949
Therapist’s Management of
Distal Radius Fractures
SUSAN MICHLOVITZ, PT, PhD, CHT AND
LYNN FESTA, OTR, CHT
CHAPTER
70
SURGEON’S MANAGEMENT OF DRFs
EXAMINATION OF THE PATIENT WITH A DRF THERAPY GUIDELINES AND PROGRESSION MOBILIZATION/MOTION PHASE
FUNCTION AND STRENGTHENING PHASE
PATIENT EDUCATION AND HOME EXERCISE PROGRAMS
THERAPY GUIDELINES BASED ON FRACTURE MANAGEMENT TECHNIQUE
CONCLUDING STATEMENTS
based on fracture fragment patterns. Using these classifica-tions should theoretically serve as a foundation for the sur-geon’s intervention. Some knowledge of the most popular fracture classification paradigms can be helpful when com-municating with surgeons. Fracture management by the surgeon may be done with the intent to reduce and stabilize fracture fragments and include the following: orthosis or plaster cast for minimally displaced fractures,1 bridging external fixation devices with or without pinning and arthroscopically assisted reduction for intra-articular fracture fragments,2 indirect reduction and percutaneous fixation3 and open reduction and internal fixation with volar fixed-angle plates with or without locking mechanisms,4 and a low-profile dorsal plate (fragment-specific fixation or inter-medullary nailing).5 The preceding chapter and this chapter should be used together to understand a comprehensive approach to the treatment of a patient with a DRF.
Progression of activity related to specific fracture manage-ment techniques is detailed later in this chapter.
Examination of the Patient
With a DRF
The principles for examining the patient and selecting outcome questionnaires for patients with hand and wrist disorders are well covered in Chapter 16. Results of an exam-ination assist the hand therapist in establishing goals for rehabilitation and determining prognosis for functional recovery. Patient values and expectations should be
CRITICAL POINTS
! Therapy techniques and activities are based on principles of fracture healing and fixation.
! Validated self-report outcome questionnaires capture dimensions of the patient that are not always obvious by more traditional physical measures such as range of motion (ROM) with a goniometer.
! Pain can be a major obstacle to the return of motion and function.
! The goal during the mobilization phase is to restore as much motion as possible, but at least functional wrist range of motion.
Management of distal radius fractures (DRFs) has evolved considerably over the past decade, mostly due to stable fixa-tion techniques that permit early mofixa-tion of the wrist (e.g., during the first 2 to 4 weeks after fracture reduction). Therapy goals after wrist fracture are to control edema and pain, restore (realistic) ROM, and promote the use of the involved extremity for grip, torque, and weight-bearing activities. This chapter discusses the therapy strategies to optimize motion and function after DRF.
Surgeon’s Management of DRFs
There are a vast number of classification systems for surgeons to use to describe the nature of DRFs, and these are generally950 PART 12 — COMMON WRIST INJURIES
pain at rest, during activity requiring repeated wrist motion, and when lifting a heavy object and ask when the pain is worst. The frequency of pain is also rated on the same 0 to 10 Likert scale. The specific activities include such items as turning a doorknob and pushing up from a chair using the involved wrist and hand. The usual activities include per-sonal care, household chores, work, and recreational activi-ties. Pain and function are weighted at 50% each, and the scale is graded from 0 (not disabled) to 100 (most disabled).7,8
Validated questionnaires such as the PRWE, Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire,6 and the Michigan Hand Outcomes Questionnaire9 can capture dimensions of the patient that are not always obvious by more traditional physical measures such as ROM with a goni-ometer.10 These measures are responsive to changes in the patient during early (within the first few months) and late (at 1 year after injury) recovery.11 The greatest changes in DASH12 and PRWE11 scores occur during the 3- to 6-month period after fracture but will continue to improve over the first year. These region-specific questionnaires can be more responsive and specific in measuring recovery after DRF than a global measure such as the SF-36 Health Survey.13 Knowledge of these time frames can assist the therapist in educating the patient about expected milestones for recovery of function and often alleviate some of the patient’s concerns about recovery.
ROM
Passive and active motion measures are indicators of impair-ment after wrist fracture. It is important to discern which structures are involved in limiting motion. Differential motion loss (e.g., active versus passive) is addressed in this chapter in the “Mobilization/Motion Phase” section. Particu-lar attention should be paid to wrist extension, supination, and pronation—motions that may be most difficult to restore after DRF. Restoration of those motions has been correlated with higher function as measured by DASH scores.14 Therapy strategies should focus on restoration of functional wrist and forearm motion. Hand function, as measured by the Jebson Taylor Hand Function Test, has been shown in normal persons to be reduced when elbow, forearm, wrist, and finger motions are limited.15 We may be able to extrapolate these results to patients after wrist fracture. Digital motion can be markedly limited after high-impact injuries, after falls in the elderly, and in patients who have osteoarthritis (Fig. 70-2). Therefore, careful monitoring of finger and thumb motion is important.
Loading Across the Wrist: Grip, Pinch,
and Push-off
Reduction in the ability to grip and twist and to bear weight on the palm, needed when pushing open a door or pushing up from a chair, are common functional complaints in persons recovering from wrist fractures. Grip and pinch strength can be tested when fracture healing has progressed to the point where weight-bearing activities are permitted on the hand. Testing can usually begin 6 to 8 weeks after considered when developing goals and therapy strategies.
Patients with minimal complications who are self-directed and motivated and have an understanding of body function-ing may be best served with one or two supervised therapy sessions with instruction in a home exercise program. Those with less body awareness, more severe injury, and complica-tions usually require a supervised therapy program. During evaluation and treatment planning, these factors should be kept in mind.
Patient Factors Including
Medical History
The therapist should collect information from the patient regarding work and daily demands including avocations, par-ticularly those activities involving weight-bearing such as pushing up from the floor or pushing heavy loads and torque-related activities or working with heavy equipment. Patients who had a high-impact injury such as a fall while snowboard-ing or ridsnowboard-ing a motorcycle may have a significant number of related soft-tissue injuries such as scapholunate ligament or triangular fibrocartilage complex tears. Co-morbidities such as diabetes mellitus, low hemoglobin, systemic lupus erythe-matosus, and immunosuppressive disorders should be docu-mented because these conditions can delay healing or reduce expectations for the final outcome. Medication use should be documented and any side effects noted (e.g., blood thinners can result in excessive bruising) (Fig. 70-1).
Patient-Rated Self-Report
Measures/Questionnaires
When assessing the patient who has had a DRF, outcomes measures should be selected that are representative of the limitations and disabilities associated with consequences of this injury. Pain scales such as the visual analogue scale, which reflects pain during rest and activity, should be admin-istered. Pain can be a major obstacle to the return of motion and function.6
Region-specific outcomes questionnaires such as the Patient-Rated Wrist Evaluation (PRWE) have a certain per-centage of questions that directly assess pain. The PRWE has 5 questions related to pain and 10 questions related to func-tion (usual and specific activities). The pain quesfunc-tions address
Figure 70-1 Volar forearm bruising in a woman who is taking warfarin.
CHAPTER 70 — THERAPIST’S MANAGEMENT OF DISTAL RADIUS FRACTURES 951
Radiographic Outcome
There is some evidence that the amount of wrist18 and forearm motion19 as well as functional outcomes gained after fracture is related to the success of anatomic reduction. In the patient who may have less demand during activities of daily living, failure to restore anatomy may not be as critical to the final outcome.7 Therefore, each patient’s outcome must be assessed individually and goals established with the patient’s input, particularly in younger adults. It is not clear whether this is as important in an elderly population.20,21
Measuring Outcome of Intervention
After Wrist Fracture
To compare therapy interventions, each clinic should con-sider a standardized battery of measures (e.g., a core outcome dataset).22 These measures should be selected from those that reflect the problems of patients with the injury, are reliable (e.g., can be reproduced with minimum amount of error), and are responsive to change (e.g., will change in response to change in patient status). A suggested testing paradigm is presented in Box 70-1. Some measures may be better to use during certain time periods after injury. For example, volu-metric or girth measures can be useful to monitor edema during the early motion phase, but are less useful later in recovery when edema has stabilized or resolved. Before it is safe to test grip strength, patient-reported outcomes such as those included on the PRWE or DASH may give information that highlights areas of concern that should be included in goal setting and addressed during therapy.13
Most recovery can be expected to occur within the first 6 months after fracture,11 although supervised therapy is often provided only during the early period after immobilization (e.g., 4 to 6 weeks). When assessing long-term outcomes in patients who have had surgical treatment for DRF, it is important to be aware of factors that have been shown to have adverse affects on recovery such as increased age and fracture, if the fracture is healed. It is prudent to check with
the surgeon regarding the status of fracture healing.
The push-off test16 is used to quantify the ability to bear weight through the palm (Fig. 70-3). This has been tested and found to be reliable in normal subjects and also in a patient population after wrist and elbow fractures.17 The uninjured side can be used for comparison because weight-bearing ability is not dependent on hand dominance.
Figure 70-2 A, Limited finger flexion after distal radius fracture in a woman with osteoarthritis affecting the hand. B, Side view of fingers during attempted flexion.
A B
Figure 70-3 Push-off test using a Jamar dynamometer. The therapist must stabilize the dynamometer on the table while the patient is applying weight on the palm onto the device.
952 PART 12 — COMMON WRIST INJURIES
shortening, dorsal angulation, and even intra-articular involvement. Implications for ultimate outcome and use of therapy techniques may be different in both examples. In the latter, it may be less likely to restore as much ROM.
Therapy techniques and activities are based on principles of fracture healing and fixation. We must consider progres-sion on an individual basis, and this can be influenced by a number of factors. These can include some of the following:
• Success of reduction of fracture and restoration of anatomy. There is some evidence that the amount of wrist18 and forearm19 motion and the functional out-comes gained after fracture are related to the success of anatomic reduction in younger adults. It is not clear whether this is as important in an elderly population.20,21
• The time frame for healing (e.g., when can wrist motion begin and when can resisted and weight-bearing exer-cises begin)?
• Complications such as nerve compression, tendon rupture, stiffness, and pain can reduce function and can lower final outcome. Some complications that occur after DRF can be addressed through a therapy program (e.g., stiffness) and some may not (e.g., fracture mal-union or delayed mal-union), resulting in debilitating pain and decreased strength. More details about complica-tions are addressed later in this chapter.
• Pain can be a major obstacle to return of motion and function.6 The source of pain, whether it be physiologic or psychosocial, should be addressed by the appropriate practitioner. If there is evidence of complex regional pain syndrome (CRPS), then pharmacologic interven-tion managed by a physician pain specialist may be used in conjunction with therapy modalities such as heat, cold, and electrical stimulation.
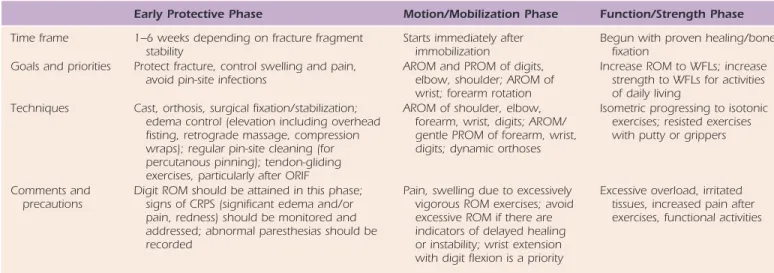
Phases of Rehabilitation
Activities during healing can be divided into the following phases: early protective phase (or during immobilization or protected motion), motion or mobilization, and function and strengthening. These are summarized in Table 70-1. Educat-ing the patient regardEducat-ing the healEducat-ing process should be done in a way that will be meaningful to that person’s status and daily life requirements. Patient compliance and its impor-tance in the rehabilitation partnership need to be empha-sized. The roles of joint mobilization and orthotic wear to increase ROM are included in the discussion.
Early Protective Phase
Ideally a hand therapist can see a patient within the first week after wrist fracture management for home instruction for symptom management (e.g., pain, edema, finger stiffness) and education regarding functional limitations and abilities. Due to a number of circumstances, we often see a patient after the first follow-up visit at the surgeon’s office and that patient has fingers that are stiff and swollen and well on the way to residual loss of motion (see Fig. 70-2).
Early on after fracture, it can be common for the patient to hold the injured upper extremity in a protected position with the shoulder adducted and internally rotated and the lower income.23 The injured worker should be assessed based
on physical work demands. Those who have jobs that involve heavy lifting, pushing, and pulling may be expected to have a longer time out of work.24 Overall outcomes can be expected to be better in patients who have higher DASH scores or patient perceived outcomes, greater grip strength, and better radiographic evidence of restoration of anatomy.25
Therapy Guidelines
and Progression
DRFs are not all the same; therefore, why should therapy be all the same? Progression of activities is discussed in each phase of rehabilitation with guidelines, techniques, and pre-cautions. These, of course, should be used as guidelines and modified for particular circumstances. Initiation of motion and progression to joint mobilization techniques, strengthen-ing, and weight-bearing activities should be undertaken when fracture healing permits. This is a determination made by the surgeon during successive follow-up office visits and reexamination and communicated to the therapist with an updated therapy referral.
Realistic goal planning for improvements in wrist ROM and hand function requires assessment of any residual defor-mities. The DRF may be a well-healed, essentially nondis-placed extra-articular fracture, but it may also be a dorsally displaced DRF with a combination of displacement, radial
Box 70-1 Suggested Therapist
Examination Components
After Distal
Radius Fracture
EARLY TESTING AFTER DRFPain using VAS or PRWE
Function with self-report outcomes questionnaires (PRWE or DASH)
Light touch with monofilaments to screen for nerve compression (median, ulnar, dorsal radial nerve) Figure-of-eight measurement or volume displacement
(if no external fixation device or cast)
ROM (if wrist is still immobilized measure adjacent joints)
LATER TESTING AFTER DRF (FROM 6 TO 8 WEEKS ONWARD)
ROM
Grip strength Pinch strength Push-off test
Function with outcomes questionnaires (PRWE or DASH)
DASH, Disability Arm, Shoulder, and Hand questionnaire; DRF, distal radius fracture; PRWE, Patient-Related Wrist Evaluation; ROM, range of motion.
CHAPTER 70 — THERAPIST’S MANAGEMENT OF DISTAL RADIUS FRACTURES 953
therapist to monitor and modify on a regular basis (e.g., weekly) to accommodate changes in edema and motion.
How do you know early on if things are not going so well for the patient? Early predictors of difficulty in recovery can include the following: metacarpophalangeal (MCP) or proxi-mal interphalangeal (PIP) joint pain and swelling, tingling in digits, and rapid change in swelling with position change. We have observed that patients who have osteoarthritis may notice their first difficulty with finger stiffness after a fall on an outstretched hand. The common comment that we hear from patients is “Well, I did not have arthritis before my injury.” In some patients, palmar nodules and bands may develop with a subsequent decrease in palmar mobility. This may be indicative of Dupuytren’s contracture.27
elbow flexed. The use of a sling should be minimized because it does not properly elevate the extremity; it promotes shoul-der and elbow stiffness and discourages functional use of the hand and arm. Slings may be used for short periods when protection is needed in crowded public situations.
Active motion of the digits, elbow, and shoulder (and wrist and forearm, if permitted) and “overhead fisting” should be encouraged to be performed several times daily. A stringent effort of elevating the hand and wrist above heart level may be successful in controlling pain and edema.26 If necessary, retrograde massage and compression wraps may be used for edema control. Cold packs or wraps may also be used after exercise if swelling has increased. But if swelling increases after each exercise session, then perhaps the vigor of the exercises should be reduced to alleviate undue stress on already inflamed tissues.
It is important to acknowledge that the pain is real for the patient. Most patients have never experienced a DRF. In addition to being frightened and in pain, the sudden interfer-ence with loss of independinterfer-ence increases the patient’s anxiety level. Along with reassurances that the pain will get better, it is important to reinforce strategies that will decrease the pain, such as elevation, massage, heat or cold modalities, and active exercises. In most patients, pain will fade as stable fracture fixation is achieved.11 However, treatment of pain is given priority if it seems to be a greater problem than the stiffness. It is difficult to use treatment techniques to address the stiffness and dysfunction if the pain cannot be controlled. Attempts should be made to determine whether the pain stems from possible median nerve compression, is sympa-thetically mediated, or is related to joint tightness or an ill-fitting immobilization cast or orthosis.
If a program of early wrist motion (e.g., within 2 to 4 weeks) is instituted, an orthosis that fits and allows full digit mobility should be worn between exercise sessions. If a well-fitted prefabricated orthosis cannot be supplied, then the therapist has the option of a custom-molded thermoplastic orthosis (Figs. 70-4 and 70-5). The orthosis may require the Table 70-1 Phases of Therapy After Distal Radius Fracture
Early Protective Phase Motion/Mobilization Phase Function/Strength Phase
Time frame 1–6 weeks depending on fracture fragment
stability Starts immediately after immobilization Begun with proven healing/bone fixation Goals and priorities Protect fracture, control swelling and pain,
avoid pin-site infections AROM and PROM of digits, elbow, shoulder; AROM of wrist; forearm rotation
Increase ROM to WFLs; increase strength to WFLs for activities of daily living
Techniques Cast, orthosis, surgical fixation/stabilization; edema control (elevation including overhead fisting, retrograde massage, compression wraps); regular pin-site cleaning (for percutanous pinning); tendon-gliding exercises, particularly after ORIF
AROM of shoulder, elbow, forearm, wrist, digits; AROM/ gentle PROM of forearm, wrist, digits; dynamic orthoses
Isometric progressing to isotonic exercises; resisted exercises with putty or grippers
Comments and
precautions Digit ROM should be attained in this phase; signs of CRPS (significant edema and/or pain, redness) should be monitored and addressed; abnormal paresthesias should be recorded
Pain, swelling due to excessively vigorous ROM exercises; avoid excessive ROM if there are indicators of delayed healing or instability; wrist extension with digit flexion is a priority
Excessive overload, irritated tissues, increased pain after exercises, functional activities
Figure 70-4 Dorsal view (A) and side view (B) of a custom circumfer-ential wrist orthosis.
A
B
Adapted from LaStayo PC, Michlovitz SM, Lee M. Wrist and hand. In: Kolt G, Snyder-Macker L, eds. Physical Therapies in Sport and Exercise. 2nd ed. 2007. AROM, active range of motion; CRPS, complex regional pain syndrome; ORIF, open reduction and internal fixation; PROM, passive range of motion; WFL, within
954 PART 12 — COMMON WRIST INJURIES
determining and prioritizing therapy strategies for improving motion, the techniques selected should to be matched with the tissues restricting motion. For example, if passive and active motion values are equally limited, then this more likely can implicate a joint as a source for the loss of motion. The motion can be lost due to obliteration of the joint space or an adaptively shortened joint capsule. The former cannot be improved by therapy, but the latter can. Selective tissue-tensioning maneuvers must be done to differentiate loss of motion. If passive PIP joint flexion is restricted, it can be caused by stiffness from osteoarthritis (which may be exac-erbated after trauma), joint contracture, or reduction of extensor tendon excursion (i.e., lengthening). Scar or pin sites on the dorsum of the hand can limit extensor tendon gliding and lead to intrinsic muscle tightness. Selective tissue tensioning is discussed in more detail in Chapters 6 and 67.
Wrist extension may be reduced because of adaptive shortening of the joint capsule, scar, or reduced flexor tendon gliding on the volar wrist. Supination loss may be attributed to volar soft-tissue injury or shortening, potentially involving the pronator quadratus, joint capsular adhesions, or distal radioulnar joint (DRUJ) malalignment. The former two can be addressed through exercise and orthotic wear. DRUJ malalignment or subluxation that limits supination and/or pronation and results in protracted pain may be addressed at a later time by surgery. Subluxation of the DRUJ can decrease forearm rotation. In particular, a severe positive ulnar vari-ance will limit supination.19
Active motion of the wrist, forearm, and digits should be emphasized early in the motion phase. Wrist extension without concurrent finger extension may be difficult to achieve early on. A priority in therapy is to overcome this substitution pattern because finger flexion, grip strength, and hand function cannot improve until the wrist extensors relearn to “work alone” without help from the digital exten-sors. If active exercise and verbal cues are not sufficient to activate wrist extensors, then neuromuscular electrical stim-ulation isolated to the wrist extensors may be helpful for muscle reeducation and to enhance tissue extensibility (Fig. 70-6).29 The electrodes would be placed over the wrist exten-sors near the elbow and middorsal forearm, the latter elec-trode to complete the circuit. A pulse rate of 25 to 35 pulses per second should be sufficient to cause a smooth titanic muscle contraction. A cycle of 10 seconds on and 20 seconds An early sign of trouble observed by the authors is the
patient who has red and painful PIP joints and fluctuating hand edema. We have observed that CRPS develops in some of these patients. Pin insertion on the radial side of the wrist may irritate the superficial branch of the radial nerve27 and result in hyperesthesia or causalgia.
Mobilization/Motion Phase
The goal during this phase is to restore as much motion as possible, but at least functional wrist ROM, defined as 40 degrees of flexion and 40 degrees of extension of the wrist and a total of 40 degrees of radial and ulnar deviation.28 Supination and wrist extension can be challenging to restore after DRF. However, regaining finger flexion can be equally if not more challenging, particularly in the person who has osteoarthritis.
Most motion gains will be made during the first 3 months after fracture.25 There can be motion gains up to 1 year, but the rate and magnitude of these changes lessen and patients would rarely need to be in supervised therapy for such an extended period of time.
Techniques to Restore Motion
A summary table (Table 70-2) is provided to assist in deci-sion making related to restoring motion after DRF. When
Figure 70-5 Custom volar forearm-based wrist orthosis.
Table 70-2 Decision Making Related to Restoring Motion After Distal Radius Fracture
Testing Characteristic Findings Suggested Intervention to Increase Motion
Joints Passive ROM (and compare
with uninjured side) Active and passive motion may be equal Joint mobilization grades 3 and 4 glides; traction grade 3 (if there was an intra-articular fracture, check imaging studies for joint space)
Tendons Compare passive with
active ROM With loss of tendon gliding, active motion will be less than passive motion in the direction of the motion measured; flexor tendon adherence can limit passive extension
Active motion of agonist; NMES of agonist muscle; resisted exercise of agonist Intrinsic
muscles Bunnell–Littler test More PIP joint flexion with MCP joints flexed, then with MCP joints extended Intrinsic muscle stretching (MCP joints extended and PIP joints flexed)
CHAPTER 70 — THERAPIST’S MANAGEMENT OF DISTAL RADIUS FRACTURES 955
and paraffin may help some patients follow their rehabilita-tion program with less discomfort and can easily be incorpo-rated into a home program. There is support in the literature for the use of heat to increase tissue extensibility in joint contracture.31
Passive stretch exercises are incorporated into the exercise program when tolerated or when fracture healing permits, as determined by radiographs and the referring surgeon. Each stretch should be held for 30 seconds for one or more repeti-tions32 several times per day. Recommendations for specific doses of passive stretch vary in the literature, and the recom-mendations are extrapolated from literature on stretching of leg muscles. Examples of passive stretches are shown in
Figures 70-8 to 70-10. The therapeutic benefits and differ-ences of both active ROM and passive stretch exercises should be explained to the patient so that there is an under-standing that one is not a substitute for the other type of exercise.
For patients with persistent joint stiffness, joint mobiliza-tion techniques can be used to assist in restoramobiliza-tion of normal joint play or accessory motion when ROM is limited.33,34 This technique has also been used as an adjunct in relieving pain. Translatory glide mobilizations of grade 3 or 4 are used to off, with a ramp up of 3 seconds should suffice to produce a
good contraction and minimize muscle fatigue. As training progresses, 10-second on and 10-second off cycling can be used. Amplitude should be set to achieve a fair plus (3+/5) muscle contraction. Electromyographic biofeedback may also be helpful for muscle reeducation of the wrist extensors, but isolated placement may be challenging because of the overlapping muscles. However, most patients can achieve independent wrist extension without the help of these modalities, but they can be useful adjuncts in selected patients.
Active wrist flexion should be performed with the fingers relaxed or extended. Radial and ulnar deviation should be done with the wrist in neutral position in the sagittal plane and the forearm pronated. It is also helpful to have the patient use the opposite hand to stabilize the forearm to prevent the elbow from moving. Forearm rotation should be done with the elbow at approximately a 90-degree angle and tucked into the side to prevent shoulder substitution. Exercises can be repeated 10 times each 3 to 4 times per day. Emphasis should be placed on moving to the end of the avail-able range, rather than just wiggling fingers and “flapping the hand about in the air.” ROM exercises through multiple planes of motion can be done with the assistance of a wand (Fig. 70-7, online).
The results of a systematic review indicated that the strongest evidence for techniques to restore passive ROM in the upper extremity include active exercise, joint mobi-lization, and the use of an orthosis.30 There were limited or no studies that examined the contribution of heat, cold, ultrasound, or electrical stimulation modalities and the use of continuous passive motion or passive stretch to increase joint motion due to joint contracture in the upper extremity.
The application of heat can have positive short-term effects on pain and tissue elasticity to facilitate ease of exer-cise. Thermal agents such as moist heat packs, Fluidotherapy,
Figure 70-6 Neuromuscular electrical nerve stimulator to increase wrist extension.
Figure 70-8 Exercises for passive stretch.
CHAPTER 70 — THERAPIST’S MANAGEMENT OF DISTAL RADIUS FRACTURES 955.e1
A
B
956 PART 12 — COMMON WRIST INJURIES
progressive orthoses by stress relaxation. Dynamic or static progressive forearm rotation orthoses can also be fabricated. Orthoses that increase wrist or forearm motion are also com-mercially available. In a retrospective review of patients after DRF, 38 of 249 patients were treated with static progressive orthoses for wrist and forearm motion. Those patients who gained wrist extension and supination had a better functional outcome as measured by grip strength and the DASH.14 Further details on specific orthotic techniques for mobiliza-tion of joints are found in Chapters 123 and 124.
Patient selection and knowledge of the healed position of the fracture are important when considering the use of one of these dynamic or static progressive orthoses. These orthotic approaches can be considered when the loss of motion is related to soft-tissue tightness due to joint capsular or mus-culotendinous unit adaptive shortening and not bony block-age or joint instability.
Good patient compliance and clear wearing instructions are required because the orthoses need to be worn several hours each day to be effective. For static progressive ortho-ses, wearing times of 30 to 60 minutes a few times each day had a positive effect on increasing wrist flexion and extension motions in a case series of patients with wrist stiffness due to DRF or other injuries who had residual joint contracture after other therapy techniques.37 Note that wear schedules in this series began for one session per day and built up to three sessions per day by 3 to 5 days. Similar positive findings were increase motion, and grades 1 and 2 are oscillations to reduce
pain. Manual traction should be applied across the joint concurrent with the glide (e.g., manual traction to the radio-carpal joint while performing a volar glide of the carpus on the distal radius to restore wrist extension). To restore wrist extension, the radiocarpal traction is accompanied by a dorsal glide of the carpus on the distal radius. Therapists using joint mobilization need to be skilled in the technique and need to make sure that there are no contraindications such as intra-articular incongruency and osteoporosis. Other contraindications for passive stretch and joint mobilization include incomplete bony union. It is not prudent to use joint mobilization in the presence of sharp, acute pain with motion and/or hypermobility.33 Further details on joint mobilization may be found in Chapter 120.
Mobilization with movement, a recently popularized tech-nique by physical therapist Brian Mulligan, may also be used to enhance passive motion and reduce pain. This combines principles of active or passive motion and joint mobilization. The therapist combines mobilization with active motion or passive motion. The goal is to increase motion in a pain-free way.33,35
Role of Orthotic Devices for Adaptively
Shortened Connective Tissue
When wrist or forearm ROM plateaus before reaching an acceptable functional level, several dynamic or static progres-sive orthotic approaches to improve pasprogres-sive ROM may be considered. Joint tightness is improved by sustained gentle stress at the end of available ROM over long periods instead of by short periods of high-intensity stretch. Low-load pro-longed stress (LLPS) is more effective than high-load brief stress.36 Knowledge of scar-tissue response to stress and bio-mechanics of the hand and wrist has led to advances in orthotic techniques that can be effective in improving wrist ROM limitations after DRF.
Static progressive orthosis use applies LLPS to the scar tissue through adjustable force application. A static progres-sive orthosis may be more effective than a dynamic orthosis when the passive ROM does not exceed the active ROM. Several types of orthoses can be fabricated with the goal of holding tissue at end ROM (Figs. 70-11 to 70-14). Dynamic orthoses work by the mechanical principle of creep and static
Figure 70-10 Exercises for passive stretch. Figure 70-11extension. (Courtesy of Kristen Valdes, OTR/L, OTD, CHT, Hand Works Orthosis to increase range of motion of wrist and finger Therapy LC, Sarasota, Florida.)
Figure 70-12 Orthosis to increase range of motion of wrist extension. (Courtesy of Paul Brach, MS, PT, CHT, Hand Center of Pittsburgh, Pitts-burgh, Pennsylvania.)
CHAPTER 70 — THERAPIST’S MANAGEMENT OF DISTAL RADIUS FRACTURES 957
that nonspecific, fluttering movements of the fingers are not effective. In addition to active flexion and extension exercises for the fingers, patients should be instructed in individual tendon-gliding exercises for the superficialis and profundus tendons. The hook-fist position isolates the digital extensors and aids interphalangeal joint flexion as well as stretches the intrinsic muscles. Particular attention needs to be paid to stretching the intrinsic muscles by extending the MCP joints while flexing the PIP and distal interphalangeal joints (Fig. 70-15).
Passive stretching of the joints should target MCP joint flexion, PIP joint extension, and stretching of the thumb webspace. There should be no painful forceful passive manipulations. The patient needs to understand the differ-ences between active ROM and passive stretch exercises and that one type of exercise is not a substitute for the other one. Like the wrist, resistant contractures of the fingers may benefit from orthotic use to provide an LLPS. Chapter 67 addresses management of the stiff hand in more detail.
Function and Strengthening Phase
When fracture healing has progressed, exercises that apply stress or load to the wrist can be initiated. It is important to emphasize that the determination of fracture healing ade-quate for initiation of stress loading and strengthening is made by the surgeon based on reexamination of the patient, either clinically or radiographically. In general, at approxi-mately 8 weeks, the wrist can typically handle progressive loading39 from light grip to wrist isometrics to progressive resisted exercises (Figs. 70-16 to 70-21) to closed chain activities. It is important to keep in mind that the patient has not used his or her involved upper extremity for any more than light activities for a considerable time. Therefore, upper extremity conditioning exercises are appropriate in both open and closed chain activities. Closed kinetic chain activi-ties include exercises performed in weight bearing such as wall push-ups, plyoball exercises, and pushing or pulling activities. Exercise tools that are used for proximal stabiliza-tion (Fig. 70-22) such as the Body Blade (www.bodyblade. com) or the B.O.I.N.G. Arm Exerciser (www.optp.com) may reported for patients who used a static progressive orthosisto restore forearm rotation.38
Orthotic devices are expensive and time-consuming to fabricate or can be costly to rent when a commercial device is used. It takes a significant commitment on the part of the patient to be compliant with wear schedules. If the patient has not been compliant in the other aspects of DRF rehabili-tation, the provision of one of these orthoses may not be indicated or justified.
What About the Hand?
Active ROM exercises of the uninvolved joints act to main-tain joint mobility, prevent tendon adherence, and help reduce edema by the “pumping” action of the muscles trans-porting the fluid proximally. Full excursion of the flexor and extensor tendons can be prohibited by the flexed position of the wrist in the cast or external fixation device. The accom-panying pain and edema may further restrict tendon gliding. Patients need to be instructed in specific exercises that need to be sustained for 10 seconds, repeated 5 to 10 times each, and performed several times each day. It must be emphasized
Figure 70-13 Orthosis to increase range of motion of wrist extension. (Courtesy of William W. Walsh, MBA, MHA, OTR/L, CHT, Hand and Reha-bilitation Specialists of North Carolina, Greensboro, North Carolina.)
Figure 70-14 Orthosis to increase range of motion wrist flexion. (Cour-tesy of Paul Brach, MS, PT, CHT, Hand Center of Pittsburgh, Pittsburgh, Pennsylvania.)
Figure 70-15 Intrinsic muscle stretching exercise. Note metacarpopha-langeal joints are extended while proximal and distal interphametacarpopha-langeal joints are flexed.
958 PART 12 — COMMON WRIST INJURIES
responsibility for their rehabilitation. Evidence in the litera-ture supports the importance of a home exercise program in patients after DRF for promoting optimal recovery.40
Therapy Guidelines Based on
Fracture Management Technique
There are a myriad of techniques used by surgeons to ana-tomically reduce and stabilize DRFs. Much has been written about the advantages and disadvantages of each, and new be useful additions to the person with high functionaldemands of the upper extremity. These exercise tools and devices can be used in a supervised therapy session and then used on a home program basis.
Patient Education and Home
Exercise Programs
Patients are usually concerned about the course of recovery from a DRF, the ultimate outcome, and whether their aches and pains are “normal.” The therapist can educate the patient about recovery milestones. MacDermid25 and colleagues pro-vided a general framework for recovery.
It seems prudent for the therapist to spend considerable time and effort designing and implementing a home program for edema control, pain modulation, and exercise. Written instructions should be in language that is not “therapy speak” but which is understandable to the specific patient. Figures or photographs that enhance the written instructions are beneficial. Digital images of the exercise session can be taken for the patient with a digital camera or with the patient’s cellular phone if it is equipped with a camera (Fig. 70-23). Therapists must be good educators, and patients with DRFs must effectively perform their home programs and accept
Figure 70-16 Progressive resisted exercises using an exercise band for wrist extension.
Figure 70-17 Progressive resisted exercises using a free weight for wrist extension.
Figure 70-18 Progressive resisted exercises using an exercise band for wrist flexion.
Figure 70-19 Progressive resisted exercises using a free weight for wrist flexion.
CHAPTER 70 — THERAPIST’S MANAGEMENT OF DISTAL RADIUS FRACTURES 959
exercise to facilitate “muscle pumping.” Tendon-gliding exer-cises and those to facilitate intrinsic stretching should be taught to the patient. Shoulder and elbow ROM should also be examined, and ROM exercises for these joints should be reviewed as part of the home exercise program.
There are some patients who are managed in this fashion who do not need formal supervised therapy. Those in whom complications develop may need ongoing supervised therapy. The older patient who did not have surgery and has a mal-union and osteoarthritis will likely have prolonged wrist pain and stiff fingers.
There are also patients whose therapy may be more focused on increasing tolerance of functional demand to allow return to premorbid activity. An example is a patient who is a violinist who required general upper extremity
Figure 70-20 Progressive resisted exercises for forearm rotation.
Figure 70-21 Grip exercises for hand strengthening.
Figure 70-22 Use of an exercise device (Body Blade) for proximal stabilization exercises.
Figure 70-23 Use of camera to document home exercise program. techniques are ever evolving. Some of the more common
techniques and postsurgical management are discussed in the following section. For individual cases, the surgeon who managed and referred the patient should be contacted to discuss specifics of fracture care and therapy management.
Closed Reduction and
Cast Immobilization
When a fracture is considered stable with good alignment and does not require surgery, a cast is applied for approxi-mately 4 to 6 weeks, depending on healing and the overall health of the patient. A properly applied cast should allow full active flexion of all digits and should be applied proximal to the distal palmar crease. When a cast is applied improp-erly, edema of the digits can occur, as well as intrinsic tight-ness, resulting in a stiff hand and potentially CRPS, even before the cast is removed. If this patient is referred to hand therapy with the cast still applied, a properly fitted cast is important. If the cast material cannot be cut down in the clinic, the patient should be referred back to the physician to have the cast reapplied. Edema control measures are intro-duced such as elevation above the level of the heart, retro-grade massage, use of self-adherent elastic wrap, and active
960 PART 12 — COMMON WRIST INJURIES
patient. The patient should be instructed to keep the pin sites dry and clean at all times because the pin is a conduit of infection.42 Daily cleaning using saline solution or diluted hydrogen peroxide applied with a clean cotton-tipped appli-cator is recommended by some, but newer evidence suggests that this may not be necessary.42 Tendon-gliding exercises performed on an hourly basis should also be emphasized. Special attention should be placed on the index finger because the dorsal pins are usually in close proximity to the extensor indicis proprius and extensor digitorum communis. Another problem seen is dorsal radial sensory nerve involvement,
Figure 70-24 Use of a therapy ball for upper extremity and core stabi-lization and endurance exercises.
Figure 70-25 A, Radiograph of distal radius fracture. B, Radiograph of external fixation device with percutaneous pinning for a distal radius fracture. (Courtesy of Daniel DiCristino, MD, Fayetteville, New York.)
A B
Figure 70-26 Radiographs of volar fixed-angle plate for a distal radius fracture. A, Lateral view of distal radius fracture. B, Lateral view of volar plate.
C, Anteroposterior view of distal radius fracture. D, Anteroposterior view of volar plate. (Courtesy of Michael Nancollas, MD, Orthopedic Associates of Central New York, Syracuse, New York.)
A B C D
conditioning and core strengthening exercises after a nondis-placed DRF. Therapy focused on conditioning and stabiliza-tion exercises including activities using a therapy ball (Fig. 70-24).
External Fixation With or Without
Percutaneous Pinning
Bridging (e.g., across the radiocarpal articulation) or non-bridging external fixation devices41 (Figs. 70-25 and 70-26) are sometimes the treatment of choice for high-energy impact injuries. The therapist must take the extent of soft-tissue injury into account when planning therapy. When a DRF is treated with external fixation, the complications most com-monly seen are pin-site infection, edema, first webspace tightness, and intrinsic tightness of the digits. If a patient is referred to hand therapy with the external fixation device still in place, examination should include the following: ROM of shoulder, elbow, and digits and edema measurement using a tape measure. Treatment is the same as with casting: retro-grade massage, use of self-adherent elasticized wrap, and active ROM. Pin-site care should also be reviewed with the
CHAPTER 70 — THERAPIST’S MANAGEMENT OF DISTAL RADIUS FRACTURES 961
gained 80% of their ROM of the wrist.44 Others also reported positive results using a dorsal Pi plate.45,46 The most common and disappointing complication seen with dorsal plate fixa-tion is rupture of the extensor pollicis longus and/or the extensor digitorum communis tendon.
Complications: When Things
Do Not Go Well
There are a variety of complications that can occur after DRF. These are outlined in Table 70-3. McKay and colleagues27 developed a complications checklist that the practitioner uses to score complications as mild (symptoms only with no specific treatment), moderate (requiring diagnostic proce-dure and/or therapeutic intervention), and severe (requiring surgery).
The highest number of complications reported in one series included median nerve compression, CRPS, tendonitis, extensor pollicis longus rupture occurring with or without dorsal plating, dorsoradial sensory neuritis, DRUJ problems, tendon adhesions, and ulnar nerve compression. Other com-plications to be aware of include flexor pollicis longus tendon rupture,46 carpal instability or subluxation, trigger finger, Dupuytren’s contracture, compartment syndrome, delayed union, and pin-site infection. In the small numbers of patients in whom CRPS develops, therapy in conjunction with phar-macologic management of pain may be prolonged. The care of the patient with CPRS is discussed in Chapters 115 and 116.
A common problem experienced is ulnar-sided wrist pain, which can affect strength and functional outcome. Most often this is due to tendinitis of the extensor carpi ulnaris tendon, DRUJ instability, or triangular fibrocartilage complex involve-ment. This pain most frequently occurs during gripping or rotational strengthening activities. Forearm pronation during gripping and loading activities increases ulnar-sided load most typically caused by placement of the proximal pin site
too close to this nerve. This can lead to CRPS; therefore, a sensory examination should also include the dorsoradial aspect of the hand. Acute carpal tunnel syndrome can also be experienced in this population, due to trauma caused by the injury and/or the position of the wrist in the fixation device. The surgeon should be contacted immediately by the patient or hand therapist if he or she experiences any sensory changes.
Open Reduction and Internal Fixation
Volar Fixed-Angle Plating
The bonus of applying a volar plate is the fixation that can be attained that allows early motion, which is advocated and encouraged by most physicians using this technique. However, literature supporting early wrist motion is equivo-cal.43 Postoperative edema can be addressed early before tendon tightness, either intrinsic or extrinsic, becomes an issue. If early motion is suggested by the surgeon, then a wrist orthosis should be worn between therapy sessions until 6 weeks after fracture. This orthosis can be prefabricated or custom fabricated depending on the fit. Scar management may be necessary using techniques such as scar massage fol-lowed by application of silicone gel pads.
Complications after volar fixed-angle plating reported in the literature include ruptures of the flexor pollicis longus and extensor tendons, but this is uncommon. If interphalan-geal joint motion of the thumb in either flexion or extension is difficult to attain, the thumb joint should be monitored closely to avoid this complication.
Dorsal Plating
This is an alternative to volar plate fixation, and in one case series, the patients were satisfied with the procedure and
Table 70-3 More Common Complications After a Distal Radius Fracture
Complication Early Late
Median nerve compression/
carpal tunnel syndrome x x Test for diminished sensibility in median distribution of hand
Complex regional pain syndrome x Early signs may be more pain than expected after injury, vasomotor instability, pain and redness in PIP joints, burning sensation in hand
Tendinitis, tenosynovitis x During remobilization may have focal pain at wrist (ulnar-sided wrist pain can implicate the ECU tendon)
Superficial branch of radial nerve
compression/neuropathy x Hyperesthesia from wrist to dorsum of thumb, index and middle fingers (to level of PIP joint) Tendon adhesions and scarring x Active motion lags behind passive motion; examine for scar adherence and observe skin for
“puckering”
Ulnar nerve compression x Paresthesia in ulnar distribution of hand and/or ulnar innervated intrinsic weakness and atrophy
Distal radioulnar joint problems x x Failure to gain forearm rotation can indicate DRUJ incongruity; note pain during forearm rotation
Ruptured EPL/FPL tendon x Symptoms of weakening of thumb extension or flexion can indicate impending rupture; loss of active extension or flexion at interphalangeal joint
Early, within the first month; late, after the first month. (Note: These are only relative time frames and can be different for each patient.) DRUJ, distal radioulnar joint; ECU, extensor carpi ulnaris; EPL, extensor pollicis longus; FPL, flexor pollicis longus.
Adapted from McKay SD, MacDermid JC, Roth JH, Richards RS. Assessment of complications of distal radius fractures and development of a complication check list. J Hand Surg 2001;26A:916–922.
962 PART 12 — COMMON WRIST INJURIES
(http://www.pedro.org.au/) and McMaster University’s Rehab+ (http://plus.mcmaster.ca/rehab/Default.aspx). Open-access journals such as BMC Musculoskeletal Disorders (http:// www.biomedcentral.com/bmcmusculoskeletdisord/) can also be accessed for information.
The importance of using current and past evidence, expert clinical opinion, and patient values to make care decisions is addressed by MacDermid in Chapter 143 (“Evidence-Based Practice”), the final chapter of this textbook.
Concluding Statements
Treatment of DRFs with better predictability, fewer compli-cations, and improved functional outcomes continues to challenge surgeons and therapists alike. There have been numerous attempts to describe and classify them and form treatment algorithms. Enhanced understanding of the anatomy and biomechanics of the wrist in the past two decades has aided the evolution of treatment for these frac-tures. The once well-established belief that deformity from this fracture can be tolerated because there are no long-term functional consequences is changing. Restoration of the anatomy can be critical to the final radiographic and func-tional outcome in many patients, particularly those with high physical demands on the involved extremity. Surgeons con-tinue to develop and refine fixation techniques to restore the distal radius anatomy with better predictability and fewer complications.
Therapists must be knowledgeable about the anatomy of the distal radius and the ramifications of any residual defor-mities and the expected time for healing after fracture. The functional effect of the accompanying soft-tissue injuries must also be factored into treatment planning because it may be just as significant as the fracture. It is the combination of soft-tissue injury, edema, and ischemia of the intrinsic muscles that leads to finger stiffness and hand dysfunction, which can remain long after the fracture has healed.
Maximum improvement from most DRFs does not occur for many months. Patients usually do not continue with therapy until maximum improvement is reached. Therefore, therapists must be good educators and patients with DRF must effectively perform their home programs and accept responsibility for their rehabilitation.
REFERENCES
The complete reference list is available online at www.expertconsult. com.
and, if painful, should be modified and performed with the forearm in neutral rotation.
Most ulnar-sided wrist pain will resolve itself within 1 year, but this problem can be debilitating to the patient’s progress, and the etiology should be determined. Because pain is the most debilitating obstacle with DRF, ulnar-sided wrist pain needs to be assessed and treated appropriately. Some ulnar-sided pain can be attributed to the change in load bearing across the wrist if there is malunion or significant shortening of the radius. If there is malalignment of fracture fragments, including a dorsal tilt of the radius, DRUJ sublux-ation or instability, or significant radial shortening that results in disabling pain, then the patient may be a candidate for a surgical procedure. This malalignment is treated with corrective osteotomy of the radius47,48 and/or ulnar shorten-ing osteotomy to alleviate ulnar impaction.49 Therapy is often required after these procedures (see Chapter 76).
Do Patients Get Better With Hand
Therapy After DRF?
Therapists recognize the value of the care and guidance that is provided to a patient through supervised therapy after a wrist fracture. Some patients with uncomplicated fractures may “figure it out on their own,” but, according to the expert clinical opinion and experience of hand therapists, patients with the typical sequelae of stiffness, edema, pain, and dis-rupted function do better with a guided and supervised program of therapy to improve the outcome. The challenge is to show which techniques are most effective through con-trolled clinical studies. As Handoll and colleagues50 wrote in their 2006 Cochrane Database Systematic Review, “there was not enough evidence available to determine the best form of rehabilitation after DRF.” It is important to note that Handoll reviewed cases where just cast immobilization had been used, and many of these patients do not require ongoing supervised therapy, rather, a well-structured home exercise plan.
Exploring Further Information on DRFs
Technologies in surgery and therapy for treating DRFs are evolving. For clinicians to remain knowledgeable, it is important to use resources from current peer-reviewed jour-nals and keep up with the evidence. Sites such as PubMed (http://www.ncbi.nlm.nih.gov/pubmed) can be used to do searches on a periodic basis. Medical Subject Headings are searched using terminology related to DRF management. PubMed provides a tutorial for those unfamiliar with this resource. There are also ratings, reviews, and articles on websites such as Physiotherapy Evidence Database (PEDro)
CHAPTER 70 — THERAPIST’S MANAGEMENT OF DISTAL RADIUS FRACTURES 962.e1
REFERENCES
1. O’Connor D, Mullett H, Doyle M, et al. Minimally displaced Colles’ fractures: a prospective randomized trial of treatment with a wrist splint or a plaster cast. J Hand Surg. 2003;28B(1):50-53. 2. Guofen C, Doi K, Hattori Y, Kitajima I. Arthroscopically assisted
reduction and immobilization of intraarticular fracture of the distal end of the radius: several options of reduction and immo-bilization. Tech Hand Up Extrem Surg. 2005;9(2):84-90.
3. Ellis A, Weiland AJ. Indirect reduction and percutaneous fixation led to a rapid recovery and better function than open reduction for intra-articular fractures of the distal end of the radius. J Bone Joint Surg Am. 2006;88:455.
4. Chung KC, Watt AJ, Kotsis SV, et al. Treatment of unstable distal radial fractures with the volar locking plating system. J Bone Joint Surg Am. 2006;88:2687-2694.
5. Simic PM, Robison J, Gardner MJ, et al. Treatment of distal radius fractures with a low-profile dorsal plating system: an outcomes assessment. J Hand Surg. 2006;31A:382-386.
6. Souer JS, Lozano-Calderón SA, Ring D. Predictors of wrist func-tion and health status after operative treatment of fractures of the distal radius. J Hand Surg. 2008;33A(2):157-163.
7. MacDermid JC. Development of a scale for patient rating of wrist pain and disability. J Hand Ther. 1996;9:178-183.
8. MacDermid JC, Tottingham V. Responsiveness of the DASH and PRWE in wrist of hand injured patients referred for rehabilitation. J Hand Ther. 2004;17:18-23.
9. Kotsis SV, Lau FH, Chung KC. Responsiveness of the Michigan Hand Outcomes Questionnaire and physical measurements in outcome studies of distal radius fracture treatment. J Hand Surg. 2007;32A(1):84-90.
10. MacDermid J, Michlovitz S. Outcomes in evidence-based practice. In: Law M, MacDermid J, eds. Evidence-Based Rehabilitation: A Guide to Practice. 2nd ed. Thorofare, NJ: Slack; 2008.
11. MacDermid JC, Roth JH, Richards RS. Pain and disability reported in the year following a distal radius fracture: a cohort study. BMC Musculoskelet Disord. 2003 Oct 31;4:24.
12. Abramo A, Kopylov P, Tagil M. Evaluation of a treatment protocol in distal radius fractures: a prospective study in 581 patients using DASH as outcome. Acta Orthop. 2008;79(3): 376-385.
13. MacDermid JC, Richards RS, Donner A, et al. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand Questionnaire, Patient-Rated Wrist Evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg. 2000;25A(2):330-340.
14. Lucado A, Li Z, Russel GB, et al. Changes in impairment and function after static progressive splinting for stiffness after distal radius fracture. J Hand Ther. 2008;21:319-325.
15. Bland MD, Beebe JA, Hardwick DD, Lang CE. Restricted active range of motion at the elbow, forearm, wrist or fingers decreases hand function. J Hand Ther. 2008;21:268-275.
16. Michlovitz SL, Ashton B, Butler M, et al. The “push off” test: assessing wrist function after fracture. Poster at American Physical Therapy Association Combined Sections Meeting, San Antonio, Texas, Feb. 2001.
17. MacDermid JC, Michlovitz S, Rafuse R, et al. Does a push-off test or other physical impairments explain disability in daily activities and work in patients with wrist and elbow pathology? Canadian Orthopaedic Conference, Montreal, Quebec, Canada, June 3-5, 2005.
18. Karnezis IA, Panagiotopoulos E, Tyllianakis M, et al. Correlation between radiological parameters and patient-rated wrist dysfunc-tion following fractures of the distal radius. Injury. 2005;36(12): 1435-1439.
19. Ishikawa J, Iwasaki N, Minami A. Influence of distal radioulnar joint subluxation on restricted forearm rotation after distal radius fracture. J Hand Surg. 2005;30A(6):1178-1188.
20. Grewal R, MacDermid JC. The risk of adverse outcomes in extra-articular distal radius fractures is increased with malalignment in patients of all ages but mitigated in older patients. J Hand Surg. 2007;32A:962-970.
21. Jaremko JL, Lambert RG, Rowe BH, et al. Do radiographic indices of distal radius fracture reduction predict outcomes in older adults receiving conservative treatment? Clin Radiol. 2007; 62(1):65-72.
22. Handoll HH, Madhok R. Conservative interventions for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2003;2:CD000314.
23. Chung KC, Kotsis SV, Kim HM. Predictors of functional outcomes after surgical treatment of distal radius fractures. J Hand Surg. 2007;32A(1):76-83.
24. MacDermid JC, Roth JH, McMurtry R. Predictors of time lost from work following a distal radius fracture. J Occup Rehabil. 2007;17:47-62.
25. MacDermid JC, Richard RS, Roth JH. Distal radius fracture: a prospective outcome study of 275 patients. J Hand Ther. 2001; 14:154-169.
26. MacDermid JC. Hand therapy management of intra-articular fractures with open reduction and Pi plate fixation: a therapist’s perspective. Tech Hand Up Extrem Surg. 2004;8:219-223. 27. McKay SD, MacDermid JC, Roth JH, Richards RS. Assessment of
complications of distal radius fractures and development of a complication checklist. J Hand Surg. 2001;26A:916-922. 28. Ryu JY, Cooney WP 3rd, Askew LJ, et al. Functional ranges of
motion of the wrist joint. J Hand Surg. 1991;16A:409-419. 29. Johnston TE. Muscle weakness and loss of motor performance.
In: Michlovitz SL, Nolan TP. Modalities for Therapeutic Interven-tion. 4th ed. Philadelphia: FA Davis; 2005:253-254.
30. Michlovitz SL, Harris BA, Watkins MP: Interventions for loss of range of motion of the upper extremity: a systematic review. J Hand Ther. 2004;17(2):118-131.
31. Usuba M, Miyanaga Y, Miyakawa S, et al. Effect of heat in increas-ing the range of knee motion after development of a joint con-tracture. Arch Phys Med Rehabil. 2006;87:247-253.
32. Youdas JW, Krause DA, Egan KS, et al. The effect of static stretch-ing of the calf muscle-tendon unit on active ankle dorsiflexion range of motion. J Orthop Sports Phys Ther. 2003;33:408-417. 33. Hertling D, Kessler RM. Wrist and hand complex. In: Hertling D,
Kessler RM. Management of Common Musculoskeletal Disorders: Physical Therapy Principles and Methods. 4th ed. Philadelphia: Lip-pincott Williams & Wilkins; 2006:430-435.
34. Kisner C, Colby LA. Therapeutic Exercise: Foundations and Tech-niques. 5th ed. Philadelphia: FA Davis; 2007:109-133.
35. Vicenzino B, Branjerdporn M, Teys P, Jordan K. Initial changes in posterior talar glide and dorsiflexion of the ankle after mobiliza-tion with movement in individuals with recurrent ankle sprain. J Orthop Sports Phys Ther. 2006;36(7):464-471.
36. Cyr LM, Ross RG. How controlled stress affects healing tissues. J Hand Ther. 1998;11(2):125-130.
37. McGrath MS, Ulrich SD, Bonutti PM, et al. Evaluation of static progressive stretch for the treatment of wrist stiffness. J Hand Surg. 2008;33A:1498-1504.
38. McGrath MS, Ulrich SD, Bonutti PM, et al. Static progressive splinting for restoration of rotational motion of the forearm. J Hand Ther. 2009;22:3-8.
39. Slutsky DJ, Herman M. Rehabilitation of distal radius fractures: a biomechanical guide. Hand Clin. 2005;21(3):455-468.
40. Lyngcoln A, Taylor N, Pizzari T, Baskus K. The relationship between adherence to hand therapy and short-term outcome after distal radius fracture. J Hand Ther. 2005;18:2-8.
41. Capo JT, Swan KG Jr, Tan V. External fixation techniques for distal radius fractures. Clin Orthop Relat Res. 2006 Apr;445:30-41. 42. Egol KA, Paksima N, Puopolo S, et al. Treatment of external
fixa-tion pins about the wrist: a prospective, randomized trial. J Bone Joint Surg. 2006;88A:349-354.
962.e2 PART 12 — COMMON WRIST INJURIES
43. Lozano-Calderón SA, Souer S, Mudgal C, et al. Wrist mobilization following volar plate fixation of fractures of the distal part of the radius. J Bone Joint Surg. 2008;90A(6):1297-1304.
44. Kamath AF, Zurakowski D, Day CS. Low-profile dorsal plating for dorsally angulated distal radius fractures: an outcomes study. J Hand Surg. 2006;31A(7):1061-1067.
45. Keller M, Steiger R. The [pi] plate: an implant for unstable exten-sion fractures of the distal radius in patients with osteoporotic bone. Tech Hand Up Extrem Surg. 2004;8(4):212-218.
46. Sánchez T, Jakubietz M, Jakubietz R, et al. Complications after Pi Plate osteosynthesis. Plast Reconstr Surg. 2006;118(1):273-274. 47. Prommersberger KJ, Ring DR, González del Pino J, et al.
Correc-tive osteotomy for intra-articular malunion of the distal part of
the radius: surgical technique. J Bone Joint Surg. 2006;88A: 202-211.
48. Viegas SF. A new modification of corrective osteotomy for treat-ment of distal radius malunion. Tech Hand Up Extrem Surg. 2006;10(4):224-230.
49. Petersen K, Breddam M, Jorgsholm P, Schroder H. Ulnar shorten-ing osteotomy after Colles fracture. Scand J Plast Reconstr Surg Hand Surg. 2005;39(3):170-177.
50. Handoll HHG, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;3: CD003324. DOI: 10.1002/14651858.CD003324.pub2.