A Green Approach to Synthesis of Nanoparticles
of Sn
3.0Ag
0.5Cu Lead-Free Solder Alloy
Siu-kwong Pang and Kam-chuen Yung
+Department of Industrial and Systems Engineering, PCB Technology Centre, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong, P. R. China
Sn3.0Ag0.5Cu nanoparticles can provide a potential solution to the high soldering temperature problem of lead-free solder alloy because the nanosize effect can depress the melting temperature. In this paper, a green approach to the synthesis of Sn3.0Ag0.5Cu nanoparticles by chemical reduction at room temperature is reported. A safe organic solvent, ethanol, was used to prevent the formation of tin oxide during synthesis without the help of capping agents and N2purging. Vigorous stirring instead of the use of capping agents was applied to the reaction
mixture to reduce agglomeration of particles during the reaction time. Owing to not having capping agents on the nanoparticles and no detection of tin oxide, the subsequent steps for eliminating them are not necessary. Ag3Sn revealed by the XRD pattern and the electron diffraction pattern
confirmed the successful alloying of Sn and Ag during synthesis. The TEM image showed that the nanoparticles were composed of a crystalline core embedded by an amorphous matrix. The average particle diameter was 53.3 nm with a standard deviation of 8.9 nm. An onset melting temperature of 187.3°C and a peak melting temperature of 212.7°C were achieved. This simple, safe and environmentally friendly method can reduce the production cost of nanosolder and may be applicable to the synthesis of other metal nanoparticles.
[doi:10.2320/matertrans.M2012112]
(Received March 22, 2012; Accepted July 31, 2012; Published September 25, 2012)
Keywords: green synthesis, lead-free nanosolder, melting temperature depression, chemical reduction, SAC solder
1. Introduction
Due to increased awareness of the toxicity of lead to humans and its harm to the environment, the European Union (EU) laid down the directive 2002/95/EC on the restriction of the use of certain hazardous substances in electrical and electronic equipment (RoHS).1) The limit for lead is set at 0.1 mass% (or 1000 ppm). Other regions such as China, the US (California) and South Korea also legislated similar laws against the use of lead in electronic products, which are commonly referred to as China RoHS (Administrative Measure on the Control of Pollution Caused by Electronic Information Products), California RoHS (California Health and Safety Code Section 25214.925214.10.2) and Korea RoHS (The Enforcement Ordinance and the Enforcement Regulation of the Act on the Recycling of Electrical and Electronic Equipment and Vehicles) respectively.24) Con-sequently, the traditional SnPb solder alloy is gradually being phased out of the electronics industry.
The alternatives to SnPb solder alloy include the systems of SnAg, SnCu, SnAgCu, SnAgCuX (X=Sb, In), SnBi, SnAgBi, SnSb and SnZn.5) The SnAgCu system is the most widely used in the electronics industry because of its relatively low melting temperature compared with other kinds of lead-free solder alloy, its high strength and low wetting angles.6,7) However, the melting point of Sn3.0Ag0.5Cu solder alloy is 220°C or higher, while the melting point of traditional SnPb solder is 183°C.6) The high soldering temperatures of lead-free solders cause adverse effects on energy consumption and thermal stress on components and substrates, leading to poor reliability of electronic assemblies.
Nanoparticles contain a large fraction of atoms at the surface, and the surface atoms have a low coordination number, which is the number of nearest neighbours. These
surface atoms facilitate fusion of the nanoparticles to increase their coordination number, resulting in forming a more stable and larger cluster, hence lowering of the melting temperature is resulted.8) Due to the importance of this nanosize effect, nanosolder particles have been chemically synthesized and studied recently.912) Jiang et al.achieved melting points of Sn3.5Ag and Sn3.0Ag0.5Cu nanoparticles as low as 194 and 199°C respectively when their average diameters of the nanoparticles were around 10 nm.5,9) Zou et al.reported the melting points of Sn3.0Ag0.5Cu and Sn3.5Ag nano-particles of around 213°C with sizes less than 100 nm.10,11) Our group reduced the melting point of Sn3.0Ag0.5Cu nanoparticles to 204.4°C when the average diameter was 20 nm.12)
Green chemistry is the design of chemical products and processes to reduce the use and generation of substances hazardous to humans and the environment.13) It can be applied to three areas in the synthesis of metal nanoparticles by chemical reduction: (1) choice of solvent, (2) selection of reducing agent, and (3) use of a capping agent for protecting from oxidation, and preventing agglomeration during the reaction time.14) However, the methods of nanosolder preparation mentioned in the previous studies were not green. Jiang et al. and Zou et al. made use of methanol and 1,10-phenanthroline as a solvent and a capping agent respectively,911)and they are toxic as described in MSDS.15) In addition, N2 purging was additionally employed in their work to prevent the nanoparticles from oxidizing. Our previous work employed water as a reaction medium and polyvinyl pyrrolidone (PVP) as a capping agent.12)Although water is safe, and the toxicity of PVP is lower than that of 1,10-phenanthroline,15) tin oxide was formed during syn-thesis even though N2 purging was implemented, thus an additional procedure for removing the tin oxide by citric acid was required.12)Therefore, a simple, cost-effective and green approach to nanosolder synthesis at room temperature and within a short time is needed.
This paper reports a green approach to synthesis of Sn3.0Ag0.5Cu nanoparticles by chemical reduction using NaBH4 as a reducing agent at room temperature to address the aforementioned problems. Since NaBH4 is a strong reducing agent, the reaction can be completed within a short time at room temperature. A safe organic solvent, ethanol, was chosen as the reaction medium to inhibit the oxide formation on the nanoparticles during synthesis. No N2 purging and toxic capping agents were applied to investigate whether ethanol alone was adequate to suppress the oxide formation, and to fulfill the green chemistry principle of chemical minimization. In order to reduce agglomeration of particles during the reaction time, without the help of capping agents, vigorous stirring was applied to the reaction mixture. Besides the relatively high cost and toxicity of the capping agents, more chemical steps may be taken to separate the capping agent from the nanoparticles due to the strong chemical bonding between the nanoparticle surface and the capping groups, since the capping agents on the nanoparticles could cause deterioration of the performance.6,16)It is another reason why getting rid of the capping agents was an aim in this study.
2. Experimental Section
2.1 Materials
Tin(II) 2-ethylhexanoate (Aldrich), silver nitrate (Sigma), copper(II) nitrate hemipentahydrate (Acros), sodium borohy-dride (Fisher) and absolute ethanol (VWR) were used as precursors, a reducing agent and a solvent respectively. They were used as received without further purification.
2.2 Synthesis
To synthesize the SnAgCu nanoparticles with a weight ratio of Sn : Ag : Cu equal to 96.5 : 3.0 : 0.5, 1.63©10¹3 moles of tin(II) 2-ethylhexanoate, 5.56©10¹5 moles of silver nitrate, and 1.57©10¹5 moles of copper(II) nitrate hemipentahydrate were mixed into 120 ml of absolute ethanol. Excess sodium borohydride was used to complete the reaction, so 4 times the mole ratio of sodium borohydride to tin(II) 2-ethylhexanoate was added to the solution.12) Vigorous stirring was applied to the solution. N2 purging was not implemented, and no capping agents were added. The reaction was held for 1 h at room temperature.12) Sn
3.0Ag0.5Cu nanoparticles formed and appeared as dark precipitates in the solution. The dark precipitates were collected and washed three times with absolute ethanol by centrifugation at 4000 rpm for 30 min. They were kept in absolute ethanol for further characterization.
2.3 Characterization
The crystal structure of the as-synthesized Sn3.0Ag 0.5Cu nanoparticles was characterized with a X-ray diffrac-tometer (XRD, Rigaku SmartLab) using the wavelength of Cu K¡ (=0.15406 nm). Ag3Sn was also verified by an electron diffraction pattern generated by a high resolution transmission electron microscope (HRTEM, JEOL JEM-2010F). Using the same TEM instrument, the morphology of the nanoparticles was revealed, and their composition was studied by means of energy dispersive X-ray spectroscopy (EDS). The particle size distribution was analyzed by measuring the particle diameters in the images taken by a scanning electron microscope (SEM, JEOL JSM-6490). DigitalMicrograph software was employed to measure the particle diameters in the SEM images. The Sn, Ag and Cu contents were quantitatively measured by SEM-EDS. The TEM specimens and the SEM specimens were prepared by dispersing the nanoparticles in absolute ethanol on a Ni grid and a Si wafer respectively. For quantitative analysis of the Sn, Ag and Cu contents using SEM-EDS, the Si wafer was fully covered by the sample, and the measuring area for each measurement ranged from 0.3 to 1 mm2. A differential scanning calorimetry (DSC) tester (PerkinElmer DSC7) was used to measure the melting temperature of the as-synthesized nanoparticles, and the heating rate of 10°C/min from 25 to 300°C was applied and nitrogen protection was used.
3. Results and Discussion
3.1 Characterization by X-ray diffraction and electron diffraction
[image:2.595.135.464.69.224.2]oxide were observed. The results prove that only a safe organic solvent, ethanol, is adequate to prevent the formation of tin oxide even though capping agents and N2purging were not applied. The poor electrical conductivity of the reaction medium due to the use of absolute ethanol as a solvent contributes to the suppression of the oxide formation during the reaction time. Since there are no capping agents on the nanoparticles and no detection of tin oxide, the subsequent steps for eliminating them are not necessary. Therefore the green chemistry principles of chemical minimization and toxicity reduction are satisfied.
Bragg’s reflections at 2ª values of 37.6 and 39.6 shown in Fig. 1 correspond to (020) and (211) lattice planes for an orthorhombic Ag3Sn structure. In addition, the electron diffraction pattern shown in Fig. 2 also confirms the formation of Ag3Sn. The interplanar spacings were measured to be 0.229 and 0.204 nm, which correspond to (211) and (121) lattice planes for the orthorhombic Ag3Sn structure respectively. Therefore, the alloying of Sn and Ag can be achieved by this green approach.
Cu6Sn5 was not observed in the XRD pattern and the electron diffraction pattern. It may be attributed to the composition of copper being less than 1%, which is below the detection limit of the techniques of X-ray diffraction and electron diffraction. This observation is consistent with the previous work by our groups and other groups.12,17,18)
3.2 Morphology and composition study by transmission electron microscopy and energy dispersive X-ray spectroscopy
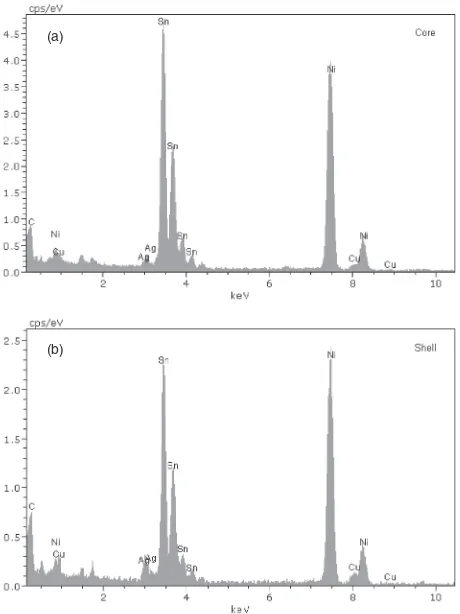
Figures 3(a) and 3(b) show the HRTEM images of Sn 3.0Ag0.5Cu nanoparticles. The particles in Fig. 3(a) appear to be close to a spherical shape. From Fig. 3(b), the particle has a coreshell structure. The lattice fringes in the dark core show good crystallinity. No lattice fringes were observed in the brighter shell, and it implies the amorphous nature of the
shell. Figures 4(a) and 4(b) are the TEM-EDS spectra arising from targeting the electron beam on the core and the shell respectively. Both spectra depict that a significant peak for L¡ of Sn atoms occurred at 3.4 keV. A small peak of L¡=3.0 keV corresponding to Ag atoms was observed in both spectra. Another small signal at 8.0 keV found in both spectra arising from the K¡ of Cu atoms. The TEM-EDS results reflect that both the crystalline core and the amorphous shell are SnAgCu alloy. It is suggested that during particle growth, defects such as kinks and ledges on the particle surface are formed. The defects cause further deposition of the Sn, Ag and Cu atoms on the non-smooth surface in a disordered noncrystalline manner, producing an amorphous shell, as observed by HRTEM. In addition, both the Sn and Cu signals in TEM-EDS spectra arising from targeting the electron beam on a crystalline nanoparticle were Fig. 2 Electron diffraction pattern of Sn3.0Ag0.5Cu nanoparticles.
(a)
(b)
[image:3.595.48.290.68.315.2] [image:3.595.309.546.70.548.2]observed, and they did not come from two individual Cu nanoparticle and Sn nanoparticle. Therefore, the results can infer the alloying of Sn and Cu in a SnAgCu nanoparticle. The C and Ni signals were observed in the spectra, resulting from the support carbonfilm on the nickel grid.
3.3 Particle size distribution, Sn, Ag and Cu contents, and melting temperature analysis by scanning electron microscopy, energy dispersive X-ray spec-troscopy and differential scanning calorimetry
Figure 5 depicts the SEM image of Sn3.0Ag0.5Cu nanoparticles. The particle sizes appear to be approximately of normal distribution (Fig. 6). By applying vigorous stirring, without the use capping agents, the average particle diameter of 53.3 nm with a standard deviation of 8.9 nm could be achieved.
The Sn, Ag and Cu contents of SnAgCu nanoparticles listed in Table 1 were quantitatively measured with SEM-EDS, and they were close to the nominal contents. Therefore, this green method can synthesize nanoparticles of lead-free solder alloy with desired Sn, Ag and Cu contents through appropriately adjusting the amounts of the reagents used in synthesis.
Figure 7 displays the DSC curve of the Sn3.0Ag0.5Cu nanoparticles. The onset melting temperature and the peak melting temperature were found to be 187.3 and 212.7°C respectively. The broad phase transition may be attributed to a wide range of the particle size distribution. Our previous work reported that the micron-sized Sn3.0Ag0.5Cu
particles supplied by Cookson (SAC 305 solder paste) showed an onset melting temperature of 219.5°C and a peak melting temperature of 222.5°C.12) The onset melting temperature of the Sn3.0Ag0.5Cu nanoparticles prepared in this study is about 30°C lower than that of micron-sized
(a)
(b)
Fig. 4 Elemental composition analysis of a Sn3.0Ag0.5Cu nanoparticle by EDS. (a) The electron beam was targeted on the core. (b) The electron beam was targeted on the shell.
Fig. 5 SEM image of Sn3.0Ag0.5Cu nanoparticles.
[image:4.595.312.541.68.242.2]Fig. 6 Particle size distribution of Sn3.0Ag0.5Cu nanoparticles.
Table 1 Sn, Ag and Cu contents of Sn3.0Ag0.5Cu nanoparticles.
Elements Mass%«standard deviation
Sn 96.06«0.76*
Ag 3.42«0.54*
Cu 0.52«0.52*
*13 measurements were carried out.
[image:4.595.54.284.69.376.2] [image:4.595.309.545.271.399.2] [image:4.595.304.549.449.503.2] [image:4.595.319.542.537.691.2]Sn3.0Ag0.5Cu particles. Similarly, the peak melting temperature of the Sn3.0Ag0.5Cu nanoparticles is around 10°C lower than that of micron-sized Sn3.0Ag0.5Cu particles.
Gao et al.reported the relationship between the size and the melting temperature for Sn3.0Ag0.5Cu particles using the following GibbsThomson equation.19)
TmðrÞ ¼Tm,bulk2ðTm,bulkHþ273:15Þ·sl m,bulkμsr
where r is the radius of a spherical particle, Tm(r) is the melting temperature of the particles, Tm,bulk is the melting temperature of the bulk alloy,¦Hm,bulkis the heat of fusion for the bulk alloy, μs is the solid phase density of the bulk
alloy, and·slis the solidliquid interfacial energy. Gaoet al. showed the dependence of the melting temperature on nano-sized Sn3.0Ag0.5Cu particles (Fig. 8), where some Sn 3.5Ag thermodynamic parameters were adopted because the corresponding parameters for Sn3.0Ag0.5Cu were not available.19) From the plot, the model predicts that particles of 53 nm diameter have a melting temperature of 214°C, which agrees well with our experimental findings that the measured peak temperature is 213°C, for measured average particle diameter of 53 nm.
4. Conclusion
In summary, Sn3.0Ag0.5Cu nanoparticles were success-fully synthesized using a green approach. It uses a one-step method for the chemical reduction of the metal compounds in absolute ethanol using NaBH4 at room temperature without the help of capping agents and N2purging. Vigorous stirring instead of the use of capping agents was applied to the reaction mixture in order to reduce agglomeration of the
diameter of 53.3 nm with a standard deviation of 8.9 nm could be achieved. No tin oxide was observed. The subsequent steps for eliminating tin oxide and capping agents are not necessary. The green chemistry principles of chemical minimization and toxicity reduction are satisfied. Lowering of the melting temperature was achieved. This simple, safe and environmentally friendly synthesis is of a great interest to the electronic materials industry, and can also reduce the production cost of nanosolder due to minimizing the chemicals used. This approach may be applicable to the synthesis of other metal nanoparticles.
REFERENCES
1) Directive 2002/95/EC. (Official Journal of the European Union, 2003) http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:037: 0019:0023:en:PDF (Accessed Mar 22, 2012).
2) Administrative Measure on the Control of Pollution Caused by Electronic Information Products, http://www.china-rohs.com.cn/laws. asp, (Accessed Mar 22, 2012).
3) California Health and Safety Code Section 25214.925214.10.2. http:// leginfo.ca.gov/cgi-bin/waisgate?WAISdocID=6440788735+0+0+0& WAISaction=retrieve (Accessed Mar 22, 2012).
4) The Enforcement Ordinance of and the Enforcement Regulation of the Act on the Recycling of Electrical and Electronic Equipment and Vehicles. http://leadfree.ipc.org/RoHS_2-1-5.asp (Accessed Mar 22, 2012).
5) H. Jiang, K.-s. (Jack) Moon and C. P. Wong:Nano-Bio-Electronic, Photonic and MEMS Packaging, (Springer, New York, 2010) pp. 217 246.
6) Y. Li, D. Lu and C. P. Wong:Electrical Conductive Adhesives with Nanotechnologies, (Springer, New York, 2010) pp. 2579.
7) M. E. Loomans and M. E. Fine:Metall. Mater. Trans. A31(2000) 11551162.
8) E. Roduner:Chem. Soc. Rev.35(2006) 583592.
9) H. Jiang, K.-s. Moon, F. Hua and C. P. Wong:Chem. Mater.19(2007) 44824485.
10) C. Zou, Y. Gao, B. Yang and Q. Zhai:Mater. Charact.61(2010) 474 480.
11) C. Zou, Y. Gao, B. Yang and Q. Zhai:J. Mater. Sci. Mater. Electron.21
(2010) 868874.
12) K. C. Yung, C. M. T. Law, C. P. Lee, B. Cheung and T. M. Yue: J. Electron. Mater.41(2012) 313321.
13) C. J.-González and D. J. C. Constable: Green Chemistry and Engineering: A Practical Design Approach, (Wiley, New Jersey, 2011) pp. 316.
14) P. Raveendran, J. Fu and S. L. Wallen:J. Am. Chem. Soc.125(2003) 1394013941.
15) The Physical and Theoretical Chemistry Laboratory Oxford Univer-sity®Chemical and Other Safety Information, http://physchem.ox. ac.uk/msds/ (Accessed Mar 22, 2012).
16) J. Liu, G. Qin, P. Raveendran and Y. Ikushima:Chem. Eur. J.12(2006) 21312138.
17) L.-Y. Hsiao and J.-G. Duhz: J. Electrochem. Soc.152(2005) J105 J109.
18) C. D. Zou, Y. L. Gao, B. Yang, X. Z. Xia, Q. J. Zhai, C. Andersson and J. Liu:J. Electron. Mater.38(2009) 351355.
19) Y. Gao, C. Zou, B. Yang, Q. Zhai, J. Liu, E. Zhuravlev and C. Schick: J. Alloy. Compd.484(2009) 777781.
[image:5.595.62.278.69.231.2]