organic papers
Acta Cryst.(2006). E62, o759–o761 doi:10.1107/S1600536806002297 Xue and Liu C
23H22N4O3
o759
Acta Crystallographica Section EStructure Reports Online
ISSN 1600-5368
2
000,2
000-Dibenzylideneisophthalohydrazide
methanol solvate
Min Xue and Shi-Xiong Liu*
Department of Chemistry, Fuzhou University, Fuzhou, Fujian 350002, People’s Republic of China
Correspondence e-mail: shixiongliu@yahoo.com
Key indicators
Single-crystal X-ray study T= 293 K
Mean(C–C) = 0.003 A˚ Rfactor = 0.047 wRfactor = 0.155
Data-to-parameter ratio = 18.3
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 5 January 2006 Accepted 18 January 2006
#2006 International Union of Crystallography
All rights reserved
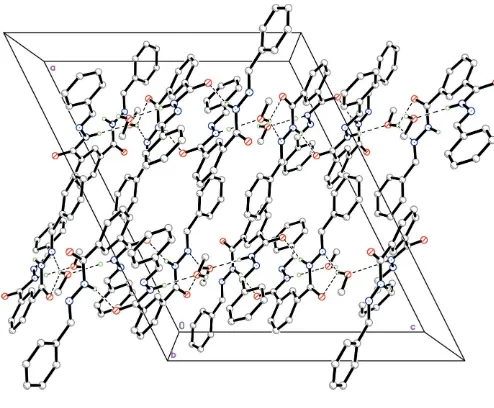
The title compound, C22H18N4O2CH3OH, was synthesized by the reaction of benzaldehyde and isophthaloyl hydrazine in methanol. The molecule is non-planar, the dihedral angles between the pairs of aromatic rings being 13.2 (1), 27.0 (1) and 18.4 (1). The hydrazide molecules are linked via hydrogen
bonds into a chain along thecaxis.
Comment
The chemistry of aroylhydrazone compounds has received increasing attention because the hydrazone group is strongly coordinated to many metal atoms and aroylhydrazone compounds possess widespread applications in the treatment of tuberculosis. They also exhibit fungicidal activity (Edwards
et al., 1975; Zhiet al., 2003; Yang & Pan, 2004). We report here the synthesis and crystal structure of the title compound, (I), obtained by the condensation of benzaldehyde with isophthaloyl hydrazine.
The molecular structure of (I) is shown in Fig. 1. The title molecule is non-planar. The dihedral angle between rings C1– C6 and C9–C14 is 13.2 (1), between rings C1–C6 and C17–
C22 is 27.0 (1) and between rings C9–C14 and C17–C22 is
[image:1.610.204.461.386.459.2] [image:1.610.215.448.548.707.2]18.4 (1). Similar C O distances (Table 1) have been
Figure 1
observed in many hydrazone compounds. The N1—C7 and N4—C16 bond lengths are close to the value of 1.280 (5) A˚ found for the imine bond length in p -dimethylenedioxy-benzaldehyde 2,4-dinitrobenzoylhydrazone (Wanget al., 2004) and shorter than the value of 1.337 (2) A˚ found for the C—N single bond in the 1:1 complex of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone and nicotinoylhydrazine (Liu et al., 2001). The N1—N2 and N3—N4 bond lengths are close to the values 1.3794 (19) and 1.388 (2) A˚ in p -dimethylenedioxy-benzaldehyde benzoylhydrazone (Funet al., 1997), indicating that a partially conjugated system operates in this hydrazone. There are four intermolecular hydrogen bonds in the crystal structure (Table 2), which link adjacent molecules to form a chain. The H atom on O3 engages in binding to atoms N1iiand O1iisimultaneously.
Experimental
Isophthaloyl hydrazine (17.8 mmol, 3.45 g) was dissolved in anhy-drous methanol (50 ml), and benzaldehyde (35.7 mmol, 3.65 ml) was added. The mixture was refluxed for 3 h and the resulting precipitate was collected by filtration and washed with methanol and diethyl ether. The product (0.37 g) was dissolved in methanol (15 ml) and CH2Cl2(15 ml), and kept at room temperature for 20 d to obtain colourless single crystals.
Crystal data
C23H22N4O3
Mr= 402.45
Monoclinic,C2=c a= 20.168 (4) A˚
b= 14.737 (3) A˚
c= 16.357 (3) A˚
= 116.54 (3)
V= 4349.0 (15) A˚3
Z= 8
Dx= 1.229 Mg m 3
MoKradiation Cell parameters from 4999
reflections
= 1.8–27.5
= 0.08 mm1
T= 293 (2) K Prism, colorless 0.550.420.36 mm
Data collection
Rigaku Weissenberg IP diffractometer
!scans
Absorption correction: multi-scan (TEXRAY; Molecular Structure Corporation, 1999)
Tmin= 0.778,Tmax= 0.970
20697 measured reflections
4999 independent reflections 3243 reflections withI> 2(I)
Rint= 0.031
max= 27.5
h= 0!26
k=19!19
l=21!19
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.047
wR(F2) = 0.155
S= 1.04 4999 reflections 273 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0901P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 0.20 e A˚
3
min=0.23 e A˚
[image:2.610.45.292.69.273.2]3
Table 1
Selected geometric parameters (A˚ ,).
C1—C7 1.454 (2) C7—N1 1.270 (2) C8—O1 1.2219 (17) C8—N2 1.351 (2) C8—C9 1.499 (2) C11—C15 1.497 (2)
C15—O2 1.2305 (18) C15—N3 1.348 (2) C16—N4 1.272 (2) C16—C17 1.463 (2) N1—N2 1.3838 (18) N3—N4 1.3837 (16)
O1—C8—N2 122.96 (15) O2—C15—N3 122.84 (14)
C7—N1—N2 114.50 (13) C16—N4—N3 115.12 (13)
O1—C8—C9—C10 153.04 (15) C10—C11—C15—O2 151.34 (15)
O1—C8—N2—N1 4.5 (2) O2—C15—N3—N4 0.7 (2)
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
N2—H2A O2i
0.86 2.17 3.0048 (17) 164 O3—H3C N1ii
0.89 2.28 3.0954 (18) 153 O3—H3C O1ii 0.89 2.30 2.9550 (19) 131 N3—H3A O3 0.86 2.05 2.8848 (19) 163
Symmetry codes: (i)xþ1 2;yþ
1
2;z; (ii)xþ 1 2;yþ
1 2;zþ
1 2.
Atoms H3Aand H3Cwere located in difference Fourier maps, but were then allowed to ride on N3 and O3, with N—H = 0.86 A˚ and O—H = 0.89 A˚ . The other H atoms were placed in idealized positions (aromatic C—H = 0.93 A˚ , methanol C—H = 0.96 A˚ and N—H = 0.86 A˚ ) and were refined using a riding model, with Uiso(H) = 1.5Ueq(C).
Data collection: TEXRAY (Molecular Structure Corporation, 1999); cell refinement: TEXRAY; data reduction: TEXSAN
(Molecular Structure Corporation, 1999); program(s) used to solve structure:SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEX (McArdle, 1995); software used to prepare material for publication:SHELXL97-2(Sheldrick, 1997).
The authors are grateful for financial support from the National Natural Science Foundation of China (Nos. 20431010 and 20171012).
organic papers
o760
Xue and Liu C23H22N4O3 Acta Cryst.(2006). E62, o759–o761
Figure 2
References
Edwards, E. I., Epton, R. & Marr, G. (1975).J. Organomet. Chem.85, C23– C25.
Fun, H.-K., Lu, Z.-L., Duan, C.-Y., Tian, Y.-P., You, X.-Z., Gong, X.-Y. & Guo, Y.-M. (1997).Acta Cryst.C53, 1454–1455.
Liu, L., Jia, D.-Z., Qiao, Y.-M. & Yu, K.-B. (2001).Acta Chim. Sin.59, 1495– 1501.
McArdle, P. (1995).J. Appl. Cryst.28, 65.
Molecular Structure Corporation (1999). TEXRAY (Version 1.10) and
TEXSAN(Version 1.10). MSC, 9009 New Trails Drive, The Woodlands, TX 77381-5209, USA.
Sheldrick, G. M. (1997). SHELXL97, SHELXS97 and SHELXL97-2. University of Go¨ttingen, Germany.
Wang, J.-L., Jia, Y.-J., Miao, F.-M. & Li, A.-X. (2004).Chin. J. Org. Chem.24, 41–49.
Yang, J.-G. & Pan, F.-Y. (2004).Acta Cryst.E60, o2009–o2010.
Zhi, J. F., Bin, Z., Su, H. W. & Zheng, M. L. (2003).Chin. J. Appl. Chem.20, 365–367.
organic papers
Acta Cryst.(2006). E62, o759–o761 Xue and Liu C
supporting information
sup-1 Acta Cryst. (2006). E62, o759–o761
supporting information
Acta Cryst. (2006). E62, o759–o761 [https://doi.org/10.1107/S1600536806002297]
2
′
,2
′
-Dibenzylideneisophthalohydrazide methanol solvate
Min Xue and Shi-Xiong Liu
2′,2′-Dibenzylideneisophthalohydrazide methanol solvate
Crystal data
C23H22N4O3
Mr = 402.45
Monoclinic, C2/c
Hall symbol: -C 2yc
a = 20.168 (4) Å
b = 14.737 (3) Å
c = 16.357 (3) Å
β = 116.54 (3)°
V = 4349.0 (15) Å3
Z = 8
F(000) = 1696
Dx = 1.229 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 4999 reflections
θ = 1.8–27.5°
µ = 0.08 mm−1
T = 293 K Prism, colorless 0.55 × 0.42 × 0.36 mm
Data collection
Rigaku Weissenberg IP diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: multi-scan
(TEXRAY; Molecular Structure Corporation, 1999)
Tmin = 0.778, Tmax = 0.970
20697 measured reflections 4999 independent reflections 3243 reflections with I > 2σ(I)
Rint = 0.031
θmax = 27.5°, θmin = 1.8°
h = 0→26
k = −19→19
l = −21→19
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.047
wR(F2) = 0.155
S = 1.04 4999 reflections 273 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0901P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.20 e Å−3
Δρmin = −0.23 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full
supporting information
sup-2 Acta Cryst. (2006). E62, o759–o761
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C1 0.44217 (9) −0.05548 (11) 0.33589 (11) 0.0529 (4)
C2 0.43306 (10) −0.13845 (12) 0.36941 (13) 0.0610 (4)
H2B 0.3877 −0.1683 0.3417 0.073*
C3 0.49100 (12) −0.17650 (16) 0.44357 (15) 0.0800 (6)
H3B 0.4848 −0.2320 0.4663 0.096*
C4 0.55846 (12) −0.1323 (2) 0.48442 (16) 0.0899 (7)
H4B 0.5974 −0.1582 0.5349 0.108*
C5 0.56849 (12) −0.05116 (19) 0.45155 (18) 0.0918 (7)
H5A 0.6141 −0.0219 0.4792 0.110*
C6 0.51095 (11) −0.01310 (14) 0.37759 (16) 0.0761 (6)
H6A 0.5179 0.0420 0.3548 0.091*
C7 0.38278 (10) −0.00983 (11) 0.25938 (12) 0.0569 (4)
H7A 0.3946 0.0409 0.2347 0.068*
C8 0.19378 (9) 0.00274 (9) 0.11527 (11) 0.0493 (4)
C9 0.14804 (9) 0.07022 (9) 0.04362 (11) 0.0475 (3)
C10 0.17282 (8) 0.15852 (9) 0.04371 (10) 0.0440 (3)
H10A 0.2169 0.1778 0.0918 0.053*
C11 0.13266 (8) 0.21787 (9) −0.02682 (10) 0.0445 (3)
C12 0.06619 (10) 0.18904 (12) −0.09731 (13) 0.0649 (5)
H12A 0.0394 0.2276 −0.1460 0.078*
C13 0.03989 (11) 0.10285 (13) −0.09500 (15) 0.0794 (6)
H13A −0.0058 0.0847 −0.1409 0.095*
C14 0.08071 (10) 0.04353 (12) −0.02534 (14) 0.0652 (5)
H14A 0.0628 −0.0145 −0.0249 0.078*
C15 0.16019 (8) 0.31091 (10) −0.03247 (11) 0.0458 (3)
C16 0.28167 (10) 0.46415 (10) 0.12448 (12) 0.0552 (4)
H16A 0.2934 0.4281 0.1758 0.066*
C17 0.31690 (9) 0.55314 (11) 0.13602 (12) 0.0542 (4)
C18 0.37162 (12) 0.57653 (13) 0.22157 (15) 0.0721 (5)
H18A 0.3843 0.5366 0.2703 0.087*
C19 0.40756 (13) 0.65904 (16) 0.23486 (18) 0.0919 (7)
H19A 0.4445 0.6743 0.2924 0.110*
C20 0.38907 (14) 0.71824 (15) 0.16387 (19) 0.0931 (7)
H20A 0.4130 0.7740 0.1733 0.112*
C21 0.33506 (13) 0.69571 (13) 0.07835 (17) 0.0804 (6)
H21A 0.3232 0.7358 0.0299 0.097*
C22 0.29830 (11) 0.61377 (11) 0.06400 (14) 0.0643 (5)
H22A 0.2612 0.5992 0.0063 0.077*
C23 0.30528 (19) 0.23499 (17) 0.29017 (17) 0.1246 (12)
supporting information
sup-3 Acta Cryst. (2006). E62, o759–o761
H23B 0.2932 0.2150 0.3378 0.187*
H23C 0.3522 0.2658 0.3167 0.187*
N1 0.31563 (7) −0.03554 (8) 0.22437 (9) 0.0512 (3)
N2 0.26757 (7) 0.01782 (8) 0.15277 (9) 0.0535 (3)
H2A 0.2847 0.0608 0.1321 0.064*
N3 0.20711 (7) 0.34913 (8) 0.04749 (9) 0.0480 (3)
H3A 0.2192 0.3210 0.0982 0.052 (5)*
N4 0.23556 (7) 0.43439 (8) 0.04670 (9) 0.0481 (3)
O1 0.16633 (7) −0.06044 (7) 0.13789 (9) 0.0647 (3)
O2 0.14186 (7) 0.34821 (8) −0.10677 (8) 0.0594 (3)
O3 0.25191 (8) 0.29256 (8) 0.23362 (9) 0.0731 (4)
H3C 0.2470 0.3409 0.2625 0.113 (9)*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C1 0.0530 (9) 0.0534 (8) 0.0523 (9) −0.0016 (7) 0.0237 (8) 0.0004 (7)
C2 0.0547 (9) 0.0610 (10) 0.0635 (11) 0.0017 (8) 0.0233 (9) 0.0126 (9)
C3 0.0744 (13) 0.0886 (13) 0.0765 (14) 0.0166 (11) 0.0332 (12) 0.0312 (12)
C4 0.0634 (12) 0.125 (2) 0.0619 (13) 0.0257 (13) 0.0104 (10) 0.0114 (13)
C5 0.0596 (12) 0.1021 (17) 0.0881 (17) −0.0089 (12) 0.0102 (12) −0.0106 (14)
C6 0.0641 (11) 0.0710 (12) 0.0842 (14) −0.0108 (10) 0.0250 (11) −0.0024 (11)
C7 0.0638 (10) 0.0463 (8) 0.0614 (11) −0.0015 (7) 0.0288 (9) 0.0101 (7)
C8 0.0616 (9) 0.0400 (7) 0.0478 (9) −0.0037 (7) 0.0257 (8) 0.0010 (6)
C9 0.0517 (8) 0.0422 (7) 0.0493 (9) −0.0009 (6) 0.0233 (7) 0.0025 (6)
C10 0.0448 (7) 0.0420 (7) 0.0433 (8) −0.0001 (6) 0.0178 (7) 0.0010 (6)
C11 0.0441 (7) 0.0423 (7) 0.0448 (8) 0.0012 (6) 0.0178 (7) 0.0019 (6)
C12 0.0574 (10) 0.0579 (10) 0.0588 (11) −0.0001 (8) 0.0074 (9) 0.0094 (8)
C13 0.0592 (11) 0.0637 (11) 0.0800 (14) −0.0137 (9) −0.0007 (10) 0.0032 (10)
C14 0.0576 (10) 0.0504 (9) 0.0744 (12) −0.0112 (8) 0.0178 (9) 0.0026 (9)
C15 0.0454 (8) 0.0425 (7) 0.0474 (9) 0.0069 (6) 0.0188 (7) 0.0073 (7)
C16 0.0644 (10) 0.0446 (8) 0.0550 (10) 0.0001 (7) 0.0253 (9) 0.0053 (7)
C17 0.0594 (9) 0.0438 (8) 0.0621 (10) −0.0010 (7) 0.0297 (9) −0.0033 (7)
C18 0.0752 (12) 0.0652 (11) 0.0675 (12) −0.0098 (9) 0.0243 (10) −0.0047 (10)
C19 0.0905 (16) 0.0802 (14) 0.0882 (17) −0.0301 (12) 0.0250 (14) −0.0150 (13)
C20 0.1007 (17) 0.0598 (11) 0.113 (2) −0.0301 (12) 0.0423 (16) −0.0086 (13)
C21 0.0999 (15) 0.0519 (10) 0.0894 (16) −0.0105 (10) 0.0422 (14) 0.0062 (10)
C22 0.0750 (11) 0.0461 (8) 0.0687 (12) −0.0017 (8) 0.0293 (10) 0.0003 (8)
C23 0.168 (3) 0.0819 (16) 0.0697 (16) 0.0374 (17) 0.0041 (17) −0.0013 (13)
N1 0.0580 (8) 0.0439 (6) 0.0490 (8) 0.0024 (6) 0.0214 (6) 0.0084 (6)
N2 0.0578 (8) 0.0446 (6) 0.0557 (8) 0.0014 (6) 0.0233 (7) 0.0156 (6)
N3 0.0566 (7) 0.0380 (6) 0.0472 (8) −0.0019 (5) 0.0213 (6) 0.0062 (6)
N4 0.0535 (7) 0.0362 (6) 0.0553 (8) 0.0016 (5) 0.0250 (7) 0.0042 (6)
O1 0.0729 (8) 0.0519 (6) 0.0663 (8) −0.0115 (6) 0.0284 (7) 0.0122 (6)
O2 0.0663 (7) 0.0536 (6) 0.0485 (7) −0.0019 (5) 0.0169 (6) 0.0121 (5)
supporting information
sup-4 Acta Cryst. (2006). E62, o759–o761
Geometric parameters (Å, º)
C1—C2 1.385 (2) C14—H14A 0.9300
C1—C6 1.391 (3) C15—O2 1.2305 (18)
C1—C7 1.454 (2) C15—N3 1.348 (2)
C2—C3 1.373 (3) C16—N4 1.272 (2)
C2—H2B 0.9300 C16—C17 1.463 (2)
C3—C4 1.382 (3) C16—H16A 0.9300
C3—H3B 0.9300 C17—C18 1.384 (3)
C4—C5 1.363 (4) C17—C22 1.390 (2)
C4—H4B 0.9300 C18—C19 1.382 (3)
C5—C6 1.367 (3) C18—H18A 0.9300
C5—H5A 0.9300 C19—C20 1.364 (3)
C6—H6A 0.9300 C19—H19A 0.9300
C7—N1 1.270 (2) C20—C21 1.375 (3)
C7—H7A 0.9300 C20—H20A 0.9300
C8—O1 1.2219 (17) C21—C22 1.381 (3)
C8—N2 1.351 (2) C21—H21A 0.9300
C8—C9 1.499 (2) C22—H22A 0.9300
C9—C14 1.379 (2) C23—O3 1.358 (3)
C9—C10 1.394 (2) C23—H23A 0.9600
C10—C11 1.384 (2) C23—H23B 0.9600
C10—H10A 0.9300 C23—H23C 0.9600
C11—C12 1.388 (2) N1—N2 1.3838 (18)
C11—C15 1.497 (2) N2—H2A 0.8600
C12—C13 1.383 (3) N3—N4 1.3837 (16)
C12—H12A 0.9300 N3—H3A 0.8600
C13—C14 1.379 (3) O3—H3C 0.8853
C13—H13A 0.9300
C2—C1—C6 118.81 (17) C9—C14—H14A 120.0
C2—C1—C7 123.03 (15) O2—C15—N3 122.84 (14)
C6—C1—C7 118.16 (16) O2—C15—C11 120.92 (14)
C3—C2—C1 119.94 (18) N3—C15—C11 116.21 (13)
C3—C2—H2B 120.0 N4—C16—C17 122.27 (15)
C1—C2—H2B 120.0 N4—C16—H16A 118.9
C2—C3—C4 120.0 (2) C17—C16—H16A 118.9
C2—C3—H3B 120.0 C18—C17—C22 119.22 (16)
C4—C3—H3B 120.0 C18—C17—C16 118.40 (16)
C5—C4—C3 120.7 (2) C22—C17—C16 122.37 (16)
C5—C4—H4B 119.7 C19—C18—C17 120.2 (2)
C3—C4—H4B 119.7 C19—C18—H18A 119.9
C4—C5—C6 119.5 (2) C17—C18—H18A 119.9
C4—C5—H5A 120.3 C20—C19—C18 120.3 (2)
C6—C5—H5A 120.3 C20—C19—H19A 119.9
C5—C6—C1 121.1 (2) C18—C19—H19A 119.9
C5—C6—H6A 119.5 C19—C20—C21 120.2 (2)
supporting information
sup-5 Acta Cryst. (2006). E62, o759–o761
N1—C7—C1 123.54 (15) C21—C20—H20A 119.9
N1—C7—H7A 118.2 C20—C21—C22 120.3 (2)
C1—C7—H7A 118.2 C20—C21—H21A 119.8
O1—C8—N2 122.96 (15) C22—C21—H21A 119.8
O1—C8—C9 122.57 (15) C21—C22—C17 119.80 (19)
N2—C8—C9 114.47 (12) C21—C22—H22A 120.1
C14—C9—C10 119.25 (15) C17—C22—H22A 120.1
C14—C9—C8 119.16 (14) O3—C23—H23A 109.5
C10—C9—C8 121.56 (14) O3—C23—H23B 109.5
C11—C10—C9 120.86 (14) H23A—C23—H23B 109.5
C11—C10—H10A 119.6 O3—C23—H23C 109.5
C9—C10—H10A 119.6 H23A—C23—H23C 109.5
C10—C11—C12 119.15 (14) H23B—C23—H23C 109.5
C10—C11—C15 122.34 (13) C7—N1—N2 114.50 (13)
C12—C11—C15 118.44 (14) C8—N2—N1 120.25 (12)
C13—C12—C11 119.88 (17) C8—N2—H2A 119.9
C13—C12—H12A 120.1 N1—N2—H2A 119.9
C11—C12—H12A 120.1 C15—N3—N4 118.98 (13)
C14—C13—C12 120.66 (17) C15—N3—H3A 120.5
C14—C13—H13A 119.7 N4—N3—H3A 120.5
C12—C13—H13A 119.7 C16—N4—N3 115.12 (13)
C13—C14—C9 120.07 (16) C23—O3—H3C 112.5
C13—C14—H14A 120.0
C6—C1—C2—C3 1.2 (3) C8—C9—C14—C13 −176.01 (18)
C7—C1—C2—C3 −178.17 (17) C10—C11—C15—O2 151.34 (15)
C1—C2—C3—C4 −0.4 (3) C12—C11—C15—O2 −25.5 (2)
C2—C3—C4—C5 −0.4 (4) C10—C11—C15—N3 −26.8 (2)
C3—C4—C5—C6 0.3 (4) C12—C11—C15—N3 156.30 (15)
C4—C5—C6—C1 0.6 (4) N4—C16—C17—C18 −173.87 (17)
C2—C1—C6—C5 −1.3 (3) N4—C16—C17—C22 4.9 (3)
C7—C1—C6—C5 178.1 (2) C22—C17—C18—C19 −0.4 (3)
C2—C1—C7—N1 9.4 (3) C16—C17—C18—C19 178.41 (19)
C6—C1—C7—N1 −169.93 (18) C17—C18—C19—C20 0.4 (4)
O1—C8—C9—C14 −28.7 (2) C18—C19—C20—C21 −0.7 (4)
N2—C8—C9—C14 151.56 (16) C19—C20—C21—C22 1.1 (4)
O1—C8—C9—C10 153.04 (15) C20—C21—C22—C17 −1.1 (3)
N2—C8—C9—C10 −26.7 (2) C18—C17—C22—C21 0.8 (3)
C14—C9—C10—C11 −3.4 (2) C16—C17—C22—C21 −177.99 (18)
C8—C9—C10—C11 174.88 (14) C1—C7—N1—N2 179.25 (14)
C9—C10—C11—C12 1.3 (2) O1—C8—N2—N1 −4.5 (2)
C9—C10—C11—C15 −175.52 (14) C9—C8—N2—N1 175.28 (13)
C10—C11—C12—C13 1.9 (3) C7—N1—N2—C8 −173.87 (15)
C15—C11—C12—C13 178.82 (18) O2—C15—N3—N4 0.7 (2)
C11—C12—C13—C14 −3.0 (3) C11—C15—N3—N4 178.84 (12)
C12—C13—C14—C9 0.8 (3) C17—C16—N4—N3 −179.83 (13)
supporting information
sup-6 Acta Cryst. (2006). E62, o759–o761
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
N2—H2A···O2i 0.86 2.17 3.0048 (17) 164
O3—H3C···N1ii 0.89 2.28 3.0954 (18) 153
O3—H3C···O1ii 0.89 2.30 2.9550 (19) 131
N3—H3A···O3 0.86 2.05 2.8848 (19) 163