organic papers
o1150
Lynch and McClenaghan C17H12N22H2O DOI: 10.1107/S160053680201735X Acta Cryst.(2002). E58, o1150±o1151 Acta Crystallographica Section EStructure Reports
Online ISSN 1600-5368
2-Phenylpyrrolo[2,3-
h
]quinoline dihydrate
Daniel E. Lyncha* and Ian
McClenaghanb
aSchool of Science and the Environment,
Coventry University, Coventry CV1 5FB, England, andbKey Organics Ltd, Highfield Industrial Estate, Camelford, Cornwall PL32 9QZ, England
Correspondence e-mail: apx106@coventry.ac.uk
Key indicators
Single-crystal X-ray study
T= 150 K
Mean(C±C) = 0.004 AÊ
Rfactor = 0.048
wRfactor = 0.093 Data-to-parameter ratio = 8.9
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
The crystal structure of the title compound, C17H12N22H2O, comprises an essentially ¯at molecule, with the phenyl ring being inclined at 18.7 (1) to the plane of the
pyrrolo[2,3-h]quinoline moiety, packed with two water molecules per asymmetric unit, all in a tetragonal lattice. One water molecule is involved in hydrogen-bonding associations with both pyrrolo-NH and quinoline-N sites while, as part of the overall hydrogen-bonding network, both water molecules and their symmetry equivalents construct a `Chinese lantern' arrange-ment.
Comment
The title compound, (I), was prepared from a two-stage procedure starting with the reaction of 8-hydrazinoquinoline dihydrochloride hydrate and acetophenone, followed by the cyclization of the intermediate product (E )-2-acetylbenzene-8-quinonylhydrozone. A structural example of this inter-mediate, as the thiophene derivative, has been previously published (Lynch & McClenaghan, 2001a). Furthermore, the structure of 2-(4-pyridyl)pyrrolo[3,2-h]quinoline, a product analogous to the title compound, has also been reported (Lynch & McClenaghan, 2001b). We are currently studying the structural aspects of derivatives of both 8-quinonyl-hydrozone and pyrrolo[3,2-h]quinoline before studying their potential as metal-chelating agents.
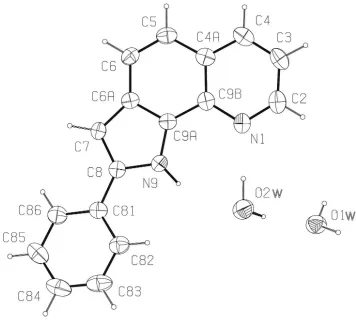
The molecule of (I) (Fig. 1) is essentially ¯at, with the phenyl ring being inclined at 18.7 (1) to the plane of the
pyrrolo[2,3-h]quinoline moiety. It crystallizes with two water molecules per asymmetric unit. One OÐH site from one water molecule resides in a hydrogen-bonded triangular arrange-ment with both pyrrolo-NH and quinoline-N sites. The other OÐH site, in conjunction with the second water molecule and their symmetry-related analogues, then forms a hydrogen-bonded `Chinese lantern' arrangement (Fig. 2).
Experimental
The title compound was obtained from Key Organics Ltd and crystals were grown from an ethanol solution.
Crystal data
C17H12N22H2O Mr= 280.32
Tetragonal,I4
a= 24.758 (4) AÊ
c= 4.6952 (9) AÊ
V= 2878.1 (8) AÊ3 Z= 8
Dx= 1.294 Mg mÿ3
MoKradiation Cell parameters from 5959
re¯ections
= 2.9±27.5 = 0.09 mmÿ1 T= 150 (2) K Block, colourless 0.200.100.10 mm
Data collection
Bruker±Nonius KappaCCD area-detector diffractometer
'and!scans
Absorption correction: multi-scan (SORTAV; Blessing, 1995)
Tmin= 0.983,Tmax= 0.991
8208 measured re¯ections
1838 independent re¯ections 1205 re¯ections withI> 2(I)
Rint= 0.079
max= 27.5 h=ÿ32!32
k=ÿ32!32
l=ÿ6!5
Re®nement
Re®nement onF2 R[F2> 2(F2)] = 0.048 wR(F2) = 0.093 S= 1.05 1838 re¯ections 206 parameters
H atoms treated by a mixture of independent and constrained re®nement
w= 1/[2(F
o2) + (0.0339P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 0.16 e AÊÿ3
min=ÿ0.20 e AÊÿ3
Table 1
Hydrogen-bonding geometry (AÊ,).
DÐH A DÐH H A D A DÐH A
N9ÐH1 O1W 0.88 (3) 2.36 (3) 3.145 (3) 148 (3) O2WÐH3W O2Wi 0.83 (2) 1.99 (2) 2.807 (3) 168 (3)
O2WÐH4W O1Wii 0.85 (2) 1.90 (2) 2.744 (3) 175 (3)
O1WÐH1W N1 0.87 (2) 1.94 (2) 2.801 (3) 172 (3) O1WÐH2W O2W 0.83 (2) 2.02 (2) 2.840 (3) 170 (3)
Symmetry codes: (i)1
2ÿy;12x;ÿ21ÿz; (ii)x;y;zÿ1.
All aromatic H atoms were included in the re®nement, at calcu-lated positions, as riding models, with CÐH set to 0.95 AÊ. All water H atoms were initially located in a difference synthesis, but were then restrained to a distance of 0.83 AÊ, with riding displacement para-meters. The remaining N-attached H atom was located in a difference synthesis; its positional and displacement parameters were re®ned. In the absence of signi®cant anomalous scattering effects, the absolute structure can not be determined; Friedel pairs were merged.
Data collection: DENZO (Otwinowski & Minor, 1997) and COLLECT(Hooft, 1998); cell re®nement:DENZOandCOLLECT; data reduction:DENZOandCOLLECT; program(s) used to solve structure:SHELXS97 (Sheldrick, 1997); program(s) used to re®ne structure: SHELXL97 (Sheldrick, 1997); molecular graphics: PLUTON94 (Spek, 1994) and PLATON97 (Spek, 1997); software used to prepare material for publication:SHELXL97.
The authors thank the EPSRC National Crystallography Service (Southampton).
References
Blessing, R. H. (1995).Acta Cryst.A51, 33±37.
Hooft, R. (1998).COLLECT. Nonius BV, Delft, The Netherlands. Lynch, D. E. & McClenaghan I. (2001a).Acta Cryst.E57, o52±o53. Lynch, D. E. & McClenaghan I. (2001b).Acta Cryst.E57, o56±o57. Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276,
Macromolecular Crystallography, Part A, edited by C. W. Carter Jr and R. M. Sweet, pp. 307±326. New York: Academic Press.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of GoÈttingen, Germany.
Spek, A. L. (1994).PLUTON94. University of Utrecht, The Netherlands. Spek, A. L. (1997).PLATON97. Version of May 1997. University of Utrecht,
The Netherlands.
Figure 2
Partial packing diagram, showing the hydrogen-bonded `Chinese lantern' arrangement of the water molecules. Hydrogen-bonding associations are shown as dotted lines.
Figure 1
supporting information
sup-1
Acta Cryst. (2002). E58, o1150–o1151
supporting information
Acta Cryst. (2002). E58, o1150–o1151 [doi:10.1107/S160053680201735X]
2-Phenylpyrrolo[2,3-
h
]quinoline dihydrate
Daniel E. Lynch and Ian McClenaghan
S1. Comment
The title compound, (I), was prepared from a two-stage procedure starting with the reaction of 8-hydrazinoquinoline
di-hydrochloride hydrate and acetophenone, followed by the cyclization of the intermediate product (E
)-2-acetylbenzene-8-quinonylhydrozone. A structural example of this intermediate, as the thiophene derivative, has been previously published
(Lynch & McClenaghan, 2001a). Furthermore, the structure of 2-(4-pyridyl)pyrrolo[3,2-h]quinoline, a product analogous
to the title compound, has also been reported (Lynch & McClenaghan, 2001b). We are currently studying the structural
aspects of derivatives of both 8-quinonylhydrozone and pyrrolo[3,2-h]quinoline before studying their potential as
metal-chelating agents.
The molecule of (I) (Fig. 1) is essentially flat, with the phenyl ring being inclined at 18.7 (1)° to the plane of the
pyrrolo[2,3-h]quinoline moiety. It crystallizes with two water molecules per asymmetric unit. One O—H site from one
water molecule resides in an hydrogen-bonded triangular arrangement with both pyrrolo-NH and quinoline-N sites. The
other O—H site, in conjunction with the second water molecule and their symmetry-related analogues, then forms an
hydrogen-bonded "Chinese lantern" arrangement (Fig. 2).
S2. Experimental
The title compound was obtained from Key Organics Ltd and crystals were grown from an ethanol solution.
S3. Refinement
All aromatic H atoms were included in the refinement, at calculated positions, as riding models, with C—H set to 0.95 Å.
All water H atoms were initially located in a difference synthesis, but were then restrained to a distance of 0.83 Å, with
riding displacement parameters. The remaining N-attached H atom was located in a difference synthesis; its positional
and displacement parameters were refined. In the absence of significant anomalous scattering effects, the absolute
Figure 1
The molecular configuration and atom-numbering scheme for the title compound, showing 50% probability ellipsoids.
Figure 2
Partial packing diagram, showing the hydrogen-bonded "Chinese lantern" arrangement of the water molecules.
[image:4.610.125.486.430.614.2]supporting information
sup-3
Acta Cryst. (2002). E58, o1150–o1151
2-phenylpyrrolo[2,3-h]quinoline dihydrate
Crystal data
C17H12N2·2H2O Mr = 280.32
Tetragonal, I4 Hall symbol: I -4
a = 24.758 (4) Å
c = 4.6952 (9) Å
V = 2878.1 (8) Å3 Z = 8
F(000) = 1184
Dx = 1.294 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 5959 reflections
θ = 2.9–27.5°
µ = 0.09 mm−1 T = 150 K Block, colourless 0.20 × 0.10 × 0.10 mm
Data collection
Bruker–Nonius KappaCCD area-detector diffractometer
Radiation source: Bruker–Nonius FR591 rotating anode
Graphite monochromator
Detector resolution: 9.091 pixels mm-1 φ and ω scans
Absorption correction: multi-scan (SORTAV; Blessing, 1995)
Tmin = 0.983, Tmax = 0.991 8208 measured reflections 1838 independent reflections 1205 reflections with I > 2σ(I)
Rint = 0.079
θmax = 27.5°, θmin = 3.3°
h = −32→32
k = −32→32
l = −6→5
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.048 wR(F2) = 0.093 S = 1.05 1838 reflections 206 parameters 4 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(Fo2) + (0.0339P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.16 e Å−3
Δρmin = −0.20 e Å−3
Special details
Geometry. Mean plane data ex SHELXL97 ###########################
Least-squares planes (x,y,z in crystal coordinates) and deviations from them (* indicates atom used to define plane) 17.1210 (0.0201) x + 0.9558 (0.0275) y + 3.3868 (0.0037) z = 1.0174 (0.0063)
* 0.0098 (0.0017) N1 * −0.0048 (0.0020) C2 * −0.0034 (0.0020) C3 * 0.0065 (0.0020) C4 * −0.0015 (0.0018) C4A * −0.0065 (0.0017) C9B
Rms deviation of fitted atoms = 0.0060
− 14.9176 (0.0246) x + 6.7625 (0.0306) y − 3.5209 (0.0037) z = 0.5527 (0.0111) Angle to previous plane (with approximate e.s.d.) = 18.73 (0.14)
* −0.0032 (0.0019) C81 * 0.0019 (0.0019) C82 * 0.0023 (0.0020) C83 * 0.0003 (0.0021) C84 * −0.0012 (0.0022) C85 * −0.0001 (0.0021) C86
Rms deviation of fitted atoms = 0.0019
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
H1 −0.0452 (12) 0.2835 (12) 0.426 (7) 0.057 (11)*
C2 0.07861 (12) 0.26321 (13) −0.1727 (7) 0.0399 (8)
H2 0.0899 0.2974 −0.2429 0.050*
C3 0.10310 (11) 0.21740 (13) −0.2832 (7) 0.0399 (8)
H3 0.1305 0.2205 −0.4241 0.050*
C4 0.08743 (11) 0.16766 (12) −0.1869 (7) 0.0366 (8)
H4 0.1042 0.1360 −0.2592 0.046*
C4A 0.04655 (10) 0.16353 (11) 0.0185 (6) 0.0311 (7)
C5 0.02781 (11) 0.11305 (11) 0.1295 (7) 0.0341 (7)
H5 0.0436 0.0805 0.0622 0.043*
C6 −0.01200 (10) 0.11030 (11) 0.3284 (7) 0.0332 (7)
H6 −0.0237 0.0763 0.3986 0.041*
C6A −0.03598 (10) 0.15882 (10) 0.4307 (6) 0.0274 (7)
C7 −0.07682 (10) 0.17015 (10) 0.6335 (6) 0.0278 (7)
H7 −0.0968 0.1443 0.7396 0.035*
C8 −0.08244 (10) 0.22546 (10) 0.6493 (6) 0.0263 (6)
N9 −0.04610 (9) 0.24847 (10) 0.4606 (5) 0.0270 (6)
C9A −0.01770 (10) 0.20845 (11) 0.3252 (6) 0.0268 (6)
C9B 0.02350 (10) 0.21262 (11) 0.1197 (6) 0.0277 (7)
C81 −0.11683 (10) 0.25757 (11) 0.8336 (6) 0.0286 (7)
C82 −0.10611 (11) 0.31220 (11) 0.8917 (7) 0.0366 (8)
H82 −0.0768 0.3298 0.7999 0.046*
C83 −0.16051 (11) 0.23337 (12) 0.9706 (7) 0.0359 (8)
H83 −0.1687 0.1966 0.9331 0.045*
C84 −0.13813 (13) 0.34066 (13) 1.0825 (7) 0.0461 (9)
H84 −0.1305 0.3776 1.1209 0.058*
C85 −0.18091 (14) 0.31576 (14) 1.2163 (7) 0.0487 (9)
H85 −0.2027 0.3354 1.3468 0.061*
C86 −0.19216 (12) 0.26215 (14) 1.1607 (7) 0.0447 (8)
H86 −0.2217 0.2450 1.2530 0.056*
O1W 0.00569 (10) 0.35901 (8) 0.2749 (5) 0.0458 (6)
H1W 0.0192 (12) 0.3296 (9) 0.205 (7) 0.057*
H2W 0.0023 (14) 0.3797 (11) 0.136 (5) 0.057*
O2W 0.00206 (9) 0.41992 (8) −0.2372 (5) 0.0424 (6)
H3W 0.0275 (9) 0.4414 (10) −0.228 (8) 0.053*
H4W 0.0047 (14) 0.4002 (11) −0.384 (5) 0.053*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
N1 0.0285 (13) 0.0382 (14) 0.0281 (14) −0.0063 (11) −0.0052 (12) 0.0025 (12)
C2 0.0356 (17) 0.0506 (19) 0.0335 (19) −0.0082 (15) −0.0038 (17) 0.0070 (17)
C3 0.0285 (16) 0.060 (2) 0.0311 (17) −0.0034 (15) 0.0030 (15) −0.0033 (17)
C4 0.0293 (16) 0.0493 (19) 0.0312 (19) 0.0022 (14) −0.0051 (15) −0.0096 (17)
C4A 0.0242 (15) 0.0392 (17) 0.0298 (17) 0.0035 (13) −0.0083 (15) −0.0021 (15)
C5 0.0300 (16) 0.0323 (16) 0.040 (2) 0.0049 (13) −0.0077 (16) −0.0061 (16)
C6 0.0330 (16) 0.0279 (15) 0.0386 (18) −0.0009 (13) −0.0027 (16) 0.0012 (15)
supporting information
sup-5
Acta Cryst. (2002). E58, o1150–o1151
C7 0.0272 (15) 0.0280 (15) 0.0283 (17) −0.0035 (12) −0.0008 (14) 0.0025 (14)
C8 0.0233 (14) 0.0300 (15) 0.0255 (15) −0.0001 (12) −0.0073 (14) 0.0009 (14)
N9 0.0285 (13) 0.0257 (13) 0.0268 (14) −0.0011 (11) −0.0025 (11) 0.0007 (12)
C9A 0.0227 (14) 0.0297 (15) 0.0278 (15) 0.0018 (12) −0.0074 (13) −0.0013 (14)
C9B 0.0245 (14) 0.0363 (16) 0.0222 (16) −0.0025 (12) −0.0088 (13) 0.0001 (14)
C81 0.0308 (15) 0.0324 (16) 0.0227 (16) 0.0042 (13) −0.0076 (14) −0.0013 (14)
C82 0.0401 (17) 0.0352 (16) 0.0345 (19) 0.0071 (14) −0.0070 (16) 0.0008 (15)
C83 0.0327 (16) 0.0377 (17) 0.0371 (19) 0.0038 (13) −0.0027 (15) −0.0004 (16)
C84 0.058 (2) 0.0399 (19) 0.040 (2) 0.0129 (17) −0.0138 (19) −0.0109 (17)
C85 0.050 (2) 0.059 (2) 0.037 (2) 0.0256 (19) −0.0044 (19) −0.0102 (18)
C86 0.0387 (18) 0.062 (2) 0.033 (2) 0.0122 (16) 0.0023 (16) −0.0013 (19)
O1W 0.0617 (15) 0.0379 (14) 0.0379 (14) 0.0026 (12) −0.0016 (12) −0.0008 (11)
O2W 0.0496 (14) 0.0365 (14) 0.0412 (13) −0.0047 (10) 0.0001 (12) −0.0056 (12)
Geometric parameters (Å, º)
N1—C2 1.332 (4) C8—C81 1.451 (4)
N1—C9B 1.367 (3) N9—C9A 1.371 (3)
C2—C3 1.387 (4) N9—H1 0.88 (3)
C2—H2 0.95 C9A—C9B 1.408 (4)
C3—C4 1.368 (4) C81—C83 1.394 (4)
C3—H3 0.95 C81—C82 1.405 (4)
C4—C4A 1.402 (4) C82—C84 1.388 (4)
C4—H4 0.95 C82—H82 0.95
C4A—C9B 1.424 (4) C83—C86 1.385 (4)
C4A—C5 1.432 (4) C83—H83 0.95
C5—C6 1.359 (4) C84—C85 1.377 (4)
C5—H5 0.95 C84—H84 0.95
C6—C6A 1.423 (4) C85—C86 1.381 (4)
C6—H6 0.95 C85—H85 0.95
C6A—C9A 1.400 (4) C86—H86 0.95
C6A—C7 1.417 (4) O1W—H1W 0.865 (17)
C7—C8 1.378 (3) O1W—H2W 0.833 (18)
C7—H7 0.95 O2W—H3W 0.825 (17)
C8—N9 1.386 (3) O2W—H4W 0.849 (18)
C2—N1—C9B 117.3 (2) C9A—N9—H1 128 (2)
N1—C2—C3 123.9 (3) C8—N9—H1 123 (2)
N1—C2—H2 118.0 N9—C9A—C6A 107.7 (2)
C3—C2—H2 118.0 N9—C9A—C9B 129.5 (3)
C4—C3—C2 119.2 (3) C6A—C9A—C9B 122.8 (3)
C4—C3—H3 120.4 N1—C9B—C9A 120.4 (2)
C2—C3—H3 120.4 N1—C9B—C4A 122.5 (2)
C3—C4—C4A 119.8 (3) C9A—C9B—C4A 117.2 (3)
C3—C4—H4 120.1 C83—C81—C82 118.1 (3)
C4A—C4—H4 120.1 C83—C81—C8 119.7 (2)
C4—C4A—C9B 117.2 (3) C82—C81—C8 122.2 (3)
C9B—C4A—C5 119.6 (3) C84—C82—H82 119.8
C6—C5—C4A 121.9 (3) C81—C82—H82 119.8
C6—C5—H5 119.0 C86—C83—C81 121.0 (3)
C4A—C5—H5 119.0 C86—C83—H83 119.5
C5—C6—C6A 119.5 (3) C81—C83—H83 119.5
C5—C6—H6 120.3 C85—C84—C82 120.4 (3)
C6A—C6—H6 120.3 C85—C84—H84 119.8
C9A—C6A—C7 107.1 (2) C82—C84—H84 119.8
C9A—C6A—C6 119.1 (3) C86—C85—C84 119.9 (3)
C7—C6A—C6 133.7 (3) C86—C85—H85 120.0
C8—C7—C6A 107.8 (2) C84—C85—H85 120.0
C8—C7—H7 126.1 C85—C86—C83 120.2 (3)
C6A—C7—H7 126.1 C85—C86—H86 119.9
C7—C8—N9 108.0 (2) C83—C86—H86 119.9
C7—C8—C81 129.5 (3) H1W—O1W—H2W 105 (3)
N9—C8—C81 122.5 (2) H3W—O2W—H4W 111 (3)
C9A—N9—C8 109.4 (2)
C9B—N1—C2—C3 −1.6 (4) C2—N1—C9B—C9A −178.7 (2)
N1—C2—C3—C4 0.3 (5) C2—N1—C9B—C4A 1.7 (4)
C2—C3—C4—C4A 0.8 (4) N9—C9A—C9B—N1 −0.5 (4)
C3—C4—C4A—C9B −0.6 (4) C6A—C9A—C9B—N1 −179.3 (2)
C3—C4—C4A—C5 179.9 (3) N9—C9A—C9B—C4A 179.0 (3)
C4—C4A—C5—C6 −179.9 (3) C6A—C9A—C9B—C4A 0.2 (4)
C9B—C4A—C5—C6 0.6 (4) C4—C4A—C9B—N1 −0.7 (4)
C4A—C5—C6—C6A 0.0 (4) C5—C4A—C9B—N1 178.9 (2)
C5—C6—C6A—C9A −0.5 (4) C4—C4A—C9B—C9A 179.8 (2)
C5—C6—C6A—C7 −179.3 (3) C5—C4A—C9B—C9A −0.7 (4)
C9A—C6A—C7—C8 −0.3 (3) C7—C8—C81—C83 −17.8 (4)
C6—C6A—C7—C8 178.6 (3) N9—C8—C81—C83 165.7 (3)
C6A—C7—C8—N9 0.1 (3) C7—C8—C81—C82 158.8 (3)
C6A—C7—C8—C81 −176.8 (3) N9—C8—C81—C82 −17.7 (4)
C7—C8—N9—C9A 0.2 (3) C83—C81—C82—C84 0.6 (4)
C81—C8—N9—C9A 177.3 (2) C8—C81—C82—C84 −176.1 (3)
C8—N9—C9A—C6A −0.4 (3) C82—C81—C83—C86 −0.6 (4)
C8—N9—C9A—C9B −179.3 (3) C8—C81—C83—C86 176.2 (3)
C7—C6A—C9A—N9 0.4 (3) C81—C82—C84—C85 −0.2 (4)
C6—C6A—C9A—N9 −178.7 (2) C82—C84—C85—C86 −0.1 (5)
C7—C6A—C9A—C9B 179.5 (2) C84—C85—C86—C83 0.0 (5)
C6—C6A—C9A—C9B 0.3 (4) C81—C83—C86—C85 0.3 (5)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
N9—H1···O1W 0.88 (3) 2.36 (3) 3.145 (3) 148 (3)
O2W—H3W···O2Wi 0.83 (2) 1.99 (2) 2.807 (3) 168 (3)
supporting information
sup-7
Acta Cryst. (2002). E58, o1150–o1151
O1W—H1W···N1 0.87 (2) 1.94 (2) 2.801 (3) 172 (3)
O1W—H2W···O2W 0.83 (2) 2.02 (2) 2.840 (3) 170 (3)