RESEARCH ARTICLE
Wnt/
β
-catenin signaling enables developmental transitions during
valvulogenesis
Fernanda M. Bosada1,2, Vidusha Devasthali1, Kimberly A. Jones1,2and Kryn Stankunas1,2,*
ABSTRACT
Heart valve development proceeds through coordinated steps by which endocardial cushions (ECs) form thin, elongated and stratified valves. Wnt signaling and its canonical effector β-catenin are proposed to contribute to endocardial-to-mesenchymal transformation (EMT) through postnatal steps of valvulogenesis. However, genetic redundancy and lethality have made it challenging to define specific roles of the canonical Wnt pathway at different stages of valve formation. We developed a transgenic mouse system that provides spatiotemporal inhibition of Wnt/β-catenin signaling by chemically inducible overexpression of Dkk1. Unexpectedly, this approach indicates canonical Wnt signaling is required for EMT in the proximal outflow tract ( pOFT) but not atrioventricular canal (AVC) cushions. Furthermore, Wnt indirectly promotes pOFT EMT through its earlier activity in neighboring myocardial cells or their progenitors. Subsequently, Wnt/β-catenin signaling is activated in cushion mesenchymal cells where it supports FGF-driven expansion of ECs and then AVC valve extracellular matrix patterning. Mice lacking Axin2, a negative Wnt regulator, have larger valves, suggesting that accumulating Axin2 in maturing valves represents negative feedback that restrains tissue overgrowth rather than simply reporting Wnt activity. Disruption of these Wnt/β-catenin signaling roles that enable developmental transitions during valvulogenesis could account for common congenital valve defects.
KEY WORDS: Heart valves, Outflow tract, Atrioventricular canal, EMT, Cushion mesenchyme, Mitral valve, Axin2, Lef1, Versican, Tenascin, Spongiosa, Chordae tendineae, Wnt/β-catenin signaling
INTRODUCTION
Heart valve defects are remarkably common, affecting 2% of the population (Pierpont et al., 2007; Go et al., 2013). Many valve defects do not become pathologic until late adulthood, contributing to the high prevalence of valve replacement surgeries (Brickner et al., 2000). However, as with severe valve defects that require childhood intervention, the underlying abnormalities originate from disruption of embryonic valvulogenesis.
The mammalian heart has four sets of valves. The two semilunar valves (SLVs)–the aortic valve (AV) and pulmonic valve (PV)– have three symmetric hemi cup-like cusps. The cusps meet to form tight seals that only open under systolic pressure, allowing unidirectional blood flow from the ventricles into the systemic and pulmonic circulations, respectively. The mitral valve (MV) and tricuspid valve (TV) form the two atrioventricular canal (AVC) valves that support blood flow between the atria and ventricles.
These valves have broad flap-like leaflets that attach to the ventricular walls by chordae tendineae (CT).
The SLVs develop from two sets of endocardial cushions, the proximal and distal outflow tract ( pOFT and dOFT) cushions, that form in the unseptated arterial pole of the looping heart. Concurrently, AVC valve development initiates from two endocardial cushions at the junction of the common atrium and ventricle. Mesenchymal cells populate the endocardial cushions soon after they form. AVC cushion mesenchyme is derived by the endocardial-to-mesenchymal transformation (EMT) of overlying endocardial cells signaled to delaminate and invade the cardiac jelly of the cushions (Markwald et al., 1977). pOFT cushion mesenchyme also is largely EMT derived, whereas the dOFT cushions are primarily populated by cardiac neural crest cells (NCCs) (Kirby et al., 1983; Waldo et al., 1998; Jiang et al., 2000). EMT initiates around embryonic day (E) 9.5 and E10.0 in the AVC and pOFT, respectively (Camenisch et al., 2002).
EMT is a multi-step process. First, the cushion fields are specified by Notch and BMP signaling to generate specialized myocardium that produces EMT-inducing ligands and a correspondingly responsive endocardium (Timmerman et al., 2004; Luna-Zurita et al., 2010; Sugi et al., 2004; Ma et al., 2005; Wang et al., 2005; McCulley et al., 2008). TGF-β and other factors then induce changes in endocardial gene expression programs that repress epithelial-state determinants and upregulate mesenchymal factors that promote ECM invasion and cell migration (reviewed by MacGrogan et al., 2014). The canonical Wnt signaling pathway, involving transcriptional changes driven by stabilized nuclear β-catenin and TCF/Lef transcription factors, might also directly promote EMT (Liebner et al., 2004). However, it is unclear whether the loss of AVC mesenchyme in endothelialβ-catenin (Ctnnb1)-null mice results from disrupted Wnt signaling or the distinct adherens junction role ofβ-catenin (Gottardi and Gumbiner, 2004; reviewed by Heuberger and Birchmeier, 2010). Earlier Wnt/β-catenin signaling roles in cardiac progenitor cells (Ai et al., 2007; Klaus et al., 2007) raises the additional possibility that Wnt/β-catenin signaling helps establish EMT-competent cushion fields.
Following EMT, cushion mesenchyme undergoes controlled proliferation in response to mitogenic signals. Wnt reporter transgenic mice suggest that canonical Wnt signaling is active in AVC and OFT cushion mesenchyme during this growth phase (Gitler et al., 2003; Alfieri et al., 2010; Klaus et al., 2012; Gillers et al., 2015; Cai et al., 2013; Cambier et al., 2014). Known mitogenic roles of Wnt/β-catenin signaling in other developmental contexts (reviewed by Niehrs and Acebron, 2012) further support a role for Wnt in cushion expansion. However, no Wnt loss-of-function studies in mice have defined a proliferative or other role of Wnt signaling in cushion mesenchyme.
As development proceeds, the valve primordia formed by the cushions elongate into thin cusps (SLVs) or leaflets (AVC valves) (reviewed by Hinton and Yutzey, 2011). The cusps/leaflets become Received 21 September 2015; Accepted 31 January 2016
1
Institute of Molecular Biology, University of Oregon, Eugene, OR 97403-1229, USA.2Department of Biology, University of Oregon, Eugene, OR 97403-1229, USA. *Author for correspondence (kryn@uoregon.edu)
DEVEL
O
stratified into layers termed the ventricularis/atrialis, spongiosa and fibrosa. Each layer acquires unique biomechanical properties conferred by the expression of distinct ECM proteins. Elastin confers flexibility to the atrialis and ventricularis layers of the AVC valves and SLVs, respectively (Vesely, 1997). The interconnecting spongiosa layer is proteoglycan enriched, notably with versican (Vcan) protein (Henderson and Copp, 1998; Mjaatvedt et al., 1998). Organized collagen fibrils provide rigidity to the fibrosa layer (Vesely, 1997; Lincoln et al., 2004). Additionally, the basal cusps of the semilunar valves are rich in tenascin-C (Tnc) (Akerberg et al., 2015). Similarly, the AVC valves have high Tnc expression where they attach to the ventricular wall, including in the chordae tendineae (CT) (Lincoln et al., 2004). Disrupted ECM patterning is one of the primary hallmarks of valve disease. While these later stages of valve formation are poorly understood, expression studies suggest canonical Wnt signaling functions during the valve patterning phase (Alfieri et al., 2010).
Models proposing that canonical Wnt signaling promotes cushion EMT, EC expansion and valve patterning have not been definitively tested using loss-of-function approaches. Although several Wnt transcripts, notablyWnt4andWnt9b, are specifically expressed in valves (Wang et al., 2013; Alfieri et al., 2010), there are no genetic studies directly implicating Wnt ligands in valve development, probably because of redundancy between the 19 Wnt family members. Wnt proteins can also signal through‘ non-canonical’ pathways – roles that cannot be distinguished by conventional genetics. Therefore, conditional deletion of floxed
Ctnnb1is the predominant approach to disrupt Wnt signaling in
mice. However, this method also abolishes the aforementioned contribution of β-catenin to adherens junctions that maintain epithelia. Furthermore, Cre-lox conditional gene deletions do not provide sufficient temporal control over pathway activity to study each of the proposed dynamic roles of Wnt during valvulogenesis. We developed an inducible loss-of-function approach using transgenic mice that provides spatiotemporal control over Wnt/β -catenin signaling during valve development. We use cell type-specific inducible expression of Dkk1, a secreted protein that binds the Wnt Lrp5/6 co-receptors to potently and specifically attenuate canonical Wnt signaling (Bafico et al., 2001; Mao et al., 2001; Semënov et al., 2001). Using this approach and refined expression studies of Wnt-responsive target genes, we show that Wnt has multiple roles throughout valve development. First, Wnt/β-catenin instructs OFT myocardium to produce EMT-inducing ligands while, surprisingly, not being directly required for EMT in either the pOFT or AVC. Second, Wnt promotes cushion expansion by supporting the responsiveness of mesenchyme to FGF signaling. This role as a mitogenic competence factor is progressively suppressed by feedback inhibition through accumulated expression of the negative Wnt regulator, Axin2. Third, Wnt/β -catenin signaling enables MV cushion mesenchyme to respond to subsequent ECM patterning cues that promote the spongiosa layer and restrain the CT-leaflet boundary. We propose that Wnt/β -catenin signaling enables transitions between the cushion establishment, expansion and patterning stages of valve development rather than directly inducing discrete developmental processes.
RESULTS
Canonical Wnt signaling is required for proximal outflow tract but not atrioventricular canal cushion EMT
β-catenin, the transcriptional effector of canonical Wnt signaling, is required in the endothelial lineage for endocardial cushion EMT
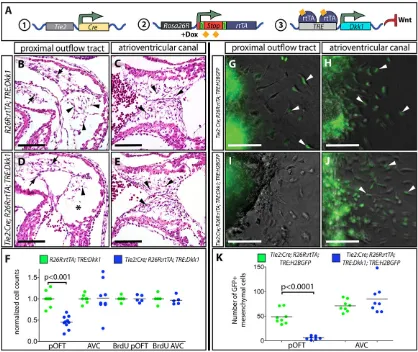
(Liebner et al., 2004). However,Ctnnb1loss-of-function studies do not distinguish between its Wnt signaling and cell adhesion roles. To examine canonical Wnt signaling roles in developing mice, we established an inducible Tet-On transgenic system that provides spatiotemporal Wnt inhibition through Dkk1 expression (Fig. S1A). Dkk1 is a secreted, potent and specific inhibitor of canonical Wnt signaling that binds to the Lrp5/6 obligate co-receptors of Wnt ligands (Bafico et al., 2001; Mao et al., 2001; Semënov et al., 2001).
Actin:rtTA;TRE:Dkk1 embryos exposed to doxycycline (Dox)
beginning at E8.5 and harvested at E10.5 had stunted limb buds and a reduced forebrain (Fig. S1B,C). These phenotypes recapitulate those in mice lacking the Wnt co-receptorLrp6(Song et al., 2009b). Further validating our approach, expression ofAxin2, which is a well-established canonical Wnt target gene (Jho et al., 2002), was reduced in embryos with ubiquitous Dkk1 expression (Fig. S1B,C, inset). Likewise,Actin:rtTA;TRE:Dkk1embryos had reduced levels of Lef1, which is transcriptionally upregulated by the canonical Wnt pathway (Filali et al., 2002), and nuclearβ-catenin in limb bud tissue (Fig. S1D-G).
E10.5 embryos ubiquitously expressingDkk1from E8.5 lacked mesenchymal cells within the pOFT cushion, consistent with the proposed role of Wnt in cushion EMT (Fig. S2A,B). However, AVC EMT proceeded normally (Fig. S2C,D). Therefore, the AVC EMT deficiency in endothelial lineage Ctnnb1-deficient mice (Liebner et al., 2004) reflect adherens junctions roles of β-catenin in maintaining the epithelial integrity of the endocardium.
The pOFT EMT deficiency upon ubiquitous Dkk1 expression could be secondary to widespread developmental defects upon global Wnt inhibition. Therefore, we used a variant Tet-on system to specifically express rtTA in the endothelial lineage (Belteki et al., 2005). In Tie2:Cre;R26R:rtTA;TRE:Dkk1 embryos, endothelial-expressed Cre recombinase excises theloxP-Stop-loxPcassette of
R26R:rtTA to drive rtTA uniquely in the endothelial/endocardial
lineage (Fig. 1A). We validated the responsiveness and specificity of this system using a surrogateTRE:H2BGFPtransgene (Tumbar et al., 2004; Fig. S3A). Dox-dependent nuclear GFP expression in endothelial, endocardial and EMT-derived mesenchymal cells of
Tie2:Cre;R26R:rtTA;TRE:H2BGFP embryos required all three
transgenes and was strong 6 h after injection (Fig. S3B-I). E10.5Tie2:Cre;R26R:rtTA;TRE:Dkk1embryos exposed to Dox from E8.5 had quantitatively reduced pOFT mesenchyme and normal dOFT and AVC mesenchyme (Fig. 1B-F). Proliferation of AVC and residual pOFT mesenchymal cells, as assessed by BrdU incorporation, was unchanged in Dkk1-expressing embryos (Fig. 1F). Therefore, Wnt/β-catenin signaling is required to generate pOFT mesenchyme but is not required for initial cell proliferation following EMT in either set of cushions. As AVC EMT precedes pOFT EMT (Camenisch et al., 2002), we tested whether earlier induction of Dkk1 would uncover an AVC EMT defect upon Wnt inhibition. However, Dkk1-expressing embryos at E8.0-E10.5 still only showed reduced pOFT mesenchyme (Fig. S4A-D).
Although dOFT and pOFT mesenchyme are primarily neural crest cell (NCC) and EMT-derived, respectively, there is some co-mingling of the two lineages (reviewed by Snarr et al., 2008). Therefore, to confirm that induction of Dkk1 reduced EMT- and not NCC-derived pOFT mesenchyme, we introduced the TRE:
H2BGFP transgene to simultaneously inhibit Wnt and trace
endocardial-derived mesenchyme. As expected, Tie2:Cre;R26R:
rtTA;TRE:H2BGFP;TRE:Dkk1 embryos expressing Dkk1 from
E8.5 to E10.5 had significantly fewer GFP+ cells in the pOFT
DEVEL
O
(Fig. S5A,B,E). Dkk1 induction had no effect on the number of GFP+cells in the AVC and non-GFP+cells (derived from NCC) in
the dOFT (Fig. S5C-E).
To evaluate the direct requirement of canonical Wnt signaling in pOFT EMT, we performed EMT explant assays using Tie2:Cre;
R26R:rtTA;TRE:H2BGFP;TRE:Dkk1 embryos. To mimic the
in vivoexperiments, we initiated Dox treatmentin utero at E8.5
and explanted cushions at E9.5 onto Dox-infused collagen gels. Dkk1-expressing OFT but not AVC cushion explants exhibited a substantial decrease in the number of GFP+ mesenchymal cells
(Fig. 1G-K). While canonical Wnt signaling therefore has a unique role in promoting pOFT EMT, the secreted nature of Dkk1 does not resolve whether Wnt signaling is required in the endocardium or in adjacent myocardium.
Myocardial Wnt signaling enables the production of pOFT EMT-inducing factors
To further explore the role of canonical Wnt signaling in pOFT EMT, we revisited expression of transgenic and endogenous
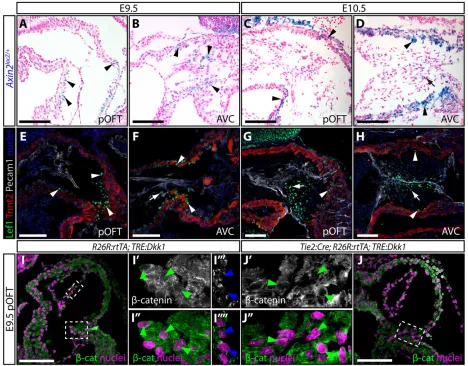
[image:3.612.97.515.58.410.2]reporters of Wnt activity in the endocardial cushions. Previous surveys of Wnt/β-catenin activity in heart development have primarily used the TOPGAL and BATGAL transgenic reporters (Alfieri et al., 2010; Cai et al., 2013; Cambier et al., 2014; Gitler et al., 2003; Maretto et al., 2003) that are non-specific in some contexts (Al Alam et al., 2011; Barolo, 2006). Therefore, we examined patterns of Wnt signaling activity using alacZknock-in allele ofAxin2, a well-established universal Wnt target gene (Jho et al., 2002; Lustig et al., 2002). Corroborating previous reports (Gillers et al., 2015; Jain et al., 2015; Klaus et al., 2012), sections of X-gal-stained Axin2lacZ/+ E9.5 embryos showed faint Axin2 expression in pOFT but not dOFT myocardium, the ventricular side of AVC myocardium and AVC mesenchyme (Fig. 2A,B). At E10.5, the highest Axin2expression persisted in pOFT and AVC myocardium, with lower levels in pOFT and AVC mesenchyme (Fig. 2C,D). We observed little to no Axin2 expression in endocardial cells of either cushion or in dOFT myocardium at either stage, arguing against a direct role for canonical Wnt signaling in promoting EMT.
Fig. 1. Canonical Wnt signaling is required for proximal outflow tract ( pOFT) but not atrioventricular canal (AVC) cushion EMT.(A) Schematic illustrating the transgenic mouse approach that uses doxycycline (Dox)-inducible expression of Dkk1 to provide spatiotemporal control over canonical Wnt signaling. (B-E) Hematoxylin and Eosin (H&E) stained sections of control (R26R:rtTA;TRE:Dkk1) (B,C) andTie2:Cre;R26R:rtTA;TRE:Dkk1(D,E) embryos treated with Dox from E8.5 to E10.5. Arrowheads show mesenchymal cells and the asterisk marks the lack of mesenchymal cells in the Dkk1-expressing pOFT cushion. (F) A quantitative comparison of the amount and proliferative rate (BrdU incorporation) of pOFT and AVC cushion mesenchyme between Dox-treatedR26R:rtTA;TRE: Dkk1andTie2:Cre;R26R:rtTA;TRE:Dkk1embryos. Numbers are normalized to the mean of the control samples in each litter. (G-J) Overlaid bright-field and fluorescence images of collagen gel cushion explants from Dox-treated E9.5Tie2:Cre;R26R:rtTA;TRE:H2BGFP(G,H) andTie2:Cre;R26R:rtTA;TRE:Dkk1;TRE: H2BGFP(I,J) embryos. EMT-derived GFP+cells (green) are highlighted with arrowheads. (K) Scatterplot graph of the number of collagen-invading GFP+ mesenchymal cells 24 h after explanting Dox-treated E9.5Tie2:Cre;R26R:rtTA;TRE:H2BGFPandTie2:Cre;R26R:rtTA;TRE:Dkk1;TRE:H2BGFPpOFT and AVC cushions.P-values indicate a significant difference determined by Student’st-tests. Scale bars: 100 µm.
DEVEL
O
Expression of the transcription factor Lef1 can mark both Wnt-responsive cells and, as a target gene, cells actively transmitting Wnt signals (Filali et al., 2002). We observed strong Lef1 expression in E9.5 pOFT and AVC myocardium and in AVC mesenchyme (Fig. 2E,F). By E10.5, Lef1 levels were decreased in both the pOFT and the AVC myocardium whereas high levels of Lef1 persisted in AVC and the now evident pOFT mesenchyme (Fig. 2G,H). These patterns largely recapitulate a previous report (Cai et al., 2013). Only sporadic endocardial cells showed low levels of Lef1 in both pOFT and AVC at E9.5 and E10.5, arguing, as with theAxin2lacZreporter, that canonical Wnt signaling does not directly induce EMT.
Consistent with active Wnt signal transduction, E9.5 pOFT myocardial cells expressed nuclearβ-catenin (Fig. 2I), which was decreased in pOFT myocardium of E8.5-E9.5 Dox-treated
Tie2:Cre;R26R:rtTA;TRE:Dkk1 embryos (Fig. 2J). Therefore,
endocardial-induced secreted Dkk1 is capable of attenuating canonical Wnt activity throughout the cushion field. In contrast to its myocardial expression,β-catenin was predominantly membrane localized and non-nuclear in pOFT endocardium (Fig. 2I).Axin2,
Lef1 and β-catenin expression all indicate that canonical Wnt signaling is mainly active in pOFT cushion myocardium during EMT. Therefore, the pOFT EMT defect upon Dkk1 induction probably reflects a cushion myocardial role for Wnt signaling in promoting neighboring endocardial cells to undergo EMT.
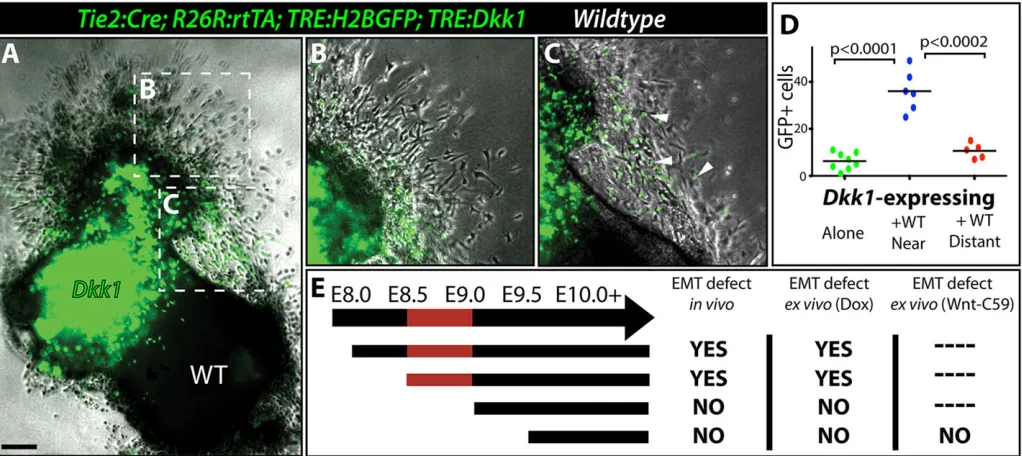
To test whether myocardial Wnt signaling promotes expression of a secreted EMT-inducing ligand, we performed EMT explant assays with E9.5 wild-type OFT tissue placed adjacent to a normally EMT-deficient Dkk1-expressing OFT. The Dkk1-expressing explant included the TRE:H2BGFP transgene to allow exclusive scoring of its EMT-derived cells otherwise intermingled with NCC-derived and wild-type explant cells. Wild-type OFTs robustly rescued EMT of immediately adjacentTie2:Cre;R26R:rtTA;TRE:
H2BGFP;TRE:Dkk1 Dox-exposed explants (Fig. 3A-D).
Therefore, Dkk1-expressing endocardium remains competent to undergo EMT and Wnt/β-catenin signaling acts in neighboring pOFT myocardium to produce an EMT inducer(s).
[image:4.612.72.540.58.424.2]We set out to determine when myocardial Wnt signaling is required for pOFT EMT by introducing Dox at different Fig. 2. Expression of canonical Wnt signaling target genes and pathway components suggests Wnt activity is predominantly myocardial at the onset of EMT.(A-D) Cryo sections showing the pOFT and AVC of X-gal stained (blue)Axin2lacZheterozygous embryos at E9.5 (A,B) and E10.5 (C,D). Sections are counterstained with Nuclear Fast Red. Black arrows and arrowheads indicateAxin2-expressing myocardium and mesenchyme, respectively. (E-H) Anti-Lef1 (green), troponin T (Tnnt2, myocardium, red) and Pecam1 (endocardium, gray) immunofluorescent stained paraffin sections of the pOFT and AVC of E9.5 (E,F) and E10.5 (G,H) wild-type embryos. Nuclei (stained with Hoechst) are in blue. White arrows and arrowheads indicate strongly expressing Lef1+myocardial and mesenchymal cells, respectively. (I,J) Anti-β-catenin stained single optical sections of pOFT myocardium ofR26R:rtTA;TRE:Dkk1(I) andTie2:Cre;R26R:rtTA; TRE:Dkk1(J) embryos treated with Dox from E8.5 to E9.5.β-catenin staining is gray/green and nuclei are purple (Hoechst). Dashed boxes outline the zoomed regions shown in the central panels. Green and blue arrowheads mark myocardial and endocardial cell nuclei, respectively. Scale bars: 100 µm.
DEVEL
O
developmental ages. Initiating Dkk1 expression at E9.5 usingTie2:
Cre;R26R:rtTA;TRE:Dkk1embryos failed to reproduce the pOFT
EMT seen with earlier inductions (Fig. S6A-C). Furthermore,Tie2:
Cre;R26R:rtTA;TRE:H2BGFP;TRE:Dkk1 OFTs explanted on
Dox-containing collagen gels without a priori in uteroexposure underwent normal EMT (Fig. S6D-F). The lack of an EMT defect in these experiments is unlikely to represent delayed Dkk1 production, because endocardial lineage-traced wild-type OFTs explanted on collagen gel containing 100 nM Wnt-C59, a potent Porcupine inhibitor (Proffitt et al., 2013) that specifically prevents Wnt secretion, also completed EMT normally (Fig. S6G-I). Conversely, the EMT defect persisted when Tie2:Cre;R26R:rtTA;TRE:
H2BGFP;TRE:DkkOFTs exposed to Doxin utero at E8.5 were
explanted onto Dox-free collagen gels (Fig. S6J-L). These timing experiments indicate that Wnt signaling acts between E8.5 and E9.0 to enable E9.5/E10.0 pOFT myocardium to produce an EMT-inducing substance (Fig. 3E). The early function of Wnt suggested an interface with either the Notch/BMP myocardial/endocardial crosstalk that render the cushion fields competent to undergo EMT. However, pOFT endocardial cells expressing Dkk1 from E8.5 to E9.5 retained Notch and BMP activity as monitored by anti-NICD and anti-pSmad1/5/8 staining, respectively (Fig. S7A-D). Further, transcript levels ofBmp2,Bmp4and Tgfb2, another myocardial-expressed EMT-inducer (Potts and Runyan, 1989; Camenisch et al., 2002), were normal in Dkk1-induced hearts (Fig. S7E).
Wnt signaling supports AVC cushion expansion
The upregulation ofAxin2and Lef1 in E10.5 OFT and AVC cushion mesenchyme (Fig. 2C,D,G,H) and previous Wnt reporter studies (Alfieri et al., 2010; Cai et al., 2013; Cambier et al., 2014; Gillers et al., 2015) indicate that Wnt signaling persists as cushions grow and elongate into mature valves. To explore Wnt signaling dynamics during the cushion/valve expansion phase, we assayed Wnt activity
by Lef1 immunostaining. At E11.5, cushion myocardial Lef1 had largely dissipated (Fig. 4A-C). As previously described (Cai et al., 2013), occasional Lef1+cushion endocardial cells were found in both
the AVC and OFT (Fig. 4A-C). However, Lef1 was most robustly expressed in cushion mesenchyme of pOFT, dOFT and AVC cushions. By E13.5, Lef1 was primarily mesenchymal with enriched expression at the distal aspects of the forming aortic and pulmonic valve cusps and mitral valve leaflets (Fig. 4D-F).
To test Wnt/β-catenin signaling requirements during cushion/ valve expansion stages, we induced global Dkk1 expression from E10.5 to E13.5 usingActin:rtTA;TRE:Dkk1mice. These embryos developed small, abnormally shaped limbs and bilateral cleft lips (Fig. S8A-C), reminiscent of other canonical Wnt loss-of-function studies (Galceran et al., 1999; Song et al., 2009a,b). Lef1 protein was largely depleted in limb sections (Fig. S8D,E), underlining the efficacy of Dkk1 induction. Hearts from Actin:rtTA;TRE-Dkk1 embryos (Dox E10.5-E13.5) had ventricular septal defects, occasional double outlet right ventricle (DORV) and quantitatively smaller and blunted mitral valves (Fig. S8F-K). To determine whether these cushion/valve defects were a direct repercussion of Wnt attenuation in the heart, we used the
Nfatc1Cre line (Wu et al., 2012) with R26R:Stop-rtTA to drive
endocardial lineage-specific Dkk1 expression. TheTRE:H2BGFP reporter showed this approach produced Dox-dependent expression in nearly all endocardial cells, most AVC cushion mesenchymal cells and a smaller subset of OFT mesenchyme (Fig. S9). Unlike
Tie2:Cre,Nfatc1Creis not active in endothelial cells outside the
heart, reducing concerns of secondary effects from Wnt attenuation throughout the vascular system.
Nfatc1Cre;R26R:rtTA;TRE:Dkk1embryos exposed to Dox from
[image:5.612.52.563.59.287.2]E10.5 to E13.5 had quantitatively smaller and hypocellular MV leaflets (Fig. 4G-I). These endocardial lineage Dkk1-expressing embryos had 23% fewer BrdU-incorporating mesenchymal cells Fig. 3. Canonical Wnt signaling enables OFT myocardium to produce an EMT-inducing factor(s).(A-C) Overlaid bright-field and fluorescence images of an explanted OFT from a Dox-exposed E9.5Tie2:Cre;R26R:rtTA;TRE:Dkk1;TRE:H2BGFPembryo cultured adjacent to the OFT from an unlabeled wild-type embryo (A). Magnified regions distant (B) and near (C) to the wild-type OFT cushion are shown. Arrowheads indicate GFP-expressing EMT-derived mesenchymal cells (green). (D) Quantification of EMT-derived mesenchymal cells from co-culture experiments. (E) Chart showing results ofin vivoandex vivo explant Dox exposure timing experiments usingTie2:Cre;R26R:rtTA;TRE:Dkk1embryos and a Wnt secretion inhibitor (Wnt-C59) to determine when pOFT EMT is sensitive to canonical Wnt signaling inhibition. The red-shaded region denotes the period of sensitivity to Wnt inhibition.P-values indicate a significant difference determined by Student’st-tests. Scale bar: 100 µm.
DEVEL
O
(Fig. 4I, P<0.04). Apoptosis, as assayed by cleaved caspase-3 (CC3) immunostaining, was unchanged (Fig. S10A-C). Therefore, canonical Wnt signaling supports the highly proliferative state of AVC cushion mesenchymal cells following EMT. Although
[image:6.612.100.513.54.575.2]Dox-exposed E13.5Nfatc1Cre;R26R:rtTA;TRE:Dkk1embryos did not have a similar defect in their SLVs (Fig. S10D-F), this negative result is likely to reflect limitedNfatc1Cre-driven recombination in SLV mesenchyme.
Fig. 4. Canonical Wnt signaling supports AVC cushion expansion following EMT.(A-F) Wide-field fluorescent images of paraffin sections showing pOFT, dOFT and AVC from E11.5 (A-C) and pulmonic (PV), aortic (AoV) and mitral (MV) valves from E13.5 (D-F) wild-type embryos stained with anti-Lef1 antibody (green). Nuclei (stained with Hoechst) are purple. White arrowheads and arrows indicate strongly expressing Lef1+mesenchymal and myocardial cells, respectively. (G,H) Hematoxylin and Eosin (H&E) stained paraffin sections showing the mitral valve (MV) of controlR26R:rtTA;TRE:Dkk1(E) and littermate Nfatc1Cre;R26R:rtTA;TRE:Dkk1(F) embryos exposed to Dox from E10.5-E13.5. (I) Scatterplot graph showing MV sectional area, leaflet length to width ratio, number of mesenchymal cells, and fraction of BrdU+proliferating cells in control andDkk1-expressing E12.5 embryos (Dox exposed beginning at E10.5). Values are normalized to the mean of control samples within each litter. (J) Timeline showing outcomes of Dox dose timing experiments in terms of gross morphology and proliferation defects. (K) Quantification of the fraction of BrdU+mesenchymal cells within the MV leaflets ofR26R:rtTA;TRE:Dkk1(control) andNfatc1Cre;R26R: rtTA;TRE:Dkk1embryos treated with Dox at E10.5-E11.5, E11.5-E12.5 or E12.5-E13.5. Solid lines outline cushion endocardium; dashed lines mark the extent of cushion myocardium. Scale bars: 100 µm.
DEVEL
O
We used the inducible Dkk1-expressing system to determine when AVC cushion mesenchyme expansion requires Wnt/β-catenin signaling (results summarized in Fig. 4J). Nfatc1Cre;R26R:rtTA;
TRE:Dkk1embryos exposed to Dox at E10.5-E11.5, E11.5-E12.5,
E12.5-E13.5 or E12.5-E15.5 did not show gross morphological defects. However, AVC mesenchyme BrdU incorporation was decreased significantly in E10.5-E11.5 and E11.5-E12.5 Dkk1-expressing embryos (Fig. 4K). Therefore, canonical Wnt signaling is uniquely required for AVC mesenchyme proliferation between E10.5 and E12.5. When Wnt/β-catenin signaling was inhibited during this‘execution period’, a reduced proliferative rate persisted to later stages. As such, E10.5-E13.5 Dkk1-expressing embryos gradually accumulated a deficit of AVC cushion mesenchymal cells, explaining why hypoplastic valves were only evident with longer Dox exposure. In agreement, E10.5-E15.5 Dox-exposed
Nfatc1Cre;R26R:rtTA;TRE:Dkk1 embryos also had smaller and
blunted mitral valves (Fig. S10G-I). The partial proliferation block and delayed phenotype manifestation upon Wnt/β-catenin inhibition suggest a supporting rather than switch-like role for Wnt signaling in AVC cushion mesenchyme proliferation.
Wnt/β-catenin signaling is a competence factor for FGF-driven AVC cushion expansion
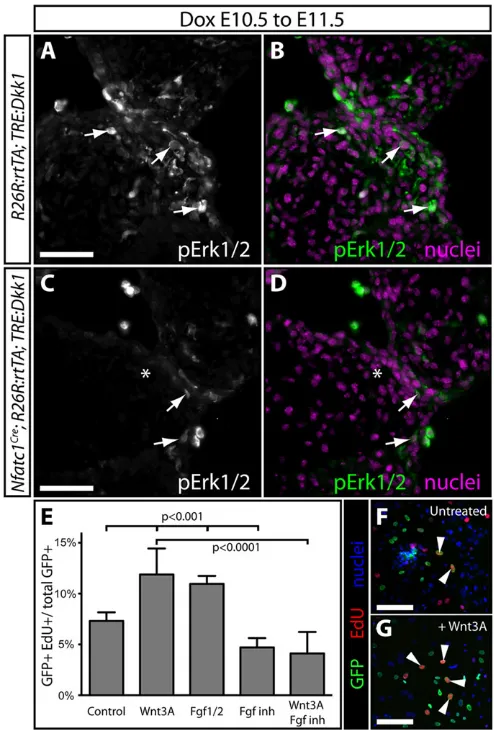
Receptor tyrosine kinases (RTKs) acting through Ras/MAPK signaling stimulate EC mesenchyme proliferation (Krenz et al., 2005, 2008). By staining for phosphorylated extracellular signal related kinase 1/2 ( pErk1/2), we observed enriched Ras/MAPK activity in distal mesenchyme of E11.5 AVC cushions (Fig. 5A,B), coincident with highest Axin2 and Lef1 expression. E10.5 to E11.5 Dox-exposed Tie2:Cre;R26R:rtTA;
TRE:Dkk1 embryos had markedly decreased pErk1/2 in AVC
mesenchyme (Fig. 5C,D), suggesting that Wnt/β-catenin promotes AVC mesenchyme proliferation by enabling mitogenic RTK signaling.
Fibroblast growth factor (FGF) RTK signaling is implicated in cushion development (Park et al., 2006; Sugi et al., 2003; Zhang et al., 2008, 2010). Moreover, Wnt and FGFs coordinate cell growth in other developmental contexts (Stulberg et al., 2012; ten Berge et al., 2008; Yin et al., 2008). Either recombinant Wnt3A or Fgf1/2 increased the fraction of EdU-incorporating GFP lineage-labeled cushion mesenchymal cells cultured fromTie2:Cre;Rosa:
rtTA;TRE:H2BGFP embryos (P<0.0001; Fig. 5E,F). The FGF
receptor inhibitor PD-173074 alone reduced EdU incorporation rates (P<0.0001; Fig. 5E). Cells treated with both Wnt3A and PD-173074 showed a significant decrease in proliferation compared with cells treated with Wnt3A alone (P<0.003; Fig. 5E). Therefore, canonical Wnt signaling directs the competency of EC mesenchyme to robustly respond to growth-promoting FGF signaling.
Axin2 suppresses Wnt-facilitated AVC cushion proliferation to prevent valve overgrowth
The absence of proliferation defects when Wnt is inhibited after E12.5 suggests that Wnt activity is actively suppressed as cushions transition from the growth to elongation phase of valve development. As Axin2 is both a target gene and negative regulator of canonical Wnt signaling, we hypothesized that a negative feedback loop restrains cushion mesenchymal Wnt activity at later stages of valve development. We observedAxin2expression, monitored using theAxin2lacZreporter allele, in E11.5 pOFT and AVC cushion myocardium and mesenchyme (Fig. 6A,B). At E13.5,
Axin2expression was largely restricted to cushion mesenchyme of
the forming semilunar and AVC valves, represented by the pulmonic and mitral valves, respectively (Fig. 6C,D). By E18.5,
Axin2 was strongly expressed throughout the mitral valves, as
previously observed (Gillers et al., 2015) (Fig. 6E). This progressively stronger and broaderAxin2expression is consistent with negative feedback inhibition of Wnt signaling as valves exit the expansion phase of their development.
We used theAxin2lacZallele, which disruptsAxin2transcription (Lustig et al., 2002), to test whether Axin2 functions as a negative regulator of Wnt signaling during later stages of embryonic valve development. WhereasAxin2lacZ/lacZembryos were present at the expected Mendelian ratio up to E16.5, homozygous null neonates were significantly underrepresented (9.4% observed versus 25% expected,n=53,P<0.009), and displayed characteristic craniofacial defects (Yu et al., 2005). E13.5 PV cusps ofAxin2lacZ/lacZembryos had normal sectional areas and length-to-width ratios. However, PV Fig. 5. Wnt/β-catenin signaling is a competence factor for FGF-driven AVC cushion expansion.(A-D) Exposure-matched immunofluorescent staining for pErk1/2 on AVC cushion sections ofTRE:Dkk1;R26R:rtTA(A,B) and Nfatc1Cre;R26R:rtTA;TRE:Dkk1(C,D) embryos exposed to Dox at E10.5-E11.5. pErk1/2 is in green/gray, and nuclei are purple (Hoechst). Arrows denote pErk1/2+mesenchymal cells. (E) Graph showing extent of EdU incorporation by GFP+primary cultured cushion mesenchymal cells left untreated (F) or treated with Wnt3A (G), Fgf1/2, FGF inhibitor (PD173074), Wnt-C59, or a combination of Wnt3A and FGF inhibitor. Bars show mean percentage of EdU+cells across three independent experiments. Error bars are one s.d. Fisher’s exact tests using pooled data were used to determine significance. Arrowheads mark GFP+/EdU+mesenchymal cells. Scale bars: 50 µm.
DEVEL
O
[image:7.612.314.561.57.422.2]cusp mesenchyme was mildly hyperplastic (Fig. 6F-H). By E16.5, PV cusp area, cell number and proliferation were all significantly increased in Axin2lacZ/lacZ embryos (Fig. 6L, Fig. S11A,B). MV leaflets ofAxin2lacZ/lacZembryos were significantly larger at E13.5 and contained more proliferative mesenchyme (Fig. 6I-K). While E16.5Axin2lacZ/lacZembryos had unchanged MV leaflet sectional area and length-to-width ratios, cell counts and proliferation remained increased, suggesting a MV volume increase not evident by sectional analysis (Fig. 6M, Fig. S11C,D). 25% of E16.5
Axin2lacZ/lacZ embryos (2/8 embryos) also had a membranous
ventricular septal defect (VSD), consistent with underlying cushion abnormalities (Fig. S11E,F).Axin2is therefore both a reporter of Wnt activity and a negative feedback regulator that helps restrict Wnt-promoted cushion growth to the expansion phase of valvulogenesis.
Wnt/β-catenin signaling enables MV cushion mesenchyme to respond to subsequent ECM patterning cues
After expanding, cushions elongate into thin valve leaflets/cusps that become stratified into three distinct ECM layers (reviewed by Hinton and Yutzey, 2011). Expression studies implicate canonical Wnt signaling in valve ECM patterning during late embryogenesis and early postnatal development (Alfieri et al., 2010). Although our
Axin2expression studies suggest canonical Wnt is largely inhibited
[image:8.612.49.432.55.555.2]during late valvulogenesis, localized Lef1 expression in E13.5 MV leaflets (Fig. 4F) indicates that a subset of mesenchyme remains Wnt-responsive to pattern the ECM of developing valves. Supporting this possibility, Lef1 expression at E15.5 and E18.5 was concentrated in interstitial and rare endocardial cells at the mid-length of the extending MV leaflets as well as the forming CT (Fig. 7A,B).
Fig. 6. Axin2 constrains Wnt-promoted growth of atrioventricular canal and semilunar valve mesenchyme.
(A-E) X-gal stained cryo-sections from E11.5 (A,B), E13.5 (C,D) and E18.5 (E)Axin2lacZheterozygous embryos. Sections are
counterstained with Nuclear Fast Red. Arrowheads and arrows indicate Axin2-expressing myocardium and mesenchyme, respectively. (F,G,I,J) H&E stained sections from E13.5 control (Axin2lacZ/+; F,I) or
Axin2-null (Axin2lacZ/lacZ; G,J) embryos. Pulmonic (PV) and mitral valves (MV) are shown. (H,K) Graphs comparing sectional area, length: width ratio, cell counting and BrdU incorporation rates between PVs and MVs of E13.5 control andAxin2-null embryos. Data are normalized to the mean of the control embryos within each litter. (L,M) Graphs show quantitative measurements as above for E16.5 PVs (L) and MVs (M). P-values indicate a significant difference determined by Student’s t-tests. Scale bars: 100 µm.
DEVEL
O
Nfatc1Cre;R26R:rtTA;TRE:Dkk1 embryos, exposed to Dox at
E15.5-E18.5 or E13.5-E18.5, did not display grossly or morphometrically misshapen mitral valves (Fig. S12A-C). However, Movat’s pentachrome staining showed that Dkk1-expressing MV leaflets had a reduced mucopolysaccharide-rich
[image:9.612.110.504.58.586.2]spongiosa layer (Fig. S13A,B). To further characterize Wnt-dependent MV patterning, we stained Dkk1-expressing embryos (E13.5-E18.5) for specific ECM proteins. Postn levels were unchanged (Fig. S13C,D). By contrast, the spongiosa component Vcan was notably decreased (Fig. 7C,D), which was also apparent Fig. 7. Spatially restricted canonical Wnt signaling supports ECM remodeling of the mitral valve during late embryonic development.(A,B) Wide-field immunofluorescence images of paraffin sections showing the mitral valves of E15.5 (A) and E18.5 (B) wild-type embryos. Anti-Lef1 immunoreactivity is green and nuclei are purple (Hoechst). Arrows and arrowheads indicate Lef1+endocardial and mesenchymal cells, respectively. (C-H) Wide-field fluorescent antibody stained MV sections fromR26R:rtTA;TRE:Dkk1(C,F) andNfatc1Cre;R26R:rtTA;TRE:Dkk1(D,G) embryos exposed to Dox from E13.5-E18.5. Anti-Vcan and anti-Tnc staining is in green, as indicated. Nuclei are purple (stained with Hoechst). Arrowheads indicate robustly expressing cells for the indicated ECM component. Asterisks indicate low-expressing regions. Western blots showing Vcan (E) or Tnc (H) expression levels in protein lysates prepared from hearts ofR26R:rtTA; TRE:Dkk1andNfatc1Cre;R26R:rtTA;TRE:Dkk1embryos that had been exposed to Dox at E13.5-E18.5. GAPDH serves as a loading control. Scale bars: 100 µm.
DEVEL
O
in whole heart western blots (Fig. 7E). Dkk1 expression also caused Tnc to expand throughout the MV leaflet in contrast to its normal concentration at the base of the MV leaflets and CT (Fig. 7F,G). Increased Tnc levels were corroborated by western blot analysis (Fig. 7H). The shorter Dkk1 induction from E15.5 to E18.5 did not produce ECM changes in the MV of Nfatc1Cre;R26R:rtTA;TRE:
Dkk1embryos (Fig. S13E,F). Therefore, canonical Wnt regulates MV ECM patterning between E13.5 and E15.5, preceding the period when the MV becomes evidently stratified. Therefore, akin to its roles in pOFT EMT and cushion growth, Wnt/β-catenin behaves as a competence factor that enables subsequent valve ECM patterning cues.
DISCUSSION
Our systematic functional study of Wnt/β-catenin signaling during mouse valve development extends and revises existing models (Fig. S14). First, we show that Wnt/β-catenin signaling does not directly promote endocardial cushion EMT, as previously surmised based largely on endothelial/endocardial-specific deletion ofCtnnb1. Rather, canonical Wnt signaling in pOFT cushion myocardium supports the production of an EMT-inducing molecule(s). Second, Wnt/β-catenin enables FGF-driven proliferation of AVC cushion mesenchyme following EMT. Negative feedback by accumulated Wnt-induced Axin2 in cushion mesenchyme progressively suppresses cushion growth as valves transition to the patterning phase of their development. Third, Wnt/β-catenin signaling in a subpopulation of AVC mesenchyme promotes formation of the Vcan-rich spongiosa layer and restricts Tnc expression to valve-muscle junctions, including the CT. In each of the establishment, expansion and patterning stages of valve development, the enabling function of Wnt/β-catenin precedes the immediate instructive cue. We propose that canonical Wnt signaling coordinates developmental transitions between steps of valvulogenesis that allow the progressive formation of these complex and congenital disease-prone tissues.
Wnt/β-catenin enables OFT myocardium to produce an EMT-inducing ligand(s) but does not directly induce pOFT or AVC EMT
Our timing, expression and co-culture experiments indicate that canonical Wnt signaling acts indirectly to promote pOFT EMT. We only observed pOFT EMT defects when initiating Wnt inhibition at least a half day prior to EMT bothin vivoandin vitro. Furthermore, Wnt activity in the OFT field at the onset of EMT was most evident in cushion myocardium, with little to no activity in cushion endocardial cells. Most importantly, adjacently placed wild-type OFT tissue rescued the EMT defect in Dkk1-expressing OFTs using explant assays. Therefore, Wnt/β-catenin-inhibited endocardium is fully competent to undergo EMT and Wnt-inhibited cushions only lack a secreted EMT-inducing substance(s). A cushion myocardial site of action for Wnt signaling is consistent with Wnt4 being sufficient to induce myocardial BMP2 expression and thereby promote AVC EMT (Wang et al., 2013). However, opposing that study’s conclusions, myocardialβ-catenin transcriptional activity is not required for EMT (Gillers et al., 2015) and we did not observe a Dkk1-induced EMT defect in the AVC cushions.
How does canonical Wnt activity in OFT cushion myocardium support EMT of the neighboring endocardial cells? In the AVC, EMT competency is conferred by myocardial/endocardial crosstalk that activates both Notch and BMP signaling (Luna-Zurita et al., 2010; Timmerman et al., 2004; Wang et al., 2005). However, NICD and pSmad1/5/8 staining showed that both pathways were intact in Dkk1-expressing OFT cushions. Tgfb2
expression levels were also unaffected by Dkk1 induction, suggesting that EMT-promoting TGFβ signaling is intact (Brown et al., 1996; Ramsdell and Markwald, 1997; Sridurongrit et al., 2008). Therefore, the Wnt-dependent EMT inducer(s) could be a novel EMT-driving factor. The source of the Wnt ligands that activate canonical Wnt signaling in OFT myocardium is probably the neighboring endocardium, given that these cells robustly express both Wnt4 and Wnt9b (Alfieri et al., 2010; Cai et al., 2013; Wang et al., 2013). This underscores the complex crosstalk between myocardium and endocardium required for EMT (de la Pompa and Epstein, 2012).
Our results argue against the widely reported conclusion that endocardial Wnt/β-catenin signaling is required for AVC EMT–a model based on potentially specious interpretations of two previous reports. First, the observation that Wnt-inhibited zebrafish embryos fail to produce primordial AVC cushions (Hurlstone et al., 2003) does not actually demonstrate an EMT defect because zebrafish AVC valve leaflets initially form through endocardial invagination (Scherz et al., 2008). Second, disrupted AVC EMT in mice with endothelial/endocardial deletion of Ctnnb1 might reflect non-transcriptional roles ofβ-catenin in forming adherens junctions that maintain the endocardium as an epithelium. Consistent with this idea, specifically disrupting the Wnt transcriptional roles of β-catenin in cardiomyocytes produces milder heart defects than complete loss of myocardial β-catenin (Gillers et al., 2015). Furthermore, deficient cushion Wnt/β-catenin activity upon conditional deletion of Tbx20 does not cause an AVC EMT defect, but instead restrains cushion growth (Cai et al., 2013). More broadly, we suggest conditional genetic studies ofCtnnb1should be interpreted cautiously before assigning phenotypes to disrupted canonical Wnt signaling.
Wnt promotes AVC cushion expansion by enhancing mitogenic FGF signaling
We observed that Wnt/β-catenin activity within cushion fields transitioned from primarily myocardial prior to and during EMT to almost entirely mesenchymal by E11.5. By using Nfatc1Cre to induce Dkk1 in endocardial cells and EMT-derived cells, we show that the first role of Wnt in AVC cushion mesenchyme is to promote cell proliferation. In agreement, endocardial Tbx20 regulates Wnt signaling to direct cushion growth (Cai et al., 2013). We probably did not observe an SLV growth defect upon endocardial lineage Dkk1 expression because theNfatc1Creline we used drives limited recombination in SLV mesenchyme. Although Dkk1 induction reduced AVC cushion mesenchyme, the effect was incomplete and resulting morphological changes relied on extended periods of canonical Wnt inhibition. These findings are most consistent with Wnt having a supporting mitogenic role rather than acting as a primary growth switch.
We show that canonical Wnt signaling promotes the responsiveness of cushion mesenchymal cells to mitogenic fibroblast growth factor (FGF) signaling. First, Ras/MAPK signaling monitored by pErk1/2 staining was reduced in Wnt-inhibited AVC mesenchyme. Second, recombinant Wnt-induced proliferation of cultured EMT-derived cushion mesenchymal cells was blocked by a specific FGF receptor inhibitor. A synergistic relationship between Wnt and FGF signals similarly promotes proliferation in the developing limb and lung mesenchyme (Stulberg et al., 2012; ten Berge et al., 2008; Yin et al., 2008). Therefore, Wnt might use a common mechanism to promote proliferation in developmental contexts (reviewed by Klaus and Birchmeier, 2008; van Amerongen and Nusse, 2009) by increasing FGF production or augmenting FGF responsiveness. Of disease
DEVEL
O
relevance, Noonan syndrome, which is caused by mutations that result in excessive Ras/MAPK activity, is characterized by hyperplastic cushions leading to pulmonic stenosis (Tartaglia et al., 2001). Therefore, our study implicates excessive developmental Wnt/β -catenin activity as a potential contributor to Noonan syndrome-like congenital valve defects characterized by valve hyperplasia.
Feedback inhibition of Wnt signaling by Axin2 provides a self-timing mechanism that prevents valve overgrowth
By expressing Dkk1 at various stages of valve development, we determined Wnt/β-catenin acts between E10.5 and E12.5 to promote cushion expansion. In a seeming contradiction, the Wnt target geneAxin2was broadly expressed in cushion mesenchyme after E12.5 and in valve interstitial cells through postnatal stages (Fang et al., 2014). Axin2 is a potent cell-intrinsic inhibitor of Wnt signaling by providing a scaffold for the cytosolic protein destruction complex involved in β-catenin catabolism.Axin2-null mice developed large valves and showed increased cushion mesenchyme proliferation as early as E13.5. We propose that Wnt/β-catenin initially transcriptionally activatesAxin2expression in a restricted pool of cushion mesenchymal cells. These cells maintain Axin2 levels independent of continuous Wnt/β-catenin input and gradually populate most of the valve interstitium. Accumulated Axin2 constrains growth-promoting canonical Wnt signaling, accounting for restricted Lef1 expression at later stages. This negative feedback establishes a self-timing mechanism whereby accumulated Wnt activity supports the transition from the cushion growth to patterning phases of valve development. A similar Wnt/Axin2 feedback repressor circuit moderates tissue growth during skull development (Yu et al., 2005), implying that this mechanism is a common logic component of organogenic networks. Our study also highlights that, like wholly synthetic Wnt/ β-catenin reporter lines, theAxin2lacZallele provides an imperfect means to monitor sites of active canonical Wnt signaling.
Wnt promotes formation of the spongiosa layer during AVC valve patterning
Canonical Wnt activity in AVC cushion mesenchyme monitored by robust Lef1 staining becomes progressively concentrated in the medial aspect of the mitral valve leaflets, coinciding with the region of abundant proteoglycan-rich spongiosa. Correspondingly, we observed that Dkk1-expressing mitral valves developed a reduced and Vcan-deficient spongiosa layer. Vcanis a known Wnt target gene (Rahmani et al., 2005) and therefore its misexpression could represent a direct effect of Wnt inhibition.Tncexpression, which is normally restricted to the rigid leaflet-muscle attachment sites including the CT, expanded to replace the diminished spongiosa in Dkk1-expressing embryos. Therefore, we anticipate that Wnt/β -catenin-inhibited valves would become inflexible, potentially leading to valve stenosis and regurgitation.
We saw no appreciable ECM patterning defects when canonical Wnt signaling was inhibited after E15.5, indicating that Wnt promotes ECM patterning prior to the first evidence of stratification. Therefore, canonical Wnt establishes tissue responsiveness to subsequent ECM patterning cues, which could include biomechanical forces provided by blood flow (Hove et al., 2003; Butcher et al., 2007), rather than directly instructing stratification. Although conditional deletion of Ctnnb1 in a subset of valve interstitial cells contradictorily does not affect valve growth or patterning (Fang et al., 2014), thePostn:Creline used in that study might not promote a sufficient loss ofCtnnb1and/or at an early enough stage to disrupt Wnt’s pre-patterning role.
Wnt signaling and developmental transitions during valvulogenesis and valve disease
Could a common mechanism underlie the seemingly disparate pOFT EMT, AVC cushion expansion and mitral valve ECM patterning roles of canonical Wnt signaling? Wnt signaling is emerging as a universal progenitor cell maintenance factor that opposes cell differentiation (reviewed by Holland et al., 2013). During valvulogenesis, Wnt/β -catenin could first act to impede OFT cushion myocardium from fully differentiating into mature muscle. As such, OFT myocardial cells would retain functional aspects of their prior status as second heart field progenitor cells, in which canonical Wnt signaling promotes expression of growth factors that could include EMT-inducing ligands (Ai et al., 2007; Klaus et al., 2007). In developing cushion/ valve mesenchyme, active Wnt signaling could maintain a progressively restricted pool of undifferentiated mesenchymal cells that produce a growth-supportive Vcan-rich microenvironment and remain responsive to additional developmental cues like FGF. As myxomatous valves are characterized by increased levels of Vcan and other proteoglycans (Gupta et al., 2009), excessive Wnt activity could promote this common manifestation of valve disease. Postnatally, specialized Wnt-responsive valve interstitial cells might serve as a stem-cell reservoir for valve homeostasis and repair. This possibility is supported by adult-onset valve disease uponCtnnb1deletion in a subset of cushion mesenchyme (Fang et al., 2014).
We propose that Wnt/β-catenin does not induce discrete events of valve development, but instead serves as a developmental timer that integrates with directly instructive signals. The molecular mechanism might not be through archetypal gene expression changes of a narrow set of target genes. Rather, Wnt signaling roles might promote, for example, a cellular metabolic state (Esen et al., 2013), or asymmetric cell divisions (Habib et al., 2013) that generally curtail differentiation. Regardless, the consequences of canonical Wnt inhibition on valve development highlight how both increased and decreased Wnt activity could cause various manifestations of congenital valve disease.
MATERIALS AND METHODS
Mouse genetics
Mouse procedures were approved and monitored by the University of Oregon’s Institutional Animal Care and Use Committee.TRE:Dkk1mice carry a transgene constructed by cloning a HA-tagged mouseDkk1cDNA into the pTRE-Tight plasmid along with a 5′signal peptide sequence. A founder line was selected that produced robust Dox-inducible Dkk1 expression and expected embryonic Wnt loss-of-function phenotypes in combination withActin:rtTA(from Dr Steven Artandi; Sarin et al., 2005). Nfatc1Cremice were shared by Dr Bin Zhou (Wu et al., 2011, 2012).Tie2: Cre (Kisanuki et al., 2001), Axin2lacZ (Lustig et al., 2002),R26R:rtTA (Belteki et al., 2005) andTRE:H2BGFP(Tumbar et al., 2004) mice were acquired from the Jackson Laboratory. Embryos were collected from timed matings staged by daily monitoring for vaginal mucus plugs. Occasional embryonic age corrections were made based on the developmental morphology of control littermate embryos.
Doxycycline treatment
Pregnant mice from timed matings received a combination of one 40 mg/kg intraperitoneal (IP) injection of Dox prepared in sterile PBS at the start of the dosing period and 100 µg/ml Dox added to the drinking water (replaced every 48 h) until the time of embryo harvesting.
Endocardial cushion explants
EMT explant assays (Runyan and Markwald, 1983) used collagen gel matrices prepared as described (Xiong et al., 2012) using Opti-MEM (Invitrogen) with 2% fetal bovine serum (FBS; HyClone), 100 units/ml penicillin, 100μg/ml streptomycin (HyClone) and 5μg/ml doxycycline (MP
DEVEL
O
Biomedicals). The medium was supplemented with 100 nM Wnt-C59 (Selleck Chemicals) where indicated. Intact outflow tracts or atrioventricular canals from E9.5 Tie2:Cre;R26R:rtTA;TRE:H2BGFPor Tie2:Cre;R26R: rtTA;TRE:H2BGFP;TRE:Dkk1 embryos (genotyped retroactively and, generally, from Dox-pretreated pregnant mice) were dissected in sterile PBS, filleted using sharp tweezers to expose the cushions and placed cushion-side down on the collagen gels. 24 h after explant culture in a dual gas 37°C incubator containing 5% carbon dioxide (CO2) and 5% oxygen (O2),
bright-field and epifluorescent images used for cell counting were captured using a Nikon Eclipse TI inverted microscope. Explant co-cultures with wild-type OFTs followed (Chang et al., 2004) using the above procedures except images were captured after 48 h. The number of GFP+cells was scored for individual explants. Data from multiple litters were combined and statistical significance determined by Student’s two-tailedt-tests.
Primary cushion mesenchymal cell culture
Cushion mesenchymal cells were prepared and cultured from E13.5Tie2: Cre;Rosa:rtTA;TRE:H2BGFP embryos. Cells were treated with Wnt3A (R&D Systems), FGF1 and FGF2 (Peprotech), and/or the FGF receptor inhibitor PD-173074 (LC Laboratories). Proliferation rates of GFP-labeled EMT-derived cells in each treatment condition were determined by 5-ethynyl-2′-deoxyuridine (EdU) incorporation. Statistical differences were assayed by one-tailed Fisher’s exact tests. Further details on the culture methods, drug treatments and EdU proliferation assays are presented in the supplementary Materials and Methods.
Immunohistochemistry
Immunostaining of paraffin sections of Nfatc1Cre, Tie2:Cre, Axin2lacZ, R26R:rtTA,TRE:H2BGFPand wild-type mice was performed at various embryonic stages as described in the supplementary Materials and Methods.
Western blotting
Western blots of whole embryo or whole heart lysates were probed with primary antibodies against Axin2, versican and tenascin-C as described in the supplementary Materials and Methods.
Acknowledgements
This project benefited from the early support of Dr Ching-Pin Chang, in whose lab theTRE:Dkk1line was validated, and Dr Calvin Kuo, whose lab generated the
TRE:Dkk1founder transgenic lines. Dr Bin Zhou shared discussions and the
Nfatc1Creline in advance of publication. Dr Steven Artandi providedActin:rtTA
mice. Dr Jill Helms sharedLef1-null tissue sections to validate the specificity of the anti-Lef1 antibody. We thank Andrew McKay, Brynn Akerberg, Astra Henner, Sarah Casper, Yujung Choi and Gene Ma for technical help and Stankunas lab members for feedback.
Competing interests
The authors declare no competing or financial interests.
Author contributions
F.M.B. and K.S. designed the experiments. F.M.B., V.D., K.A.J. and K.S. performed experiments. F.M.B. and K.S. prepared and wrote the manuscript.
Funding
F.M.B. was supported by a National Institutes of Health (NIH) training grant [5T32HD007348]; and an NIH/NRSA graduate fellowship [5F31HL117617]. The March of Dimes (Basil O’Connor Award) and the NIH [1R01HL115294] provided research funding (K.S. lab). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at
http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.130575/-/DC1
References
Ai, D., Fu, X., Wang, J., Lu, M.-F., Chen, L., Baldini, A., Klein, W. H. and Martin, J. F.(2007). Canonical Wnt signaling functions in second heart field to promote right ventricular growth.Proc. Natl. Acad. Sci. USA104, 9319-9324.
Akerberg, B. N., Sarangam, M. L. and Stankunas, K.(2015). Endocardial Brg1 disruption illustrates the developmental origins of semilunar valve disease.Dev. Biol.407, 158-172.
Al Alam, D., Green, M., Tabatabai Irani, R., Parsa, S., Danopoulos, S., Sala, F. G., Branch, J., El Agha, E., Tiozzo, C., Voswinckel, R. et al.(2011). Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2LacZ during murine lung development and repair.PLoS ONE6, e23139.
Alfieri, C. M., Cheek, J., Chakraborty, S. and Yutzey, K. E.(2010). Wnt signaling in heart valve development and osteogenic gene induction.Dev. Biol. 338, 127-135.
Bafico, A., Liu, G., Yaniv, A., Gazit, A. and Aaronson, S. A.(2001). Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow.Nat. Cell Biol.3, 683-686.
Barolo, S.(2006). Transgenic Wnt/TCF pathway reporters: all you need is Lef?
Oncogene25, 7505-7511.
Belteki, G., Haigh, J., Kabacs, N., Haigh, K., Sison, K., Costantini, F., Whitsett, J., Quaggin, S. E. and Nagy, A.(2005). Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction.Nucleic Acids Res.33, e51.
Brickner, M. E., Hillis, L. D. and Lange, R. A.(2000). Congenital heart disease in adults.N. Engl. J. Med.342, 256-263.
Brown, C. B., Boyer, A. S., Runyan, R. B. and Barnett, J. V.(1996). Antibodies to the type II TGFβreceptor block cell activation and migration during atrioventricular cushion transformation in the heart.Dev. Biol.174, 248-257.
Butcher, J. T., McQuinn, T. C., Sedmera, D., Turner, D. and Markwald, R. R. (2007). Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition.Circ. Res.100, 1503-1511.
Cai, X., Zhang, W., Hu, J., Zhang, L., Sultana, N., Wu, B., Cai, W., Zhou, B. and Cai, C.-L.(2013). Tbx20 acts upstream of Wnt signaling to regulate endocardial cushion formation and valve remodeling during mouse cardiogenesis.
Development140, 3176-3187.
Cambier, L., Plate, M., Sucov, H. M. and Pashmforoush, M.(2014). Nkx2-5 regulates cardiac growth through modulation of Wnt signaling by R-spondin3.
Development141, 2959-2971.
Camenisch, T. D., Molin, D. G. M., Person, A., Runyan, R. B., Gittenberger-de Groot, A. C., McDonald, J. A. and Klewer, S. E.(2002). Temporal and distinct TGFβ ligand requirements during mouse and avian endocardial cushion morphogenesis.Dev. Biol.248, 170-181.
Chang, C.-P., Neilson, J. R., Bayle, J. H., Gestwicki, J. E., Kuo, A., Stankunas, K., Graef, I. A. and Crabtree, G. R.(2004). A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis.Cell118, 649-663. de la Pompa, J. L. and Epstein, J. A. (2012). Coordinating tissue
interactions: notch signaling in cardiac development and disease. Dev. Cell22, 244-254.
Esen, E., Chen, J., Karner, C. M., Okunade, A. L., Patterson, B. W. and Long, F. (2013). WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation.Cell Metab.17, 745-755.
Fang, M., Alfieri, C. M., Hulin, A., Conway, S. J. and Yutzey, K. E.(2014). Loss of β-catenin promotes chondrogenic differentiation of aortic valve interstitial cells.
Arterioscler. Thromb. Vasc. Biol.34, 2601-2608.
Filali, M., Cheng, N., Abbott, D., Leontiev, V. and Engelhardt, J. F.(2002). Wnt-3A/β-catenin signaling induces transcription from the LEF-1 promoter.J. Biol. Chem.277, 33398-33410.
Galceran, J., Fariñas, I., Depew, M. J., Clevers, H. and Grosschedl, R.(1999). Wnt3a−/−-like phenotype and limb deficiency in Lef1−/−Tcf1−/−mice.Genes Dev.13, 709-717.
Gillers, B. S., Chiplunkar, A., Aly, H., Valenta, T., Basler, K., Christoffels, V. M., Efimov, I. R., Boukens, B. J. and Rentschler, S. (2015). Canonical Wnt signaling regulates atrioventricular junction programming and electrophysiological properties.Circ. Res.116, 398-406.
Gitler, A. D., Lu, M. M., Jiang, Y. Q., Epstein, J. A. and Gruber, P. J.(2003). Molecular markers of cardiac endocardial cushion development.Dev. Dyn.228, 643-650.
Go, A. S., Mozaffarian, D., Roger, V. L., Benjamin, E. J., Berry, J. D., Borden, W. B., Bravata, D. M., Dai, S., Ford, E. S., Fox, C. S. et al.(2013). Heart disease and stroke statistics–2013 update: a report from the American Heart Association.
Circulation127, e6-e245.
Gottardi, C. J. and Gumbiner, B. M.(2004). Distinct molecular forms ofβ -catenin are targeted to adhesive or transcriptional complexes.J. Cell Biol.167, 339-349.
Gupta, V., Barzilla, J. E., Mendez, J. S., Stephens, E. H., Lee, E. L., Collard, C. D., Laucirica, R., Weigel, P. H. and Grande-Allen, K. J.(2009). Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves.Cardiovasc. Pathol.18, 191-197.
Habib, S. J., Chen, B.-C., Tsai, F.-C., Anastassiadis, K., Meyer, T., Betzig, E. and Nusse, R.(2013). A localized Wnt signal orients asymmetric stem cell division in vitro.Science339, 1445-1448.
Henderson, D. J. and Copp, A. J.(1998). Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart.Circ. Res.83, 523-532.
DEVEL
O
Heuberger, J. and Birchmeier, W.(2010). Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol.2, a002915.
Hinton, R. B. and Yutzey, K. E.(2011). Heart valve structure and function in development and disease.Annu. Rev. Physiol.73, 29-46.
Holland, J. D., Klaus, A., Garratt, A. N. and Birchmeier, W.(2013). Wnt signaling in stem and cancer stem cells.Curr. Opin. Cell Biol.25, 254-264.
Hove, J. R., Köster, R. W., Forouhar, A. S., Acevedo-Bolton, G., Fraser, S. E. and Gharib, M.(2003). Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis.Nature421, 172-177.
Hurlstone, A. F. L., Haramis, A.-P. G., Wienholds, E., Begthel, H., Korving, J., van Eeden, F., Cuppen, E., Zivkovic, D., Plasterk, R. H. A. and Clevers, H. (2003). The Wnt/β-catenin pathway regulates cardiac valve formation.Nature425, 633-637.
Jain, R., Li, D., Gupta, M., Manderfield, L. J., Ifkovits, J. L., Wang, Q., Liu, F., Liu, Y., Poleshko, A., Padmanabhan, A. et al.(2015). Integration of Bmp and Wnt signaling by Hopx specifies commitment of cardiomyoblasts. Science 348, aaa6071.
Jho, E.-h., Zhang, T., Domon, C., Joo, C.-K., Freund, J.-N. and Costantini, F. (2002). Wnt/β-Catenin/Tcf signaling induces the transcription of Axin2, a Negative regulator of the signaling pathway.Mol. Cell. Biol.22, 1172-1183.
Jiang, X., Rowitch, D. H., Soriano, P., McMahon, A. P. and Sucov, H. M. (2000). Fate of the mammalian cardiac neural crest. Development 127, 1607-1616.
Kirby, M. L., Gale, T. F. and Stewart, D. E.(1983). Neural crest cells contribute to normal aorticopulmonary septation.Science220, 1059-1061.
Kisanuki, Y. Y., Hammer, R. E., Miyazaki, J.-i., Williams, S. C., Richardson, J. A. and Yanagisawa, M. (2001). Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo.Dev. Biol.230, 230-242.
Klaus, A. and Birchmeier, W.(2008). Wnt signalling and its impact on development and cancer.Nat. Rev. Cancer8, 387-398.
Klaus, A., Saga, Y., Taketo, M. M., Tzahor, E. and Birchmeier, W.(2007). Distinct roles of Wnt/β-catenin and Bmp signaling during early cardiogenesis.Proc. Natl. Acad. Sci. USA104, 18531-18536.
Klaus, A., Müller, M., Schulz, H., Saga, Y., Martin, J. F. and Birchmeier, W. (2012). Wnt/β-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc. Natl. Acad. Sci. USA 109, 10921-10926.
Krenz, M., Yutzey, K. E. and Robbins, J.(2005). Noonan syndrome mutation Q79R in Shp2 increases proliferation of valve primordia mesenchymal cells via extracellular signal-regulated kinase 1/2 signaling. Circ. Res. 97, 813-820.
Krenz, M., Gulick, J., Osinska, H. E., Colbert, M. C., Molkentin, J. D. and Robbins, J.(2008). Role of ERK1/2 signaling in congenital valve malformations in Noonan syndrome.Proc. Natl. Acad. Sci. USA105, 18930-18935.
Liebner, S., Cattelino, A., Gallini, R., Rudini, N., Iurlaro, M., Piccolo, S. and Dejana, E. (2004). β-Catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol.
166, 359-367.
Lincoln, J., Alfieri, C. M. and Yutzey, K. E.(2004). Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos.Dev. Dyn.230, 239-250.
Luna-Zurita, L., Prados, B., Grego-Bessa, J., Luxán, G., del Monte, G., Bengurı́a, A., Adams, R. H., Pérez-Pomares, J. M. and de la Pompa, J. L. (2010). Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation.J. Clin. Invest.
120, 3493-3507.
Lustig, B., Jerchow, B., Sachs, M., Weiler, S., Pietsch, T., Karsten, U., van de Wetering, M., Clevers, H., Schlag, P. M., Birchmeier, W. et al.(2002). Negative feedback loop of Wnt signaling through upregulation of conductin/Axin2 in colorectal and liver tumors.Mol. Cell. Biol.22, 1184-1193.
Ma, L., Lu, M.-F., Schwartz, R. J. and Martin, J. F.(2005). Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning.
Development132, 5601-5611.
MacGrogan, D., Luxán, G., Driessen-Mol, A., Bouten, C., Baaijens, F. and de la Pompa, J. L.(2014). How to make a heart valve: from embryonic development to bioengineering of living valve substitutes.Cold Spring Harb. Perspect. Med.4, a013912.
Mao, B., Wu, W., Li, Y., Hoppe, D., Stannek, P., Glinka, A. and Niehrs, C.(2001). LDL-receptor-related protein 6 is a receptor for Dickkopf proteins.Nature411, 321-325.
Maretto, S., Cordenonsi, M., Dupont, S., Braghetta, P., Broccoli, V., Hassan, A. B., Volpin, D., Bressan, G. M. and Piccolo, S.(2003). Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors.Proc. Natl. Acad. Sci. USA100, 3299-3304.
Markwald, R. R., Fitzharris, T. P. and Manasek, F. J. (1977). Structural development of endocardial cushions.Am. J. Anat.148, 85-119.
McCulley, D. J., Kang, J.-O., Martin, J. F. and Black, B. L.(2008). BMP4 is required in the anterior heart field and its derivatives for endocardial cushion
remodeling, outflow tract septation, and semilunar valve development.Dev. Dyn.
237, 3200-3209.
Mjaatvedt, C. H., Yamamura, H., Capehart, A. A., Turner, D. and Markwald, R. R.(1998). The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation.Dev. Biol. 202, 56-66.
Niehrs, C. and Acebron, S. P.(2012). Mitotic and mitogenic Wnt signalling: mitotic and mitogenic Wnt signalling.EMBO J.31, 2705-2713.
Park, E. J., Ogden, L. A., Talbot, A., Evans, S., Cai, C.-L., Black, B. L., Frank, D. U. and Moon, A. M.(2006). Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling.Development133, 2419-2433.
Pierpont, M. E., Basson, C. T., Benson, D. W., Gelb, B. D., Giglia, T. M., Goldmuntz, E., McGee, G., Sable, C. A., Srivastava, D. and Webb, C. L.(2007). Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association congenital cardiac defects committee, council on cardiovascular disease in the young: endorsed by the American Academy of Pediatrics.Circulation115, 3015-3038.
Potts, J. D. and Runyan, R. B.(1989). Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factorβ.
Dev. Biol.134, 392-401.
Proffitt, K. D., Madan, B., Ke, Z., Pendharkar, V., Ding, L., Lee, M. A., Hannoush, R. N. and Virshup, D. M. (2013). Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer.
Cancer Res.73, 502-507.
Rahmani, M., Read, J. T., Carthy, J. M., McDonald, P. C., Wong, B. W., Esfandiarei, M., Si, X., Luo, Z., Luo, H., Rennie, P. S. et al.(2005). Regulation of the versican promoter by theβ-catenin-t-cell factor complex in vascular smooth muscle cells.J. Biol. Chem.280, 13019-13028.
Ramsdell, A. F. and Markwald, R. R.(1997). Induction of endocardial cushion tissue in the avian heart is regulated, in part, by TGFβ-3-mediated autocrine signaling.Dev. Biol.188, 64-74.
Runyan, R. B. and Markwald, R. R.(1983). Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue.Dev. Biol.95, 108-114.
Sarin, K. Y., Cheung, P., Gilison, D., Lee, E., Tennen, R. I., Wang, E., Artandi, M. K., Oro, A. E. and Artandi, S. E.(2005). Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436, 1048-1052.
Scherz, P. J., Huisken, J., Sahai-Hernandez, P. and Stainier, D. Y. R.(2008). High-speed imaging of developing heart valves reveals interplay of morphogenesis and function.Development135, 1179-1187.
Semënov, M. V., Tamai, K., Brott, B. K., Kühl, M., Sokol, S. and He, X.(2001). Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6.Curr. Biol.11, 951-961.
Snarr, B. S., Kern, C. B. and Wessels, A.(2008). Origin and fate of cardiac mesenchyme.Dev. Dyn.237, 2804-2819.
Song, L., Li, Y., Wang, K. and Zhou, C. J.(2009a). Cardiac neural crest and outflow tract defects in Lrp6 mutant mice.Dev. Dyn.239, 200-210.
Song, L., Li, Y., Wang, K., Wang, Y.-Z., Molotkov, A., Gao, L., Zhao, T., Yamagami, T., Wang, Y., Gan, Q. et al.(2009b). Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development 136, 3161-3171.
Sridurongrit, S., Larsson, J., Schwartz, R., Ruiz-Lozano, P. and Kaartinen, V. (2008). Signaling via the Tgf-βtype I receptor Alk5 in heart development.Dev. Biol.322, 208-218.
Stulberg, M. J., Lin, A., Zhao, H. and Holley, S. A.(2012). Crosstalk between Fgf and Wnt signaling in the zebrafish tailbud.Dev. Biol.369, 298-307.
Sugi, Y., Ito, N., Szebenyi, G., Myers, K., Fallon, J. F., Mikawa, T. and Markwald, R. R.(2003). Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation.Dev. Biol.258, 252-263.
Sugi, Y., Yamamura, H., Okagawa, H. and Markwald, R. R. (2004). Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice.Dev. Biol.269, 505-518. Tartaglia, M., Mehler, E. L., Goldberg, R., Zampino, G., Brunner, H. G., Kremer,
H., van der Burgt, I., Crosby, A. H., Ion, A., Jeffery, S. et al.(2001). Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome.Nat. Genet.29, 465-468.
ten Berge, D., Brugmann, S. A., Helms, J. A. and Nusse, R.(2008). Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development.Development135, 3247-3257.
Timmerman, L. A., Grego-Bessa, J., Raya, A., Bertrán, E., Pérez-Pomares, J. M., Dıez, J., Aranda, S., Palomo, S., McCormick, F., Izpisú ́a-Belmonte, J. C. et al. (2004). Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation.Genes Dev.18, 99-115.
Tumbar, T., Guasch, G., Greco, V., Blanpain, C., Lowry, W. E., Rendl, M. and Fuchs, E.(2004). Defining the epithelial stem cell niche in skin.Science303,
359-363.
DEVEL
O
van Amerongen, R. and Nusse, R.(2009). Towards an integrated view of Wnt signaling in development.Development136, 3205-3214.
Vesely, I.(1997). The role of elastin in aortic valve mechanics.J. Biomech.31, 115-123.
Waldo, K., Miyagawa-Tomita, S., Kumiski, D. and Kirby, M. L.(1998). Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure.Dev. Biol.196, 129-144.
Wang, J., Sridurongrit, S., Dudas, M., Thomas, P., Nagy, A., Schneider, M. D., Epstein, J. A. and Kaartinen, V. (2005). Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart.Dev. Biol.
286, 299-310.
Wang, Y., Wu, B., Chamberlain, A. A., Lui, W., Koirala, P., Susztak, K., Klein, D., Taylor, V. and Zhou, B.(2013). Endocardial to myocardial Notch-Wnt-Bmp axis regulates early heart valve development.PLoS ONE8, e60244.
Wu, B., Wang, Y., Lui, W., Langworthy, M., Tompkins, K. L., Hatzopoulos, A. K., Baldwin, H. S. and Zhou, B. (2011). Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation.Circ. Res.109, 183-192.
Wu, B., Zhang, Z., Lui, W., Chen, X., Wang, Y., Chamberlain, A. A., Moreno-Rodriguez, R. A., Markwald, R. R., O’Rourke, B. P., Sharp, D. J. et al.(2012).
Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling.Cell151, 1083-1096.
Xiong, Y., Zhou, B. and Chang, C.-P.(2012). Analysis of the endocardial-to-mesenchymal transformation of heart valve development by collagen gel culture assay. InCardiovascular Development(ed. X. Peng and M. Antonyak), pp. 101-109. Totowa, NJ: Humana Press.
Yin, Y., White, A. C., Huh, S.-H., Hilton, M. J., Kanazawa, H., Long, F. and Ornitz, D. M.(2008). An FGF–WNT gene regulatory network controls lung mesenchyme development.Dev. Biol.319, 426-436.
Yu, H.-M. I., Jerchow, B., Sheu, T.-J., Liu, B., Costantini, F., Puzas, J. E., Birchmeier, W. and Hsu, W.(2005). The role of Axin2 in calvarial morphogenesis and craniosynostosis.Development132, 1995-2005.
Zhang, J., Lin, Y., Zhang, Y., Lan, Y., Lin, C., Moon, A. M., Schwartz, R. J., Martin, J. F. and Wang, F.(2008). Frs2α-deficiency in cardiac progenitors disrupts a subset of FGF signals required for outflow tract morphogenesis.Development
135, 3611-3622.
Zhang, J., Chang, J. Y. F., Huang, Y., Lin, X., Luo, Y., Schwartz, R. J., Martin, J. F. and Wang, F.(2010). The FGF-BMP signaling axis regulates outflow tract valve primordium formation by promoting cushion neural crest cell differentiation.Circ. Res.107, 1209-1219.