inorganic papers
i256
Abdelhediet al. Cs2(SO4)0.57(SeO4)0.43Te(OH)6 doi:10.1107/S1600536805034574 Acta Cryst.(2005). E61, i256–i258 Acta Crystallographica Section E
Structure Reports Online
ISSN 1600-5368
Cs
2(SO
4)
0.57(SeO
4)
0.43Te(OH)
6, an adduct between
dicaesium sulfate selenate and telluric acid
Mohamed Abdelhedi,a,b Mohamed Dammak,b* Alain Cousson,aMartine Nierlichcand Kolsi Abdelwahebb
aLaboratoire Le´on Brillouin, CE/Saclay, Baˆt. 563,
91191 Gif-sur-Yvette Cedex, France,
bLaboratoire de l’Etat Solide, Faculte´ des
Sciences de Sfax, 3018 Sfax, Tunisia, andcSCM,
CE/Saclay, Baˆt. 125, 91191 Gif-sur-Yvette Cedex, France
Correspondence e-mail: meddammak@yahoo.fr
Key indicators
Single-crystal X-ray study
T= 298 K
Mean(S–O) = 0.009 A˚ H-atom completeness 0% Disorder in main residue
Rfactor = 0.037
wRfactor = 0.035
Data-to-parameter ratio = 10.7
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
The title compound, dicaesium sulfate selenate–telluric acid adduct, Cs2(SO4)0.57(SeO4)0.43Te(OH)6, is a solid solution in the series Cs2(SO4)Te(OH)6/Cs2(SeO4)Te(OH)6. It crystal-lizes in the same structure as the end member Cs2(SeO4)Te(OH)6 in space group P21/c, whereas the corresponding sulfate adopts another structure type and crystallizes in space groupR3. The structure contains planes of statistically distributed SO4/SeO4 tetrahedra alternating with planes of Te(OH)6 octahedra, and with Cs
+
cations situated between the planes. Both Te atoms lie on centres of symmetry.
Comment
Pursuing our study of adducts between sulfate and/or selenate salts with telluric acid, among which we have solved the crystal structures of Cs2SO4Te(OH)6, Cs2SeO4Te(OH)6(Dammaket
al., 2001) and Rb2(SO4)0.5(SeO4)0.5Te(OH)6(Abdelhediet al., 2005), we have grown crystals of Cs2(SO4)0.57(SeO4)0.43 -Te(OH)6, (I), from a solution of Cs2SO4, Cs2SeO4and telluric acid.
Compound (I) is a solid solution in the series Cs2(SO4)Te(OH)6/Cs2(SeO4)Te(OH)6and crystallizes in the same space group as the end member Cs2(SeO4)Te(OH)6, whereas the sulfate end member adopts another structure type in space groupR3 (Dammaket al., 2001). In (I), the S and Se atoms are statistically distributed over the same site. The crystal structure can be regarded as being built up of planes of Te(OH)6octahedra (atx= 0 and12) alternating with planes of
XO4tetrahedra (atx=14and 3
4;X= S and Se), with Cs +
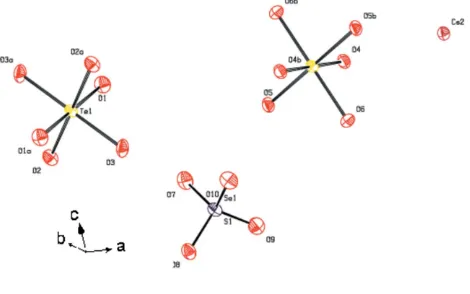
cations intercalated between the planes (Figs. 1 and 2). In the XO4 tetrahedra, the X—O distances range from 1.511 (9) A˚ to
[image:1.610.217.445.551.728.2]Received 16 September 2005 Accepted 24 October 2005 Online 31 October 2005
Figure 1
1.558 (9) A˚ , intermediate between those observed in the end members Cs2SO4Te(OH)6 [1.399 (10)–1.405 (7) A˚ ] and Cs2SeO4Te(OH)6 [1.630 (4)–1.649 (4) A˚ ; Dammak et al., 2001]. The Te atoms occupy two sites, giving rise to two kinds of octahedra,viz. Te1O6and Te2O6, having bond lengths and angles similar to those in K2SO4Te(OH)6(Zilberet al., 1980) and Tl2SO4Te(OH)6 (Zilber et al., 1982) (Table 1). The coordination environments around both Cs cations are slightly different, with Cs1 having ten coordination partners and Cs2 having 11 coordination partners (Table 1; Figs. 3 and 4). In contrast, the Rb cations in Rb2(SO4)0.5(SeO4)0.5Te(OH)6 (Abdelhedi et al., 2005) and the Cs cations in Cs2SeO4Te(OH)6 (Dammak et al., 2001) are ninefold coor-dinate.
Experimental
Crystals were grown at room temperature by evaporating an aqueous solution of telluric acid, H6TeO6(Aldrich, 99%), caesium carbonate, Cs2CO3(Aldrich 99.9%), selenic acid, H2SeO4(Aldrich 94%) and caesium sulfate Cs2SO4, (Aldrich 99.999%) (in the stoichiometric ratio 1:0.5:0.5:0.5). After a few days, colourless single crystals of Cs2(SO4)0.57(SeO4)0.43Te(OH)6were obtained.
Crystal data
Cs2(SO4)0.57(SeO4)0.43Te(OH)6
Mr= 611.68
Monoclinic,P21=c a= 12.2646 (4) A˚
b= 7.3926 (4) A˚
c= 12.6354 (6) A˚ = 111.095 (3) V= 1068.84 (9) A˚3
Z= 4
Dx= 3.765 Mg m 3
MoKradiation Cell parameters from 986
reflections = 1.8–25.8
= 11.12 mm1
T= 298 K Prism, colourless 0.100.050.05 mm
Data collection
Nonius KappaCCD diffractometer ’scans
Absorption correction: multi-scan (using intensity measurements) MULABS inPLATON(Spek, 2003)
Tmin= 0.541,Tmax= 0.573
2044 measured reflections
1881 independent reflections 1216 reflections withI> 3(I)
Rint= 0.042
max= 25.8
h=14!14
k=8!0
l= 0!14
Refinement
Refinement onF R[F2> 2(F2)] = 0.037
wR(F2) = 0.035
S= 0.94 1216 reflections 114 parameters
H-atom parameters not refined Weighting scheme: Chebychev
polynomial (Watkin, 1994; Prince, 1982)
w= [1(Fo2Fc)2/362(Fo)]2/
[2.38T0(x)2.95T1(x) +
2.50T2(x)1.02T3(x) +
0.340T4(x)], wherex=F/Fmax;
robust weighting (Prince, 1982)
(/)max< 0.001
max= 2.43 e A˚
3
min=2.54 e A˚
3
Extinction correction: Larson (1970)
Extinction coefficient: 17.2 (14)
Table 1
Selected geometric parameters (A˚ ,).
Xis disordered S/Se on a single site.
Te1—O1i 1.935 (8) Te1—O2i 1.917 (8) Te1—O3i 1.896 (8) Te2—O4ii 1.937 (8) Te2—O6ii 1.925 (8) Te2—O5ii 1.905 (8)
X1—O7 1.511 (9)
X1—O8 1.526 (8)
X1—O9 1.558 (9)
X1—O10 1.546 (8) Cs1—O5iii 2.988 (9) Cs1—O1iii 3.065 (9) Cs1—O3iv 3.116 (9) Cs1—O2v 3.147 (9) Cs1—O7v 3.255 (9) Cs1—O9vi 3.292 (9) Cs1—O8iv 3.334 (9) Cs1—O1vii 3.413 (9) Cs1—O6viii 3.460 (9) Cs1—O7iii 3.698 (8) Cs2—O6ix 3.098 (8) Cs2—O4 3.109 (8) Cs2—O2x 3.133 (9) Cs2—O3ii 3.153 (9) Cs2—O9iv 3.165 (9) Cs2—O8xi 3.248 (9) Cs2—O4iv 3.301 (8) Cs2—O5iv 3.337 (8) Cs2—O10ii 3.404 (8) Cs2—O10ix 3.639 (10) Cs2—O6iv 3.675 (9) O1i —Te1—O2i 86.5 (4) O1i —Te1—O3i 91.3 (4) O2i —Te1—O3i 88.5 (4) O2i—Te1—O1 93.5 (4) O3i
—Te1—O1 88.7 (4) O3i
—Te1—O2 91.5 (4) O4ii—Te2—O6ii 87.9 (4) O4ii —Te2—O5ii 88.5 (3) O6ii —Te2—O5ii 87.8 (4) O6ii
—Te2—O4 92.1 (4) O5ii
—Te2—O4 91.5 (3) O6ii
—Te2—O5 92.2 (4) O7—X1—O8 110.8 (5) O7—X1—O9 107.3 (4) O8—X1—O9 108.6 (5) O7—X1—O10 108.1 (5) O8—X1—O10 108.6 (5) O9—X1—O10 113.4 (5)
Symmetry codes: (i) x;y;zþ1; (ii) xþ1;yþ1;zþ1; (iii)
xþ1;y1 2;zþ
1
2; (iv) xþ1;yþ 1 2;zþ
1
2; (v) xþ1;y;z; (vi) xþ1;y;z;
(vii)xþ1;yþ1 2;z
1
2; (viii)x;yþ 1 2;z
1
2; (ix)x;yþ1;z; (x)xþ1;yþ1;z; (xi) xþ1;yþ3
2;zþ 1 2.
inorganic papers
Acta Cryst.(2005). E61, i256–i258 Abdelhediet al. Cs
[image:2.610.46.281.73.220.2]2(SO4)0.57(SeO4)0.43Te(OH)6
i257
Figure 2
Part of the crystal structure of (I), with displacement parameters drawn at the 50% probability level. [Symmetry codes: (a)x,y, 1z; (b) 1x,
1y, 1z.] Figure 3
For the refinement of the occupation factors for S and Se, their sum was restrained to be equal to 1. The highest peak is situated 1.00 A˚ from Cs2, and the deepest hole 0.63 A˚ from S/Se. H atoms could not be located.
Data collection: COLLECT (Nonius, 2001); cell refinement:
DENZO (Otwinowski & Minor, 1997); data reduction: DENZO;
program(s) used to solve structure: SHELXS86 (Sheldrick, 1985); program(s) used to refine structure:CRYSTALS(Betteridgeet al., 2003); molecular graphics: DIAMOND (Brandenburg & Berndt, 1999); software used to prepare material for publication: CRYS-TALS.
References
Abdelhedi, M., Dammak, M., Cousson, A. & Kolsi, A. W. (2005).J. Alloys Compd.398, 55–61.
Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K. & Watkin, D. J. (2003).J. Appl. Cryst.36, 1487.
Brandenburg, K. & Berndt, M. (1999).DIAMOND. Version 2.1.b. Crystal Impact GbR, Bonn, Germany.
Dammak, M., Mhiri, T., Jaud, J. & Savariault, J. M. (2001).Int. J. Inorg. Mater.
3, 861–873.
Larson, A. C. (1970).Crystallographic Computing, edited by F. R. Ahmed, S. R. Hall & C. P. Huber, p. 29. Copenhagen: Munksgaard.
Nonius (2001).COLLECT. Nonius BV, Delft, The Netherlands.
Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276,
Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
Prince, E. (1982).Mathematical Techniques in Crystallography and Materials Science.New York: Springer-Verlag.
Sheldrick, G. M. (1985).SHELXS86. University of Go¨ttingen, Germany. Spek, A. L. (2003).PLATON. University of Utrecht, The Netherlands. Watkin, D. J. (1994).Acta Cryst.A50, 411–437.
Zilber, R., Durif, A. & Averbuch-Pouchot, M. T. (1980).Acta Cryst.B36, 2743– 2745.
Zilber, R., Durif, A. & Averbuch-Pouchot, M. T. (1982).Acta Cryst.B38, 1554– 1556.
inorganic papers
i258
Abdelhediet al. Cs [image:3.610.58.267.72.257.2]2(SO4)0.57(SeO4)0.43Te(OH)6 Acta Cryst.(2005). E61, i256–i258
Figure 4
supporting information
sup-1
Acta Cryst. (2005). E61, i256–i258
supporting information
Acta Cryst. (2005). E61, i256–i258 [https://doi.org/10.1107/S1600536805034574]
Cs
2(SO
4)
0.57(SeO
4)
0.43·
Te(OH)
6, an adduct between dicaesium sulfate selenate
and telluric acid
Mohamed Abdelhedi, Mohamed Dammak, Alain Cousson, Martine Nierlich and Kolsi
Abdelwaheb
Dicesium sulfate-selenate tellurate
Crystal data
Cs2(SO4)0.57(SeO4)0.43·Te(OH)6 Mr = 611.68
Monoclinic, P21/c
Hall symbol: -P 2ybc
a = 12.2646 (4) Å
b = 7.3926 (4) Å
c = 12.6354 (6) Å
β = 111.095 (3)°
V = 1068.84 (9) Å3 Z = 4
F(000) = 1063.2
Dx = 3.765 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 986 reflections
θ = 1.8–25.8°
µ = 11.12 mm−1 T = 298 K Prism, colourless 0.10 × 0.05 × 0.05 mm
Data collection
Nonius KappaCCD diffractometer
Graphite monochromator
φ scans
Absorption correction: empirical (using intensity measurements)
MULABS in PLATON (Spek, 2003)
Tmin = 0.541, Tmax = 0.573
2044 measured reflections 1881 independent reflections 1216 reflections with I > 3σ(I)
Rint = 0.042
θmax = 25.8°, θmin = 1.8° h = −14→14
k = −8→0
l = 0→14
Refinement
Refinement on F
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.037 wR(F2) = 0.035 S = 0.94 1216 reflections 114 parameters 1 restraint
Primary atom site location: heavy-atom method H-atom parameters not refined
Weighting scheme: Chebychev polynomial (Watkin, 1994; Prince, 1982) w = 1/[2.38T0(x)-2.95T1(x) + 2.50T2-1.02T3 +
0.340Tn-1(x)],
where x = F /Fmax; robust weighting (Prince,
1982): W = w[1-(δF/6σF)2]2
(Δ/σ)max = 0.000204
Δρmax = 2.43 e Å−3
Δρmin = −2.54 e Å−3
supporting information
sup-2
Acta Cryst. (2005). E61, i256–i258
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq Occ. (<1)
Te1 0.0000 0.0000 0.5000 0.0126
Te2 0.5000 0.5000 0.5000 0.0125
Cs1 0.86573 (7) 0.03842 (10) 0.10885 (7) 0.0157
Cs2 0.63706 (7) 0.99099 (11) 0.35694 (6) 0.0147
S1 0.24815 (10) −0.00046 (10) 0.24890 (10) 0.0182 0.566 (7)
Se1 0.24815 (10) −0.00046 (10) 0.24890 (10) 0.0182 0.434 (7)
O1 −0.0176 (8) 0.2415 (11) 0.4370 (7) 0.0224
O2 −0.1109 (8) −0.0746 (11) 0.3562 (7) 0.0198
O3 0.1211 (7) −0.0524 (12) 0.4446 (7) 0.0218
O4 0.4726 (8) 0.6954 (10) 0.3913 (7) 0.0173
O5 0.3627 (8) 0.3851 (11) 0.3997 (7) 0.0177
O6 0.5857 (8) 0.3786 (11) 0.4197 (7) 0.0169
O7 0.1425 (8) 0.1012 (11) 0.2521 (7) 0.0222
O8 0.2189 (9) −0.1993 (11) 0.2202 (8) 0.0203
O9 0.2838 (8) 0.0861 (11) 0.1537 (8) 0.0240
O10 0.3449 (8) 0.0117 (12) 0.3679 (7) 0.0236
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Te1 0.01131 (16) 0.01085 (16) 0.01222 (16) 0.00000 (16) 0.00004 (16) 0.00000 (16)
Te2 0.01232 (16) 0.01082 (16) 0.01294 (16) 0.00030 (16) 0.00275 (16) 0.00025 (16)
Cs1 0.0132 (4) 0.0164 (3) 0.0178 (3) 0.0005 (3) 0.0058 (3) 0.0013 (3)
Cs2 0.0129 (4) 0.0147 (3) 0.0162 (3) −0.0007 (3) 0.0049 (3) 0.0002 (3)
S1 0.01767 (10) 0.01767 (10) 0.01767 (10) −0.00276 (10) 0.00445 (10) −0.00276 (10)
Se1 0.01767 (10) 0.01767 (10) 0.01767 (10) −0.00276 (10) 0.00445 (10) −0.00276 (10)
O1 0.023 (6) 0.017 (4) 0.026 (5) 0.002 (4) 0.008 (4) 0.004 (3)
O2 0.014 (5) 0.017 (4) 0.024 (5) 0.001 (3) 0.002 (4) 0.000 (3)
O3 0.013 (5) 0.023 (4) 0.032 (5) 0.001 (4) 0.011 (4) 0.003 (4)
O4 0.017 (5) 0.017 (4) 0.018 (4) −0.004 (3) 0.006 (4) −0.001 (3)
O5 0.014 (5) 0.021 (4) 0.016 (4) −0.005 (3) 0.004 (4) −0.001 (3)
O6 0.018 (5) 0.018 (4) 0.017 (4) 0.002 (3) 0.009 (4) 0.000 (3)
O7 0.025 (6) 0.015 (4) 0.026 (5) 0.001 (3) 0.008 (4) 0.008 (3)
O8 0.017 (5) 0.015 (4) 0.028 (5) 0.000 (4) 0.007 (4) 0.001 (3)
O9 0.018 (5) 0.021 (4) 0.031 (5) 0.003 (4) 0.007 (4) 0.000 (4)
O10 0.026 (5) 0.019 (4) 0.022 (4) 0.002 (4) 0.004 (4) 0.005 (4)
Geometric parameters (Å, º)
Te1—O1i 1.935 (8) Cs1—O1iii 3.065 (9)
Te1—O2i 1.917 (8) Cs1—O3iv 3.116 (9)
Te1—O3i 1.896 (8) Cs1—O2v 3.147 (9)
Te1—O1 1.935 (8) Cs1—O7v 3.255 (9)
Te1—O2 1.917 (8) Cs1—O9vi 3.292 (9)
supporting information
sup-3
Acta Cryst. (2005). E61, i256–i258
Te2—O4ii 1.937 (8) Cs1—O1vii 3.413 (9)
Te2—O6ii 1.925 (8) Cs1—O6viii 3.460 (9)
Te2—O5ii 1.905 (8) Cs1—O7iii 3.698 (8)
Te2—O4 1.937 (8) Cs2—O6ix 3.098 (8)
Te2—O5 1.905 (8) Cs2—O4 3.109 (8)
Te2—O6 1.925 (8) Cs2—O2x 3.133 (9)
S1—O7 1.511 (9) Cs2—O3ii 3.153 (9)
S1—O8 1.526 (8) Cs2—O9iv 3.165 (9)
S1—O9 1.558 (9) Cs2—O8xi 3.248 (9)
S1—O10 1.546 (8) Cs2—O4iv 3.301 (8)
Se1—O7 1.511 (9) Cs2—O5iv 3.337 (8)
Se1—O8 1.526 (8) Cs2—O10ii 3.404 (8)
Se1—O9 1.558 (9) Cs2—O10ix 3.639 (10)
Se1—O10 1.546 (8) Cs2—O6iv 3.675 (9)
Cs1—O5iii 2.988 (9)
O1i—Te1—O2i 86.5 (4) O4ii—Te2—O5 91.5 (3)
O1i—Te1—O3i 91.3 (4) O6ii—Te2—O5 92.2 (4)
O2i—Te1—O3i 88.5 (4) O5ii—Te2—O5 179.994
O1i—Te1—O1 179.994 O4—Te2—O5 88.5 (3)
O2i—Te1—O1 93.5 (4) O4ii—Te2—O6 92.1 (4)
O3i—Te1—O1 88.7 (4) O6ii—Te2—O6 179.994
O1i—Te1—O2 93.5 (4) O5ii—Te2—O6 92.2 (4)
O2i—Te1—O2 179.994 O4—Te2—O6 87.9 (4)
O3i—Te1—O2 91.5 (4) O5—Te2—O6 87.8 (4)
O1—Te1—O2 86.5 (4) O7—S1—O8 110.8 (5)
O1i—Te1—O3 88.7 (4) O7—S1—O9 107.3 (4)
O2i—Te1—O3 91.5 (4) O8—S1—O9 108.6 (5)
O3i—Te1—O3 179.994 O7—S1—O10 108.1 (5)
O1—Te1—O3 91.3 (4) O8—S1—O10 108.6 (5)
O2—Te1—O3 88.5 (4) O9—S1—O10 113.4 (5)
O4ii—Te2—O6ii 87.9 (4) O7—Se1—O8 110.8 (5)
O4ii—Te2—O5ii 88.5 (3) O7—Se1—O9 107.3 (4)
O6ii—Te2—O5ii 87.8 (4) O8—Se1—O9 108.6 (5)
O4ii—Te2—O4 179.994 O7—Se1—O10 108.1 (5)
O6ii—Te2—O4 92.1 (4) O8—Se1—O10 108.6 (5)
O5ii—Te2—O4 91.5 (3) O9—Se1—O10 113.4 (5)