mir-101-3p is a key regulator of tumor metabolism in triple negative breast cancer targeting AMPK
Full text
Figure
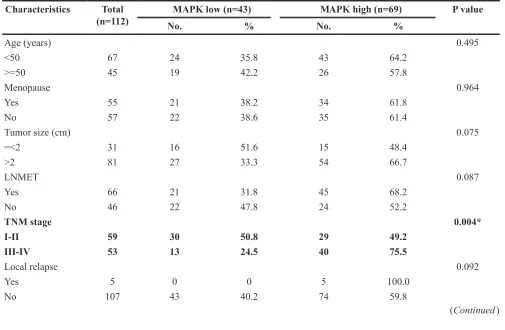
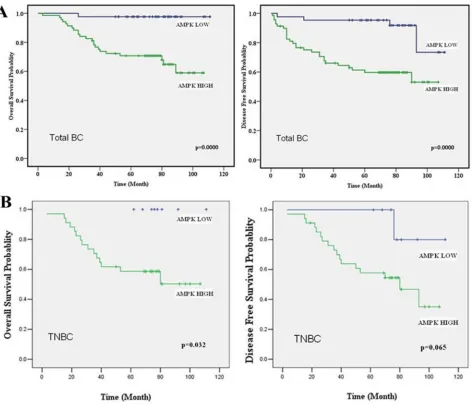
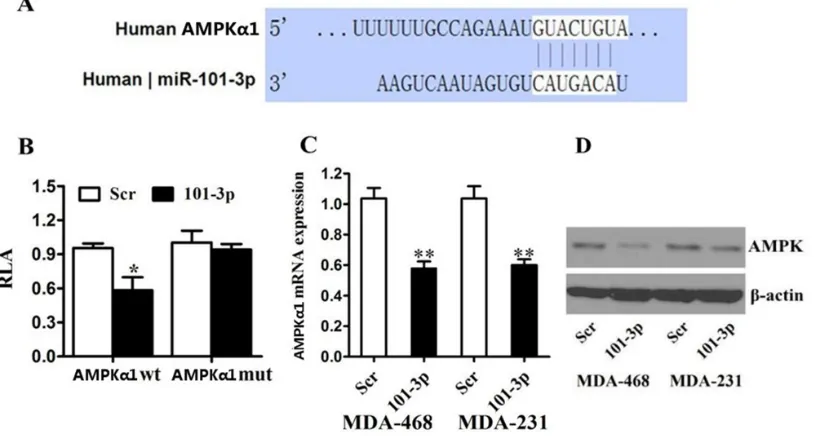
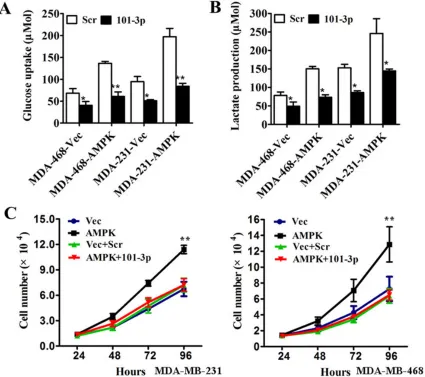
Related documents
Plasmid-encapsulated polyethylene glycol-grafted polyethylenimine nanoparticles for gene delivery into rat mesenchymal stem cells.. Xiao-Ai
This is to certify that research study entitled “ How does the use of an Interactive White Board impact an algebra teacher’s implementation of effective mathematics teaching
In this paper, we will adopt the idea of C˘adariu and Radu [ 12 ] and prove the Hyers- Ulam-Rassias stability and the Hyers-Ulam stability of the Volterra integral equation ( 1.2
Computed tomography scan (coronal plane) showing the foreign body located in the supero medial aspect of the maxillary sinus and partial mucosal thickening of the sinus upon the
Abstract: Fokker-Planck PDEs (incl. diffusions) for stable Lévy processes (incl. Wiener processes) on the joint space of positions and orientations play a major role in
equi isolates from foals with respiratory problems from various parts of Haryana and Rajasthan were used.. Nasal swabs from each case were collected in
The Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) study involved high-risk patients with coronary, peripheral arterial,
In-Vitro release studies proved that the prepared niosomes loaded with rivastigmine tartrate is considered to be a successful intranasal drug delivery system