Smith et al., 1981).
In both B. subtilis and S. pneumoniae the amount of DNA bound to the cell is proportional to the molecular size of the DNA (Dabnau, 1976). In B. subtilis the proton motive force and especially the ΔpH component work as a driving force for DNA uptake in transformation (van Nieuwenhoven et al., 1982).
1.8.2 Binding and uptake of DNA in Gram-negative bacteria
In transformation studies, two naturally competent Gram-negative bacteria Haemophilus influenza and Neisseria gonorrhoeae have been well studied (Snyder and Champness, 1997). Specific uptake sequences are required to take up DNA efficiently by these two Gram-negative bacteria (Dabnau, 1999). Smith et al. (1995) demonstrated that the 10 bp sequence (GCCGTCTGAA) is a recognition sequence which is required for efficient DNA uptake in N. gonorrhoeae (Smith et al., 1995). On the other hand, in Haemophilus efficient uptake requires the presence of another specific nucleotide sequence on the incoming DNA (5'- AAGTGCGGTCA-3'). The uptake signal sequences (USSs) for H. influenzae are often found as inverted-repeats between genes and formed stem-loop configurations in mRNA for transcription termination (Smith et al., 1995). However, not all Gram-negative bacteria show uptake sequence specificity. For instance, Acinobacter calcoaceticus can take up DNA from any source (Smith et al., 1995).
1.8.3 Transport of DNA in Gram-positive and Gram-negative Bacteria
Although many Gram-positive and Gram-negative bacteria differ in various cell surface properties, it is believed that the mechanism of DNA transport across the cytoplasmic membrane is conserved in both types of bacteria. In Gram-positive bacteria, after binding and fragmentation a single strand of DNA is translocated across the cytoplasmic membrane and achieves resistance to exogenously added DNase (Mejean and Claverys, 1984). In Gram-negative organisms double-stranded DNA crosses the outer membrane and achieves protection from the DNase. After uptake, the DNA follows the Gram-positive path, transported across the inner membrane as a single-stranded molecule. After entering into the cell the single-stranded transforming DNA might synthesize the complementary strand and reestablish itself as a plasmid. It could also stably integrate into the chromosome by homologous recombination of the translocated strand into the chromosome or the recipient DNA, or be degraded (Chen and Dubnau 2004). In both systems, the non-transported strand is degraded to acid-soluble products, which are released into the medium or periplasm (Chen and Dubnau, 2004).
1.8.4 Function of DNA uptake