RESEARCH
Impact of acute diabetes decompensation
on outcomes of diabetic patients admitted
with ST-elevation myocardial infarction
Mayada Issa
1†, Fahad Alqahtani
2†, Chalak Berzingi
2, Mohammad Al‑Hajji
2, Tatiana Busu
2and Mohamad Alkhouli
2,3*Abstract
Background: Acute hyperglycemia is associated with worse outcomes in diabetic patients admitted with ST‑eleva‑ tion myocardial infarction (STEMI). However, the impact of full‑scale decompensated diabetes on STEMI outcomes has not been investigated.
Methods: We utilized the national inpatient sample (2003–2014) to identify adult diabetic patients admitted with STEMI. We defined decompensated diabetes as the presence of diabetic ketoacidosis (DKA) or hyperglycemic hyper‑ osmolar state (HHS). We compared in‑hospital morbidity and mortality and cost between patients with and without diabetes decompensation before and after propensity‑score matching.
Results: A total of 73,722 diabetic patients admitted with STEMI were included in the study. Of those, 1131 (1.5%) suf‑ fered DKA or HSS during the hospitalization. After propensity‑score matching, DKA/HHS remained associated with a significant 32% increase in in‑hospital mortality (25.6% vs. 19.4%, p = 0.001). The DKA/HHS group also had higher inci‑ dences of acute kidney injury (39.4% vs. 18.9%, p < 0.001), sepsis (7.3% vs. 4.9%, p = 0.022), blood transfusion (11.3% vs. 8.2%) and a non‑significant trend towards higher incidence of stroke (3.8% vs. 2.4%, p = 0.087). Also, DKA/HHS diagnosis was associated with lower rates of referral to coronary angiography (51.5% vs. 55.5%, p = 0.023), coronary stenting (26.1% vs. 34.8%, p < 0.001), or bypass grafting (6.2% vs. 8.7%, p = 0.033). Referral for invasive angiography was associated with lower odds of death during the hospitalization (adjusted OR 0.66, 95%CI 0.44‑0.98, p = 0.039).
Conclusions: Decompensated diabetes complicates ~ 1.5% of STEMI admissions in diabetic patients. It is associated with lower rates of referral for angiography and revascularization, and a negative differential impact on in‑hospital morbidity and mortality and cost.
Keywords: Myocardial infarction, Diabetic ketoacidosis, Hyperosmolar hyperglycemic state, Coronary angiography, Coronary stenting
© The Author(s) 2018. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creat iveco mmons .org/licen ses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creat iveco mmons .org/ publi cdoma in/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Open Access
*Correspondence: Mohamad.Alkhouli@wvumedicine.org
†Mayada Issa and Fahad Alqahtani contributed equally to this manuscript 3 West Virginia University Heart & Vascular Institute, 1 Medical Drive,
Morgantown, WV 26505, USA
Background
The impact of acute hyperglycemia on clinical out-comes of diabetic and non-diabetic patients admitted with ST-elevation myocardial infarction (STEMI) has been extensively studied. High admission blood glucose among STEMI patients is associated with higher short-term incidences of failed reperfusion, acute kidney injury, stent thrombosis, myocardial damage and death among [1–12]. In addition, hyperglycemia during STEMI hospi-talizations in diabetics predicted left ventricular remod-eling and survival during long-term follow-up [13–18]. However, large-scale outcomes data of STEMI patients with acutely decompensated diabetes manifesting as dia-betic ketoacidosis (DKA) or hyperglycemic hyperosmo-lar state (HHS) are scarce [11]. We utilized a nationwide representative sample to assess the contemporary trends in the incidence and in-hospital morbidity and mortality and cost of decompensated diabetes (defined as DKA or HSS) among diabetic patients admitted with STEMI.
Methods
Study data
The National Inpatient Sample (NIS) was used to derive patient relevant information between January, 1st 2003 and December, 31st 2014. The NIS is the largest publicly available all-payer administrative claims-based database and contains information about patient discharges from approximately 1000 non-federal hospitals in 45 states. It contains clinical and resource utilization information on 5–8 million discharges annually, with safeguards to pro-tect the privacy of individual patients, physicians, and hospitals. These data are stratified to represent approxi-mately 20% of US inpatient hospitalizations across dif-ferent hospital and geographic regions (random sample). National estimates of the entire US hospitalized popula-tion were calculated using the Agency for Healthcare Research and Quality sampling and weighting method.
Study population
Patients > 18-year-old with a principle discharge diag-nosis of ST-elevation myocardial infarction (Interna-tional Classification of Diseases-Ninth Revision-Clinical
Modification [ICD-9-CM] codes 410.×1 except 410.71)
between 2003 and 2014 were identified in the NIS. Non-diabetic patients were then excluded yielding a cohort of diabetic patients admitted with STEMI. Those were then stratified into patients with or without decompensated diabetes. Similar to prior studies, we defined decom-pensated diabetes was defined as: diabetes with ketoaci-dosis (ICD-9-CM codes 250.10-250.13) or diabetes with hyperosmolaity (ICD-9-CM codes 250.20-250.23) [19, 20]. Myocardial infarction codes used in this study have a negative predictive value of 96.1%, and a positive
predictive value of 95.9% and are similar to what has been reported in other studies [21, 22].
Outcomes analysis and study endpoints
A comparative analysis was performed between diabetic patients admitted with STEMI with and without DKA/ HSS before and after propensity score matching for the primary end point of in-hospital mortality, and for the secondary end points of in-hospital complications, length of stay, post-discharge intermediate care utilization and cost.
To account for potential confounding factors and reduce the effect of selection bias, a propensity score-matching model was developed using logistic regression to derive two matched groups for the comparative out-comes analysis. Patients admitted with STEMI with or without DKA/HHS were entered into a nearest neighbor 1:1 variable ratio, parallel, balanced propensity-match-ing model uspropensity-match-ing a caliper of 0.01 without replacement to ensure perfect matching. Variables included in the propensity match model are listed in Additional file 1: Table S1.
We anticipated that decompensated diabetes will have a differential impact on referral patterns for invasive angiography. We hence examined the impact of inva-sive angiography referral on in-hospital mortality among patients with decompensated diabetes using a multivari-able logistical regression analysis model.
Statistical analysis
Descriptive statistics presented as frequencies with per-centages for categorical variables and as means with standard deviations for continuous variables. Base-line characteristics were compared using a Pearson Chi squared test and Fisher’s exact test for categorical vari-ables and an independent-samples t test for continuous variables. Matched categorical variables were presented as frequencies with percentages and compared using McNamar’s test. Matched continuous variables were pre-sented as means with standard deviations and compared using a paired-samples t-test. A type I error rate of < 0.05 was considered statistically significant. To assess mono-tonic trends of utilization and outcomes we employed the non-parametric Mann-Kendal trend method. All statisti-cal analyses were performed using SPSS version 24 (IBM Corporation, Armonk, NY) and R, version 3.3.1.
Results
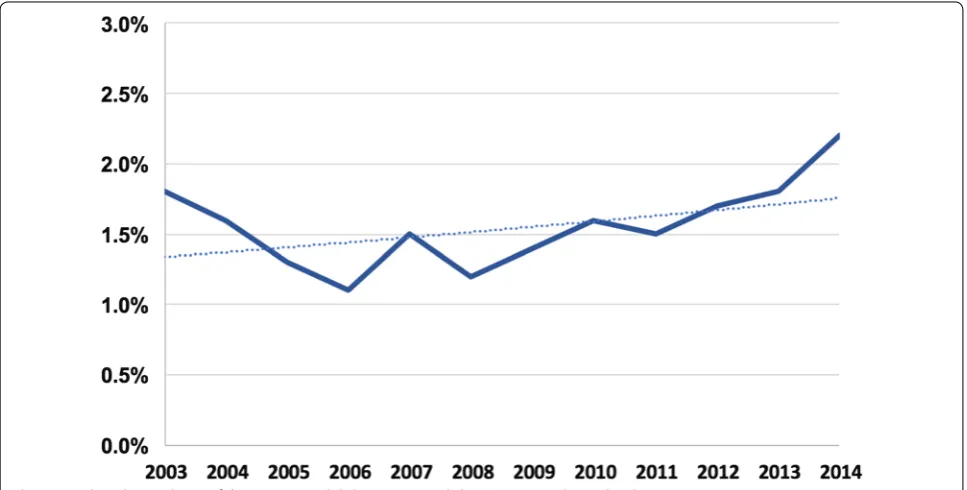
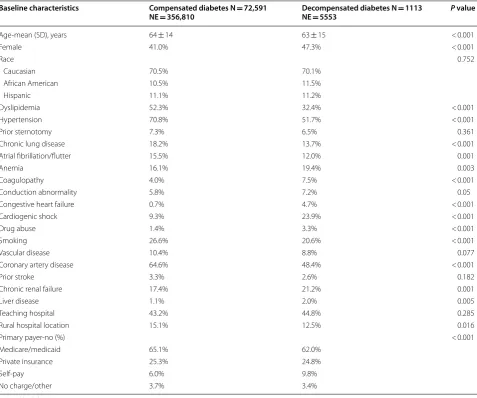
during the hospitalization with modest temporal increase in this incidence during the study period (Fig. 1). Patients with DKA/HHS were younger (63 ± 15 vs. 67 ± 15 years, p < 0.001) and a distinctive risk profile compared with diabetic patients without DKA/HHS, characterized with lower incidences of hypertension, hyperlipidemia, smok-ing, chronic obstructive lung disease, known coronary artery disease, and prior atrial fibrillation (Table 1).
Patients with decompensated diabetes had higher inci-dences of cardiogenic shock (23.9% vs. 9.3%), and cardiac arrest (11.8% vs. 5.6%), and were less likely to undergo coronary angiography (51.4% vs. 61.1%), percutaneous coronary intervention (PCI) (25.8% vs. 40%), or coronary bypass grafting (6.2% vs. 9.2%) (P < 0.001 for all) (Tables 1,
2). However, referral to coronary angiography and PCI
increased overtime (Fig. 2).
Outcomes of the unmatched cohorts
Compared with patients with decompensated diabetes, those with DKA/HHS had over twofold higher in-hos-pital mortality (26.4% vs. 11.9%, p < 0.001) and higher incidences of acute kidney injury (40% vs. 13.3%), stroke (3.7% vs. 1.6%), sepsis (7.5% vs. 1.9%), blood transfu-sion (11.3% vs. 8.2%) and mechanical ventilation (8.6% vs. 2.9%), (p < 0.001 for all). They also had longer hospi-talizations (7 ± 8 vs. 5 ± 6 days, p < 0.001), higher hos-pital charges (87,382 ± 132,119$ vs. 64,396 ± 81,185$, p < 0.001), and were less likely to be discharged directly to home (58.7% vs. 68.4%, p < 0.001) (Table 3).
Outcomes of the matched cohorts
Following propensity-score matching, baseline character-istics between the two sets of STEMI patients with and without DKA/HHS became well matched (Additional
file 1: Table 2 and Figure S1). The presence of DKA/
HHS remained associated with significantly lower rates of referral to coronary angiography (52.2% vs. 57.1%, p = 0.023), and PCI (29.5% vs. 38.4%, p < 0.001)
(Addi-tional file 1: Table S3). In these propensity-matched
cohorts, DKA/HHS remained associated with a sig-nificant 32% increase in in-hospital mortality (25.6% vs. 19.4%, p = 0.001). The DKA/HHS group also had signifi-cantly higher incidences of acute kidney injury (39.4% vs. 18.9%, p < 0.001), sepsis (7.3% vs. 4.9%, p = 0.022), and a non-significant trend towards higher stroke (3.8% vs. 2.4%, p = 0.087). Similar to the unmatched cohorts, they also had longer hospitalizations, higher hospital charges and were less likely to be discharged directly to home (Table 3).
Given that patients in the decompensated diabetes group were less likely to be referred for invasive angiog-raphy and they also had higher in-hospital mortality, the impact of invasive angiography referral on in-hospital mortality among these patients was examined using a multivariable logistical regression analysis model. This model adjusted for demographics, baseline clinical risk factors, insurance status and hospital characteristic, and showed that referral for invasive angiography was associ-ated with lower odds of death during the hospitalization
(adjusted OR 0.66, 95%CI 0.44–0.98, p = 0.039). Variables included in this regression models are the same variables used for propensity score matching outlined in Addi-tional file 1: Table S1.
Discussion
The main findings of the present investigation are: (1) acute diabetes decompensation occur in 1.5% of diabetics admitted with STEMI, (2) those patients have less preva-lence of previously diagnosed coronary artery disease, but higher incidences of cardiogenic shock and cardiac arrest, (3) patients with decompensated diabetes were less likely to undergo coronary angiography and revas-cularization compared with patients with compensated diabetes, (4) referral to angiography was independently associated with lower risk-adjusted odds of in-hospital
Table 1 Baseline characteristics of the study’s population
SD standard deviation
Baseline characteristics Compensated diabetes N = 72,591
NE = 356,810 Decompensated diabetes N NE = 5553 = 1113 P value
Age‑mean (SD), years 64 ± 14 63 ± 15 < 0.001
Female 41.0% 47.3% < 0.001
Race 0.752
Caucasian 70.5% 70.1%
African American 10.5% 11.5%
Hispanic 11.1% 11.2%
Dyslipidemia 52.3% 32.4% < 0.001
Hypertension 70.8% 51.7% < 0.001
Prior sternotomy 7.3% 6.5% 0.361
Chronic lung disease 18.2% 13.7% < 0.001
Atrial fibrillation/flutter 15.5% 12.0% 0.001
Anemia 16.1% 19.4% 0.003
Coagulopathy 4.0% 7.5% < 0.001
Conduction abnormality 5.8% 7.2% 0.05
Congestive heart failure 0.7% 4.7% < 0.001
Cardiogenic shock 9.3% 23.9% < 0.001
Drug abuse 1.4% 3.3% < 0.001
Smoking 26.6% 20.6% < 0.001
Vascular disease 10.4% 8.8% 0.077
Coronary artery disease 64.6% 48.4% < 0.001
Prior stroke 3.3% 2.6% 0.182
Chronic renal failure 17.4% 21.2% 0.001
Liver disease 1.1% 2.0% 0.005
Teaching hospital 43.2% 44.8% 0.285
Rural hospital location 15.1% 12.5% 0.016
Primary payer‑no (%) < 0.001
Medicare/medicaid 65.1% 62.0%
Private insurance 25.3% 24.8%
Self‑pay 6.0% 9.8%
No charge/other 3.7% 3.4%
Table 2 Management patterns in the unmatched cohorts
PTCA percutaneous transluminal coronary angiography, IV intravenous, PCI
percutaneous coronary intervention, BMS bare metal stent, DES drug eluting stent
Management Compensated
diabetes N = 72,591 NE = 356,810 (%)
Decompensated diabetes N = 1113 NE = 5553 (%)
P value
Coronary angiography 62.9 52.3 < 0.001
Coronary intervention 43.2 29.6 < 0.001
IV thrombolysis 1.8 1.3 0.218
Underwent PTCA 3.2 3.7 0.308
Underwent PCI 40.0 25.8 < 0.001
BMS 27.4 15.4 < 0.001
death in patients with decompensated diabetes, and (5) acute diabetes decompensation was associated with 32% higher in-hospital mortality, higher incidences of certain key morbidities, more resource utilization, and higher cost.
Prior studies have shown that the hyperglycemia dur-ing STEMI admissions is not uncommon in both diabet-ics and non-diabetdiabet-ics, and hyperglycemia in this setting is associated with worse short and long-term outcomes [1–18]. Nonetheless, to our knowledge, the incidence of full-scale uncontrolled hyperglycemia (decompensated diabetes) manifesting as DKA or HHS and its impact on
clinical outcomes among STEMI patients have not been previously investigated.
Our analysis reveals that the incidence of DKA or HHS among diabetic patients admitted with STEMI is low (1.5%) but is associated with lower odds of under-going revascularization, and higher morbidity and mortality and cost. Albeit intuitive, the findings of our study deserve more scrutiny:
1. The lower prevalence of known coronary artery dis-ease and the worse initial presentation (evident by Fig. 2 Trends in percutaneous coronary revascularization among diabetic patients admitted with STEMI
Table 3 In-hospital outcomes of STEMI patients with and without DKA/HHS
UTI urinary tract infection, IC intermediate care facility, SD standard deviation, LOS length of stay
Clinical outcome Unmatched cohorts Matched cohorts
Compensated diabetes N = 72,591 NE = 356,810 (%)
Decompensated diabetes
N = 1113 NE = 5553 (%) Pvalue Compensated diabetes N = 1103 NE = 5410 (%) Decompensated diabetes N = 1103 NE = 5410 (%) P value
Death 11.9 26.4 < 0.001 19.4 25.6 0.001
Blood transfusion 8.2 11.3 < 0.001 11.7 11.1 0.689
Acute kidney injury 13.3 40.0 < 0.001 18.9 39.4 < 0.001
New dialysis 0.4 0.5 0.521 0.5 0.5 0.99
Stroke 1.6 3.7 < 0.001 2.4 3.8 0.087
UTI 7.4 13.1 < 0.001 8.3 13.1 0.001
Sepsis 1.9 7.5 < 0.001 4.9 7.3 0.022
Acquired pneumonia 6.6 12.0 < 0.001 7.5 12.1 < 0.001
Mechanical ventilation 2.9 8.6 < 0.001 7.1 8.0 0.456
Tracheostomy 0.6 1.5 <0.001 2.6 1.0 0.006
Discharged home 60.3 43.3 < 0.001 51.0 44.1 0.01
Discharged to IC 26.9 29.1 28.3 29.2
LOS (mean ± SD) 5 ± 6 7 ± 8 < 0.001 6 ± 7 7 ± 7 0.02
the higher incidence of cardiogenic shock and car-diac arrest) in the DKA/HHS population are absorb-ing. Although speculative, these patients may have constituted a different cohort of patients at baseline that is characterized with worse chronic glycemic control, attenuated symptoms due to diabetic neu-ropathy, delayed presentations, and possibly larger infarcts [23, 24].
2. The interrelation between DKA/HHS and worse out-comes in STEMI patients is rather complex and may be difficult to unravel from cohort analyses like the present one. Several studies have shown than uncon-trolled hyperglycemia is associated with high plasma catecholamine levels, oxidative stress, and endothe-lial and microvascular dysfunction possibly leading to higher incidence of no reflow, larger infarcts and hence worse STEMI outcomes [1, 25, 26]. Vice versa, the occurrence of DKA/HHS during a STEMI admis-sion may be related to worse STEMI profile in these patients (cardiogenic shock or cardiac arrest). These patients may experience more intense hemody-namic and hormonal derangements and hence may develop DKA or HHS as a result for their ‘higher-risk’ STEMI. Further studies are needed to deline-ate the impact of variable degrees of hyperglycemia on STEMI outcomes and to identify the associated underlying mechanisms.
3. Patients with DKA/HHS were less likely to be referred to angiography, although this has improved with time. Reasons for this ‘less invasive’ practice are not known and can not be ascertained in our study. Nonetheless, referral for angiography was indepen-dently associated with improved in hospital survival suggesting a potential opportunity for improvement in this cohort.
4. The excess associated mortality of DKA/HHS was significantly attenuated after rigorous propensity score matching: 26.4% vs. 11.9%, p < 0.001 before matching, and 25.6% vs. 19.4%, p = 0.001 after match-ing. This suggests that baseline and hospital charac-teristics played a key role in the variance of outcomes between the two groups.
Limitations
Our study has a number of limitations. (1) The NIS is an administrative database that gathers data for billing pur-poses and can be limited by erroneous coding. Neverthe-less, the Healthcare Cost and Utilization Project’s quality control minimizes the discrepancies related to diagnosis coding. Also, both the procedures and the hard clinical endpoints reported in our study are hard to miscode. (2) Angiographic data, and information on peri-procedural hemodynamics, medications use, are not available in the
NIS. (3) Similarly, granular data on blood glucose levels, and insulin use as well as the timing of the DKA/HHS diagnoses are not captured in NIS. (5) Pre-admission diabetic control and anti-diabetic medications are not included in this dataset. Several studies have suggested a possible impact of Metformin and other anti-diabetics on infarct size and outcomes in diabetic patients admit-ted with STEMI [27–29]. This potential effect can not be assessed with the current study design. (4) Significant dif-ferences in baseline risk profiles were noted between the two groups. The impact of unmeasured confounders on the comparative analysis cannot be ruled out. Nonethe-less, we believe that our rigorous propensity score match-ing should minimize that risk.
Conclusions
Decompensated diabetes complicates ~ 1.5% of STEMI admissions in diabetic patients. It is associated with lower rates of referral for angiography and revascularization, and a negative differential impact on in-hospital morbid-ity and mortalmorbid-ity and cost. Further studies are needed to identify preventative and management strategies to miti-gate the excess morbidity and mortality in these patients.
Authors’ contributions
MI and FA designed the study, acquired the database and performed the statistical analysis. CB, MAH and TB performed the background search, and drafted the manuscript. MA supervised the study and made critical revisions of the manuscript. All authors read and approved the final manuscript.
Author details
1 Department of Medicine, West Virginia University, Morgantown, WV, USA. 2 Division of Cardiology, West Virginia University, Morgantown, WV, USA. 3 West
Virginia University Heart & Vascular Institute, 1 Medical Drive, Morgantown, WV 26505, USA.
Acknowledgements None.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data will be made available upon request for other research for the pur‑ pose of reproducing the study’s results.
Consent for publication
Was also waived because the data are derived from a nationwide de‑identified database.
Additional file
Disclosures
None of the authors have any competing interests or disclosures relevant to this study.
Ethics approval and consent to participate
The institutional review board approved this study. Informed consent require‑ ments were waived because the data are derived from a nationwide publically available de‑identified database.
Funding
No funding was received for this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in pub‑ lished maps and institutional affiliations.
Received: 30 April 2018 Accepted: 6 July 2018
References
1. Hao Y, Lu Q, Li T, Yang G, Hu P, Ma A. Admission hyperglycemia and adverse outcomes in diabetic and non‑diabetic patients with non‑ST‑ elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. 2017;17:6.
2. Bessonov IS, Kuznetsov VA, Potolinskaya YV, Zyrianov IP. Sapozhnikov SS [Impact of hyperglycemia on the results of percutaneous coronary interventions in patients with acute ST‑segment elevation myocardial infarction]. Ter Arkh. 2017;89:25–9.
3. Chen PC, Chua SK, Hung HF, Huang CY, Lin CM, Lai SM, Chen YL, Cheng JJ, Chiu CZ, Lee SH, Lo HM, Shyu KG. Admission hyperglycemia predicts poorer short‑ and long‑term outcomes after primary percutaneous coro‑ nary intervention for ST‑elevation myocardial infarction. J Diab Invest. 2014;5:80–6.
4. Ota S, Tanimoto T, Orii M, Hirata K, Shiono Y, Shimamura K, Matsuo Y, Yamano T, Ino Y, Kitabata H, Yamaguchi T, Kubo T, Tanaka A, Imanishi T, Akasaka T. Association between hyperglycemia at admission and micro‑ vascular obstruction in patients with ST‑segment elevation myocardial infarction. J Cardiol. 2015;65:272–7.
5. Kocas C, Abaci O, Halil GS, Arslan S, Cetinkal G, Bostan C, Coskun U, Yildiz A, Ersanli M. Admission hyperglycemia is associated with failed reperfu‑ sion following fibrinolytic therapy in patients with STEMI: results of a retrospective study. Am J Cardiovasc Drugs. 2015;15:35–42. 6. Chang J, Zhang G, Zhang L, Hou YP, Liu XL, Zhang L. High admission
glucose levels increase Fas apoptosis and mortality in patients with acute ST‑elevation myocardial infarction: a prospective cohort study. Cardiovasc Diabetol. 2013;12:171.
7. Planer D, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Xu K, Fahy M, Mehran R, Stone GW. Impact of hyperglycemia in patients with ST‑seg‑ ment elevation myocardial infarction undergoing percutaneous coronary intervention: the HORIZONS‑AMI trial. Int J Cardiol. 2013;167:2572–9. 8. Zhang JW, Zhou YJ, Cao SJ, Yang Q, Yang SW, Nie B. Impact of stress
hyperglycemia on in‑hospital stent thrombosis and prognosis in nondiabetic patients with ST‑segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24:352–6.
9. Tsuchida K, Nakamura N, Soda S, Sakai R, Nishida K, Hiroki J, Kashiwa A, Fujihara Y, Kimura S, Hosaka Y, Takahashi K, Oda H. Relationship between glucose fluctuations and ST‑segment resolution in patients with ST‑ elevation acute myocardial infarction. Int Heart J. 2017;58:328–34. 10. Eitel I, Hintze S, de Waha S, Fuernau G, Lurz P, Desch S, Schuler G, Thiele H.
Prognostic impact of hyperglycemia in nondiabetic and diabetic patients with ST‑elevation myocardial infarction: insights from contrast‑enhanced magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:708–18. 11. Birkhead J, Weston C, Timmis A, Chen R. The effects of intravenous insulin
infusions on early mortality for patients with acute coronary syndromes who present with hyperglycaemia: a matched propensity analysis using data from the MINAP database 2008–2012. Eur Heart J Acute Cardiovasc Care. 2015;4:344–52.
12. Hoebers LP, Damman P, Claessen BE, Vis MM, Baan J Jr, van Straalen JP, Fischer J, Koch KT, Tijssen JG, de Winter RJ, Piek JJ, Henriques JP. Predictive value of plasma glucose level on admission for short and long term mor‑ tality in patients with ST‑elevation myocardial infarction treated with pri‑ mary percutaneous coronary intervention. Am J Cardiol. 2012;109:53–9. 13. Djordjevic‑Radojkovic D, Koracevic G, Stanojevic D, Damjanovic M, Apos‑
tolovic S, Pavlovic M. Stress hyperglycemia in acute ST‑segment elevation myocardial infarction is a marker of left ventricular remodeling. Acute Card Care. 2013;15:38–43.
14. Jensen CJ, Eberle HC, Nassenstein K, Schlosser T, Farazandeh M, Naber CK, Sabin GV, Bruder O. Impact of hyperglycemia at admission in patients with acute ST‑segment elevation myocardial infarction as assessed by contrast‑enhanced MRI. Clin Res Cardiol. 2011;100:649–59.
15. Sarazawa K, Nakano A, Uzui H, Mitsuke Y, Geshi T, Okazawa H, Ueda T, Lee JD. Acute hyperglycemia causes microvascular damage, leading to poor functional recovery and remodeling in patients with reperfused ST‑ segment elevation myocardial infarction. J Nucl Cardiol. 2012;19:507–14. 16. Ertelt K, Brener SJ, Mehran R, Ben‑Yehuda O, McAndrew T, Stone GW.
Comparison of outcomes and prognosis of patients with versus without newly diagnosed diabetes mellitus after primary percutaneous coronary intervention for st‑elevation myocardial infarction (the HORIZONS‑AMI Study). Am J Cardiol. 2017;119:1917–23.
17. Savonitto S, Morici N, Cavallini C, Antonicelli R, Petronio AS, Murena E, Olivari Z, Steffenino G, Bonechi F, Mafrici A, Toso A, Piscione F, Bolognese L, De Servi S. One‑year mortality in elderly adults with non‑ST‑elevation acute coronary syndrome: effect of diabetic status and admission hyper‑ glycemia. J Am Geriatr Soc. 2014;62:1297–303.
18. Lazaros G, Tsiachris D, Vlachopoulos C, Chrysohoou C, Milkas A, Papageor‑ giou N, Tousoulis D, Stefanadis C. Distinct association of admission hyper‑ glycemia with one‑year adverse outcome in diabetic and non‑diabetic patients with acute ST‑elevation myocardial infarction. Hell J Cardiol. 2013;54:119–25.
19. Hritani A, Jan MF, Schleis G, Zehrer T, Olet S, Ammar KA, Allaqaband S. Clinical features and prognosis of type ii myocardial infarction in acutely decompensated diabetes patients. Am J Med. 2018;131(7):820–8. 20. Eubanks A, Raza F, Alkhouli M, Glenn AN, Homko C, Kashem A, Bove A.
Clinical significance of troponin elevations in acute decompensated diabetes without clinical acute coronary syndrome. Cardiovasc Diabetol. 2012;11:154.
21. Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, Ghali WA, Investigators I. Assessing validity of ICD‑9‑CM and ICD‑10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–41.
22. Agarwal M, Agrawal S, Garg L, Mohananey D, Garg A, Bhatia N, Lavie CJ. National trends in the incidence, management, and outcomes of heart failure complications in patients hospitalized for ST‑segment elevation myocardial infarction. Mayo Clin Proc. 2017;1:26–36.
23. Berman N, Jones MM, De Coster DA. ‘Just like a normal pain’, what do people with diabetes mellitus experience when having a myocardial infarction: a qualitative study recruited from UK hospitals. BMJ Open. 2017;7:e015736.
24. Reinstadler SJ, Stiermaier T, Eitel C, Metzler B, de Waha S, Fuernau G, Desch S, Thiele H, Eitel I. Relationship between diabetes and ischaemic injury among patients with revascularized ST‑elevation myocardial infarc‑ tion. Diab Obes Metab. 2017;19:1706–13.
25. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentra‑ tions are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72.
26. Marfella R, Verrazzo G, Acampora R, La Marca C, Giunta R, Lucarelli C, Paolisso G, Ceriello A, Giugliano D. Glutathione reverses systemic hemo‑ dynamic changes induced by acute hyperglycemia in healthy subjects. Am J Physiol. 1995;268:E1167–73.
27. Hesen NA, Riksen NP, Aalders B, Ritskes‑Hoitinga M, El Messaoudi S, Wever KE. A systematic review and meta‑analysis of the protective effects of metformin in experimental myocardial infarction. PLoS ONE. 2017;12:e0183664.
•fast, convenient online submission •
thorough peer review by experienced researchers in your field • rapid publication on acceptance
• support for research data, including large and complex data types •
gold Open Access which fosters wider collaboration and increased citations maximum visibility for your research: over 100M website views per year •
At BMC, research is always in progress.
Learn more biomedcentral.com/submissions
Ready to submit your research? Choose BMC and benefit from:
Smet BJ, van der Harst P, van Veldhuisen DJ, Investigators G‑I. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS‑III randomized clinical trial. JAMA. 2014;311:1526–35.