P
EDIATRICS
Oct 2000VOL. 106 NO. 4
䡠䡠䡠 䡠䡠䡠 䡠䡠䡠 䡠䡠䡠 䡠䡠
Cerebral Intravascular Oxygenation Correlates With Mean Arterial
Pressure in Critically Ill Premature Infants
Miles Tsuji, MD*; J. Philip Saul, MD‡; Adre du Plessis, MD§; Eric Eichenwald, MD*; Jamil Sobh, BS‡; Robert Crocker, BS§; and Joseph J. Volpe, MD§
ABSTRACT. Objectives. Premature infants
experi-ence brain injury, ie, germinal matrix–intraventricular hemorrhage (GMH-IVH) and periventricular leukomala-cia (PVL), in considerable part because of disturbances in cerebral blood flow (CBF). Because such infants are sus-ceptible to major fluctuations in mean arterial blood pressure (MAP), impaired cerebrovascular autoregula-tion would increase the likelihood for the changes in CBF that could result in GMH-IVH and PVL. The objec-tives of this study were to determine whether a state of impaired cerebrovascular autoregulation could be iden-tified reliably and conveniently at the bedside, the fre-quency of any such impairment, and the relation of the impairment to the subsequent occurrence of severe GMH-IVH and PVL.
Patients and Methods. To monitor the cerebral circu-lation continuously and noninvasively, we used near-infrared spectroscopy (NIRS) to determine quantitative changes in cerebral concentrations of oxygenated hemo-globin (HbO2) and deoxygenated hemoglobin (Hb) from
the first hours of life. Our previous experimental study showed a strong correlation between a measure of cere-bral intravascular oxygenation (HbD), ie, HbDⴝHbO2
ⴚ Hb, determined by NIRS, and volemic CBF, deter-mined by radioactive microspheres. We studied 32 very low birth weight premature infants (gestational age: 23–31 weeks; birth weight: 605-1870 g) requiring mechan-ical ventilation, supplemental oxygen, and invasive blood pressure monitoring by NIRS from 1 to 3 days of age. MAP measured by arterial catheter pressure
trans-ducer and arterial oxygen saturation measured by pulse oximetry were recorded simultaneously. The relation-ship of MAP to HbD was quantitated by coherence anal-ysis.
Results. Concordant changes (coherence scores >.5) in HbD and MAP, consistent with impaired cerebrovas-cular autoregulation, were observed in 17 of the 32 in-fants (53%). Eight of the 17 inin-fants (47%) developed se-vere GMH-IVH or PVL or both. Of the 15 infants with apparently intact autoregulation, ie, coherence scores <.5, only 2 (13%) developed severe ultrasonographic le-sions. Thus, for the entire study population of 32 infants, 8 of the 10 with severe lesions exhibited coherence scores >.5.
Conclusions. We conclude that NIRS can be used in a noninvasive manner at the bedside to identify premature infants with impaired cerebrovascular autoregulation, that this impairment is relatively common in such in-fants, and that the presence of this impairment is associ-ated with a high likelihood of occurrence of severe GMH-IVH/PVL.Pediatrics 2000;106:625– 632; near-infra-red spectroscopy, cerebrovascular autoregulation, prema-turity, intraventricular hemorrhage, periventricular leu-komalacia.
ABBREVIATIONS. CBF, cerebral blood flow; GMH-IVH, germinal matrix–intraventricular hemorrhage; PVL, periventricular leu-komalacia; MAP, mean arterial blood pressure; NIRS, near-infrared spectroscopy; HbO2, oxygenated hemoglobin; Hb,
hemo-globin; HbD, cerebral intravascular oxygenation; HbT, total hemoglobin; Sao2, oxygen saturation.
A
large body of experimental and clinical ob-servations indicates that disturbances in ce-rebral blood flow (CBF) are important in the pathogenesis of germinal matrix–intraventricular hemorrhage (GMH-IVH) and periventricular leu-komalacia (PVL), the 2 most important forms of brain injury in premature infants.1–3The human in-fant seems to have a particular propensity for devel-opment of disturbances in CBF for 2 major reasons. First, because of their very high incidence ofrespi-From the *Joint Program in Neonatology, Harvard Medical School, Boston, Massachusetts; and the Departments of ‡Cardiology and §Neurology, Chil-dren’s Hospital and Harvard Medical School, Boston, Massachusetts. Dr Tsuji is currently with St Joseph’s Hospital, Milwaukee, Wisconsin. Dr Saul is currently with Children’s Heart Center of South Carolina, Charleston, South Carolina.
Jamil Sobh is currently with Microwave Medical Systems, Acton, Massa-chusetts.
Received for publication Mar 8, 2000; accepted Jun 22, 2000.
Reprint requests to (J.J.V.) Neurology Department, Fegan 1103, Children’s Hospital, 300 Longwood Ave, Boston, MA 02115. E-mail: volpe_j@ l.tch.harvard.edu
ratory disease, need for mechanical ventilation, the complications of such ventilation, and a variety of other adverse medical conditions, alterations in mean arterial blood pressure (MAP) are very com-mon in such infants. Second, considerable experi-mental data and some clinical results indicate that cerebrovascular autoregulation, the mechanism by which CBF is maintained constant despite alterations in MAP, is either defective or absent in at least some infants (see the “Discussion” section). Several excel-lent studies using the xenon-133 clearance technique to measure CBF have shown impaired tion in sick premature infants and intact autoregula-tion in clinically stable infants. One such study has shown a clear relationship between impaired auto-regulation and the subsequent occurrence of GMH-IVH.4,5However, the xenon-133 clearance technique to determine CBF involves radiation exposure and cannot provide the minute-to-minute information re-quired for optimal delineation of impaired cerebro-vascular autoregulation.
Near-infrared spectroscopy (NIRS) is a relatively recently developed technique that can provide con-tinuous bedside quantitation of changes in cerebral concentration of oxygenated hemoglobin (HBO2) and deoxygenated hemoglobin (Hb).6 –9 We have shown recently in a piglet model that declines in CBF (measured by radioactive microspheres) caused by decreases in MAP can be detected by simultaneous measurements of cerebral intravascular oxygenation (HbD), measured by NIRS as the difference between HbO2 and Hb, ie, HbD ⫽ HbO2 ⫺ Hb.1 Thus, we undertook this study of premature infants from the first hours of life to determine whether we could identify infants with the hallmark of impaired cere-brovascular autoregulation, ie, concordant changes in cerebral perfusion, assessed by NIRS measure-ments of HbD, and in MAP, assessed by conven-tional techniques. Moreover, because there is increas-ing evidence that autoregulation is not a simple static system but rather a more complex rate-sensitive sys-tem, we used coherence analysis to evaluate autoreg-ulation as a dynamic process. We then determined whether the likelihood of occurrence of cranial ultra-sonographic evidence for severe hemorrhagic and/or ischemic disease (GMH-IVH/PVL) was re-lated to the previous occurrence of impaired cerebro-vascular autoregulation. The data indicate that NIRS can be used to identify premature infants with con-cordant changes in HbD and MAP, ie, impaired ce-rebrovascular autoregulation, that this abnormality is relatively common in such infants, and that the presence of the abnormality is associated with a high likelihood for development of GMH-IVH/PVL.
METHODS Patient Population
Thirty-two preterm infants with gestational age ⬍32 weeks were enrolled in the study from the neonatal intensive care units of the Children’s Hospital, Boston, and the Brigham and Women’s Hospital, Boston, between November 1993 and August 1995. Total enrollment was determined by the entry criteria, consent rate, and enrollment period. Average gestational age was 27.1⫾2.5 weeks, and average birth weight was 1023⫾341 g. There were 10 female infants and 22 male infants. To study a cohort of premature infants
at high risk for brain injury, infants were recruited on the day of birth from those admissions with gestational age⬍33 weeks and respiratory distress syndrome requiring intubation, mechanical ventilation, supplemental oxygen, and placement of umbilical arterial catheters for blood pressure monitoring and arterial blood gas sampling. All infants had lung disease by clinical examination and by chest radiographs consistent with hyaline membrane dis-ease or pulmonary insufficiency of extreme prematurity. Infants with congenital anomalies or known intracranial hemorrhage or other lesions at the time of diagnosis were excluded. The study was approved by the human studies committees of each hospital. Informed consent was obtained from the parents of each infant.
NIRS Studies
NIRS measurements were collected with a 4-wavelength (776, 828, 848, 913 nm) spectrophotometer (NIRO-500, Hamamatsu Photonics, Hamamatsu, Japan). The instrument transmits laser diode light via a fiber-optic bundle to the head and thereby to the intracranial compartment. An identical bundle receives transmit-ted light from the intracranial compartment and conveys the light to a sensitive photomultiplier tube. The end of each fiber-optic bundle (optode) was affixed to a thin, flexible foam pad and the optodes were spaced 3 cm apart. The foam pads were applied to the skin of the scalp on a line 1 cm above the supraorbital ridge with the transmitting optode 1 cm lateral to the midsagittal plane on either the right or left forehead and the receiving optode 3 cm lateral to the transmitting optode. This placement ensured sam-pling of cerebral hemoglobin signals from fronto-parietal brain tissue and avoided the sagittal sinus. The optode pads were se-cured to the head with a flexible bandage wrapped around the head. An opaque hat was then placed on the infant’s head to further shield the optodes from ambient light. Optode position was alternated between right and left scalp positions between recordings to minimize skin irritation. Because the skin of ex-tremely premature infants is very thin and easily abraded, no adhesive tape was used.
Changes in the relative concentrations of HbO2and Hb were
calculated from changes in light absorption at each of the trans-mitted laser frequencies according to the Beer-Lambert law.8,10
The calculations were corrected for the interoptode distance and the effect of light scattering, using a differential pathlength factor of 4.39.11Concentration changes in HbO
2and Hb were expressed
in Umol/L. HbD was calculated as HbO2⫺Hb.
During monitoring periods, NIRS measurements were re-corded simultaneously with arterial Sao2 monitored by pulse
oximetry (N-200, Nellcor, Hayward, CA) and with MAP (HP 78801A neonatal monitor, Hewlett Packard, Palo Alto, CA). Blood pressure was measured from a pressure transducer connected to the umbilical arterial catheter. Positioning of the tip of the umbil-ical catheter in the descending aorta was confirmed by chest radiograph. Analog outputs from the blood pressure monitor and pulse oximeter were sampled by an analog to digital converter built into the 500. The values were sampled by the NIRO-500 instrument every .5 seconds, and the average value for non-overlapping 5- or 10-second intervals was written to a computer file with calculated values for HbO2and Hb. The averaging
inter-vals, which represented⬃10 to 30 heart beats, allowed accurate assessment of MAP with less high frequency noise. To eliminate episodes of artifact, the data were separated into 30-minute blocks of uninterrupted recordings for coherence analysis. Only periods in which Sao2fluctuated⬍5% were considered to minimize the
influence of Sao2on HbD.
Study Protocol
Following informed consent, the NIRS instrument optodes were positioned on the study infant and recordings were initiated. During the study the infant and the NIRS instrument were con-tinually observed by an investigator or study technician. Clinical care of the infant continued under the direction of the infant’s attending physician without interruption or modification by the investigators. Clinical events that could influence the NIRS signal baseline, such as head positioning changes, suctioning, or sponta-neous movement, were noted on a log sheet.
Infants were studied during the first 3 hospital days. Hemody-namic instability thought to be associated with GMH-IVH and PVL is often most acute during the first few days of age,2,4,12–14
hospital days.2,14Recording periods of up to 6 hours on each of the
study days were conducted as nearly continuously as possible. However, NIRS recordings were interrupted if movement artifacts significant enough to cause a baseline shift occurred. In practice this constraint resulted in interruptions during physical examina-tions, chest radiographs, intravenous line procedures, and other clinical events requiring manipulation of the infant. Spontaneous head movements by the infants also interrupted the NIRS mea-surements and arterial blood gas sampling additionally interfered with recordings attributable to loss of the arterial pressure signal. To eliminate episodes of artifact, the data were separated into 30-minute blocks of uninterrupted recordings for coherence anal-ysis.
Cranial Ultrasound Examinations
Cranial ultrasound examinations were performed routinely on day 3, at 10 to 14 days, and at 1 month of age according to usual clinical practice in the respective neonatal intensive care units. Weekly cranial ultrasound examinations were obtained if abnor-malities were detected. Standard coronal and sagittal views were recorded. GMH-IVH was scored according to the system of Papile et al.15Ultrasound criteria for PVL consisted of bilateral
echolu-cencies in white matter areas and/or the presence of ventriculo-megaly without grade 3 or 4 GMH-IVH and without evidence of increased intracranial pressure (macrocephaly, abnormally rapid head growth, tense anterior fontanelle, rapid progressive ventric-ular dilation). Infants were classified by their ultrasonographic findings into 2 outcome groups: 1) those with severe abnormalities (grade 3 or 4 GMH-IVH or PVL); and 2) those with minimal (grade 1–2 GMH-IVH) or no abnormalities.
Coherence Analysis
Examination of the raw recordings suggested that slow changes in HbD and MAP were correlated in some, but not all, records. Because the cerebrovascular autoregulatory response may be fre-quency specific,16,17 the correlation of HbD and MAP in each
recording was quantitated in a frequency specific manner by calculating coherence scores. This approach uses transfer function analysis. Transfer function analysis uses Fourier transforms to compute the statistical correlation of the harmonic components of 2 waveforms. The results can be quantitated as a coherence score, which describes the degree of correlation between the waveforms at a given frequency or frequency range. A coherence of 1.0 indicates perfect frequency-specific correlation, and a coherence of 0 indicates a complete lack of frequency-specific correlation. Co-herence scores between .5 and 1.0 indicate increasing concordance between the waveforms. According to the Nyquist theorem of digital sampling, a sampling interval of 5 seconds allows detection of frequencies up to .1 Hz and a sampling interval of 10 seconds allows detection of frequencies up to .05 Hz.18Coherence scores
were computed on each 30-minute block of continuous data for bandwidths covering the limits of the data-sampling interval (0 – .01 Hz, .01–.05 Hz, .05–.1 Hz for ultralow, very low, and low frequency ranges, respectively). As described in the “Discussion” section, major physiologic importance can be attributed to the ultralow frequency bandwidth. The .05- to .1-Hz range was only computed when the sampling interval was at least 5 seconds. It should be noted that the nonoverlapping sampling intervals lead to a small potential for aliased information at lower frequencies. However, importantly, because the method was used uniformly for all subjects and epochs, this method should not introduce any intersubject/intrasubject or group bias. Computations were per-formed with a combination of custom software and a scientific computation software package (MatLab, Natick, MA).
Statistics
The data were analyzed by grouping according to no/minimal versus severe ultrasound abnormalities (grades 0 –2 GMH-IVH vs grades 3– 4 GMH-IVH/PVL) and by high (⬎.5) versus low coher-ence scores (⬍.5). Differences between groups were compared with Student’sttest or by2testing using commercial statistical
software (Systat, SPSS, Chicago, IL). APvalue of⬍.05 was con-sidered significant.
RESULTS Cerebral Hemodynamic Features
We studied 32 premature infants, as characterized in the “Methods” section. After exclusion of records containing movement artifacts and other baseline shifts, an average of 6.9⫾4.5 30-minute records were examined by coherence analysis for each infant over the first 3 hospital days. Considered as a total group, average coherence values were low for all infants (.32⫾.10, .32⫾.12, and .33⫾.13 for ultralow, very low, and low frequency ranges). However, examina-tion of the data indicated the presence of concordant HbD and MAP changes in the ultralow frequency band for some infants. Ultralow frequency coherence values⬎.5, indicative of concordant MAP and HbD changes, were observed in 17 of the 32 infants (53%). Notably, in these 17 infants, not all records exhibited high coherence values, suggesting that the degree of CBF regulation in response to blood pressure changes varied at different times in the same infant. An example of NIRS and MAP data recorded from a premature infant and indicative of ultralow coher-ence is shown in Fig 1. The blood pressure recording shows spontaneous fluctuations in MAP over the 30 minutes of recording. Although modest variability in the NIRS HbD recording is also apparent during this period, changes in MAP are not associated with changes in HbD. The ultralow frequency coherence score for this recording is .22. In contrast, the record-ings in Fig 2 show spontaneous fluctuations in MAP that are associated with parallel changes in HbD. Thus, pronounced increases and decreases in HbD occurred in parallel with identical changes in the systemic circulation. The ultralow frequency coher-ence score for this recording is .75, reflecting the concordant nature of the changes in HbD and MAP. This infant subsequently developed ultrasono-graphic evidence of PVL. Similar concordance of changes in HbD and MAP in 2 other infants are shown in Figs 3 and 4. For the infant whose data are shown in Fig 4, selected time segments showed a high correlation (rvalues:⬎.90) by linear regression, although the slope and intercept varied over time. Coherence analysis yielded a markedly elevated ul-tralow frequency coherence score of .87. During the sampling intervals in all infants, simultaneous mea-surements of Sao2 by transcutaneous monitoring showed normal values and no appreciable changes (see Figs 1 and 3 for examples).
Ultrasonographic Abnormalities as a Function of Cerebral Hemodynamic Characteristics
ultra-sound abnormalities were 25.7 ⫾ 1.5 weeks and 878⫾217 g, and for the 22 infants with minimal or no abnormalities, 27.7⫾2.6 weeks and 1089⫾370 g (P values were not significant for both gestational age and birth weight).
To evaluate a possible relationship between im-paired cerebrovascular regulation and the occur-rence of severe lesions, we compared the maximum ultralow frequency coherence scores in the infants
with and without severe ultrasonographic abnormal-ities. The maximum ultralow frequency coherence score for infants with severe ultrasound abnormali-ties was .64⫾.19, and the maximum score for infants with normal or minimal ultrasound findings was .45⫾ .19 (P⫽.023). The infants who had coherence values ⬎.5 had a higher rate of severe ultrasono-graphic abnormalities (8/17 or 47%), compared with infants with coherence scores⬍.5 (2/15 or 13%;P⫽
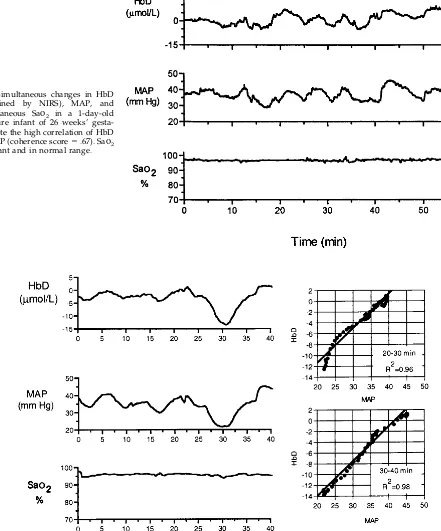
Fig 1. Simultaneous changes in HbD (determined by NIRS, MAP,
and transcutaneous Sao2in a
1-day-old premature infant of 28 weeks’ gestation. Despite abundant sponta-neous changes in MAP, there are only small changes in HbD, and there is poor correlation of HbD and MAP (ultralow frequency coherence
score⫽.20). Subsequent cranial
ul-trasound scans were normal.
Fig 2. Simultaneous changes in HbD (determined by NIRS) and MAP in a 1-day-old premature infant of 27 weeks’ gestation who subsequently de-veloped ultrasonographic evidence for
PVL. HbO2and MAP are highly
corre-lated (ultralow frequency coherence
.04; Fig 5). For the entire population, 8 of the 10 infants (80%) with severe GMH-IVH or PVL or both exhibited coherence scores⬎.5.
DISCUSSION
This study of 32 premature infants, assessed from the first hours of life, demonstrates concordant changes in HbD, assessed by NIRS, and MAP, as-sessed by intravascular catheter, in approximately one half of the population. Moreover, a strong
rela-tionship between this concordance and the develop-ment of ultrasonographic evidence for severe GMH-IVH/PVL was documented. The findings have implications for the occurrence of impaired cerebro-vascular autoregulation in the premature newborn and the relation of such impairment to the develop-ment of severe GMH-IVH/PVL.
Concerning the occurrence of impaired cerebro-vascular autoregulation in the premature newborn, the results of this study suggest that such impair-Fig 3. Simultaneous changes in HbD
(determined by NIRS), MAP, and
transcutaneous Sao2 in a 1-day-old
premature infant of 26 weeks’ gesta-tion. Note the high correlation of HbD
and MAP (coherence score⫽.67). Sao2
is constant and in normal range.
Fig 4. Simultaneous changes in HbD (determined by NIRS) and MAP in a 1-day-old premature infant of 27 weeks’ gestation who subsequently developed ultrasonographic evidence for PVL. Note the prolonged HbD and MAP correlation (ultralow frequency
ment can be identified by simultaneous measure-ments of HbD and MAP. Thus, in 53% of the infants an ultralow frequency coherence score of ⬎.5 could be identified between HbD and MAP in the ultralow frequency bandwidth. When the coherence function between 2 parameters, here HbD and MAP, is ⬎.5, the changes in 1 signal are linearly related to changes in the other signal at that frequency. We chose to focus on the ultralow frequency (0 –.01 Hz) response range because these frequencies correspond to changes occurring over several minutes. Although higher frequency coherence of CBF velocity and MAP have been noted in previous studies of prema-ture infants with relatively brief cerebral Doppler ultrasound recordings,19 NIRS allows analysis of longer recording intervals and much lower frequen-cies. Changes in MAP increase in amplitude as fre-quency decreases.20Because prolonged cerebral isch-emia (eg, several minutes to hours) is likely to be more injurious than transient (eg, several seconds) reductions in CBF, prolonged correlations of CBF to these larger changes in MAP are more likely to be pathologically significant. These prolonged correla-tions are captured by ultralow frequency coherence. A relatively high incidence of ultralow frequency CBF and MAP correlation in premature infants is suggested also by the study of von Siebenthal et al17 of cerebral blood volume (as measured by total Hb [HbT] ⫽ HbO2⫹ Hb) and MAP. However, the co-herence scores were not as high when HbT was used to monitor cerebral perfusion in that study as when HbD was used in the present study. This discrepancy
is consistent with the findings of our previous exper-imental study in piglets which showed that HbD is a better measure of CBF than is HbT during hypoten-sive episodes.21
We propose that the concordance between HbD and MAP reflects impaired cerebrovascular autoreg-ulation in considerable part because of our previous study of these parameters in the neonatal piglet.21 Thus, as noted earlier, under conditions of hypoten-sion severe enough to cause a decrease in CBF, si-multaneous measurements of HbD showed a parallel decrease. Importantly, the decrease in HbD was shown to correlate with a decrease in volemic CBF measured simultaneously by radioactive micro-spheres. The fact that HbD, a measure of cerebral intravascular oxygenation and thereby a reflection of cerebral oxygen delivery, correlates with CBF per se is supported further by the demonstration in our infants by transcutaneous monitoring that arterial Sao2(changes in which could alter HbD) was in the normal range and did not change appreciably during the study. Thus, under such conditions, changes in cerebral oxygen delivery should reflect changes in CBF. This conclusion is stated with the assumption that major changes in cerebral oxygen utilization did not occur concordantly with the changes in MAP. We have no reason to believe that this was likely in our infants.
Our demonstration of impaired cerebrovascular autoregulation or a pressure-passive cerebral circu-lation in the human premature newborn is not novel but is important because of the relative ease of ap-plication of the methodology. The first indication of impaired cerebrovascular autoregulation in at least a subset of premature infants was established in 1979 by Lou and coworkers22 using the xenon-133 clear-ance technique for measurement of CBF. Subsequent studies with this technique have shown, in general, intact cerebrovascular autoregulation in clinically stable premature infants and a pressure-passive ce-rebral circulation in sick preterm infants.5,23–27 How-ever, the xenon-133 clearance technique involves ra-diation exposure and is difficult to use routinely for detection of impaired cerebrovascular autoregula-tion because only a very few time points can be used. Indeed, any technique for measuring cerebral perfu-sion with such limited temporal features is of some-what limited value in detection of a dynamic process such as cerebrovascular autoregulation. This limita-tion may underlie the lack of deteclimita-tion of a concor-dance between MAP and single measurements of CBF by NIRS in a recent study.28 Utilization of the continuous measurement of HbD by NIRS, simulta-neous with measurement of MAP, circumvents the limitations of previous approaches and suggests that impaired cerebrovascular autoregulation is relatively common in the premature newborn.
Concerning the relationship of impaired cerebro-vascular autoregulation to the development of severe GMH-IVH/PVL, the results of this study suggest a direct relationship. Thus, approximately one half of the infants with the hemodynamic impairment de-veloped severe ultrasonographic abnormalities, and among all infants with the severe lesions, 80% had
maximum coherence scores consistent with impaired cerebrovascular autoregulation. Because infants did not have systematic ultrasound scans before the third day of life, our findings do not prove that the im-paired cerebrovascular autoregulation was present before the occurrence of severe GMH-IVH/PVL. However, a decisive demonstration of a relationship between a pressure-passive cerebral circulation and the occurrence of IVH was made initially in a study of 57 preterm infants by the xenon-133 clearance technique.4 –5This cerebrovascular regulatory abnor-mality may account in considerable part for the pre-viously reported relationships between the subse-quent occurrence of IVH and increases in MAP or decreases in MAP followed by reperfusion.13,29 –33A similar explanation may account for the relations shown between the subsequent occurrence of PVL and decreases in MAP or the presence of related markers of circulatory insufficiency (eg, hypoplastic left heart syndrome, extracorporeal membrane oxy-genation, fetal/neonatal acidemia, hypovolemia, el-evations of plasma uric acid on the first postnatal day).32,34 – 42
CONCLUSION
The findings of this preliminary study suggest that continuous measurements of HbD by NIRS, simul-taneous with measurements of MAP, provide the capability to define reliably and conveniently the presence of impaired cerebrovascular autoregulation in the human premature newborn. Moreover, the data indicate that this abnormality is relatively com-mon in premature infants, and more importantly has a strong relationship with the subsequent occurrence of severe cranial ultrasonographic abnormalities. Taken together, the results raise the possibilities that the infant at highest risk for the development of GMH-IVH/PVL can be identified by NIRS before the occurrence of the lesions and that correction of the cerebral circulatory disturbance might lead to pre-vention of the lesions. Clearly what is needed now is confirmation of these findings in a larger study; de-termination of whether the temporal characteristics and the severity of the cerebral circulatory abnormal-ity provide more refined predictive value for the development of the lesions; definition of the causes of the cerebral circulatory abnormality and the means of correction thereof; and determination of whether correction of the abnormality leads to pre-vention of the brain injury.
ACKNOWLEDGMENTS
This work was supported by research Grants NS32570, HD18655, and NS-38475 (to J.J.V.), Grant HD01010 (to M.T.); Grant NS01721 (to A.D.P.); and Grants HL-59212 and HD-96007 (to J.P.S.) from the National Institutes of Health.
The near infrared spectroscopy instrument was provided by Hamamatsu Photonics, KK, Hamamatsu, Japan.
Dr Timothy Watkins contributed to the coherence analyses.
REFERENCES
1. Tsuji M, du Plessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets.
Pediatr Res. 1998;44:591–595
2. Volpe JJ.Neurology of the Newborn. Philadelphia, PA: WB Saunders; 1995 3. Volpe JJ. Brain injury in the premature infant: neuropathology, clinical
aspects, and pathogenesis.Ment Retard Dev Disabil Res Rev. 1997;3:3–12 4. Pryds O, Greisen G, Lou H, Friis-Hansen B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation.
J Pediatr. 1989;115:638 – 645
5. Pryds O. Control of cerebral circulation in the high-risk neonate.Ann Neurol. 1991;30: 321–329
6. Delpy DT, Cope MC, Cady EB. Cerebral monitoring in newborn infants by magnetic resonance and near infrared spectroscopy.Scand J Clin Lab Invest. 1987;47:9 –17
7. Tsuji M. Cerebral monitoring by near-infrared spectroscopy.J Intern Care Med. 1996;11:162–172
8. Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EO. Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry.Lancet. 1986;2:1063–1066
9. Adcock LM, Wafelman LS, Hegemier S, et al. Neonatal intensive care applications of near-infrared spectroscopy. Clin Perinatol. 1999;26: 893–903
10. Wray S, Cope M, Delphy T, Wyatt JS, Reynolds EOR. Characterization of the near infrared absorption spectra of cytochrome aa3and
haemo-globin for the non-invasive monitoring of cerebral oxygenation.Biochim Biophys Acta. 1988;933:184 –192
11. Wyatt JS, Cope M, Delpy DT, et al. Meaurement of optical pathlength for cerebral near-infrared spectroscopy in newborn infants.Dev Neuro-sci. 1990;12:140 –144
12. Funato M, Tamai H, Noma K, Kurita T, Kajimoto Y, Yoshioka Y, Shimada S. Clinical events in association with timing of intraventricular hemorrhage in preterm infants.J Pediatr. 1992;121:614 – 619
13. Bada HS, Korones SB, Perry EH, et al. Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage.J Pediatr. 1990;117:607– 614
14. Lou HC. Pathogenesis of hemodynamic brain lesions in the newborn. In: Wasterlain CG, Vert P, eds.Neonatal Seizures. New York, NY: Raven Press; 1990:123–128
15. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm.J Pediatr. 1978;92:529 –534
16. Giller C. The frequency-dependent behavior of cerebral autoregulation.
Neurosurgery. 1990;27:362–368
17. von Siebenthal K, Beran J, Wolf M, Keel M, Dietz V, Kundu S, Bucher HU. Cyclical fluctuations in blood pressure, heart rate and cerebral blood volume in preterm infants.Brain Dev. 1999;21:529 –534 18. Bracewell RN.The Fourier Transform and Its Applications. New York, NY:
McGraw-Hill; 1978
19. Reynolds KJ, Panerai RB, Kelsall AWR, Rennie JM, Evans DH. Spectral pattern of neonatal cerebral blood flow velocity: comparison with spec-tra from blood pressure and heart rate.Pediatr Res. 1997;41:276 –284 20. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood
pressure and heart rate variability in evaluating cardiovascular regulation: a critical appraisal.Hypertension. 1995;25:1276 –1286 21. Soul JS, du Plessis AJ. New technologies in pediatric neurology:
near-infrared spectroscopy.Semin Pediatr Neurol. 1999;6:101–110
22. Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cere-bral blood flow in the distressed newborn infant.J Pediatr. 1979;94: 118 –21
23. Pryds O, Greisen G. Effect of Paco2and haemoglobin concentration on
day to day variation of CBF in preterm neonates.Acta Paediatr Scand Suppl. 1989;360:33–36
24. Greisen G. Cerebral blood flow in preterm infants during the first week of life.Acta Paediatr Scand. 1986;75:43–51
25. Greisen G, Trojaborg W. Cerebral blood flow, Paco2changes, and visual
evoked potentials in mechanically ventilated, preterm infants. Acta Paediatr Scand. 1987;76:394 – 400
26. Pryds O, Edwards AD. Cerebral blood flow in the newborn infant.Arch Dis Child. 1996;74:F63–F69
27. Muller AM, Morales C, Briner J, Baenziger O, Duc G, Bucher HU. Loss of CO2reactivity of cerebral blood flow is associated with severe brain
damage in mechanically ventilated very low birth weight infants.Eur J Paediatr Neurol. 1997;5:157–163
28. Tyszczuk L, Meek J, Elwell C, Wyatt J. Cerebral blood flow is indepen-dent of mean arterial blood pressure in preterm infants undergoing intensive care.Pediatrics. 1998;102:337–341
29. Perry EH, Bada HS, Ray JD, Korones SB, Arheart K, Magill HL. Blood pressure increases, birth weight-dependent stability boundary, and in-traventricular hemorrhage.Pediatrics. 1990;85:727–732
31. Kuban K, Volpe JJ. Intraventricular hemorrhage: an update.J Intensive Care Med. 1993;8:157–176
32. Miall-Allen VM, de Vries LS, Whitelaw AG. Mean arterial blood pres-sure and neonatal cerebral lesions.Arch Dis Child. 1987;62:1068 –1069 33. Watkins AM, West CR, Cooke RW. Blood pressure and cerebral
haem-orrhage and ischaemia in very low birthweight infants.Early Hum Dev. 1989;19:103–110
34. Low JA, Froese AF, Galbraith RS, Sauerbrei EE, McKinven JP, Karchmar EJ. The association of fetal and newborn metabolic acidosis with severe periventricular leukomalacia in the preterm newborn.Am J Obstet Gy-necol. 1990;162:977–981
35. Greisen G, Munck H, Lou H. May hypocarbia cause ischaemic brain damage in the preterm infant?Lancet. 1986;2:460
36. Greisen G, Pryds O, Rosen I, Lou H. Poor reversibility of EEG abnor-mality in hypotensive, preterm neonates.Acta Paediatr Scand. 1988;77: 785–790
37. Perlman JM, Volpe JJ. Neurologic complications of captopril treatment
of neonatal hypertension.Pediatrics. 1989;83:47–52
38. Shortland DB, Gibson NA, Levene MI, Archer LN, Evans DH, Shaw DE. Patent ductus arteriosus and cerebral circulation in preterm infants.Dev Med Child Neurol. 1990;32:386 –393
39. Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Acquired neuropatho-logic lesions associated with the hypoplastic left heart syndrome. Pedi-atrics. 1990;85:991–1000
40. Low JA, Froese AB, Galbraith RS, Smith JT, Sauerbrei EE, Derrick EJ. The association between preterm newborn hypotension and hypoxemia and outcome during the first year.Acta Paediatr. 1993;82:433– 437 41. Jarjour IT, Ahdab-Barmada A. Cerebrovascular lesions in infants and
children dying after extracorporeal membrane oxygenation. Pediatr Neurol. 1994;10:13–19
42. Perlman JM, Risser R. Relationship of uric acid concentrations and severe intraventricular hemorrhage/leukomalacia in the premature in-fant.J Pediatr. 1998;132:436 – 439
ERRATUM
In the August 2000 issue ofPediatrics, the article by Ploin et al1contains an error on Page 313. On the right-hand side of Figure 2, the two vertically positioned treatment-group boxes (“Nebulization Group” and “MDI⫹ASD Group”) should be reversed.
REFERENCE
1. Ploin D, Chapuis FR, Stamm D, et al. High-dose albuterol by metered-dose inhaler plus a spacer device versus nebulization in preschool children with recurrent wheezing: a double-blind, randomized equivalence trial.Pediatrics.
2000;106;625
Pediatrics
Crocker and Joseph J. Volpe
Miles Tsuji, J. Philip Saul, Adre du Plessis, Eric Eichenwald, Jamil Sobh, Robert
Critically Ill Premature Infants
Cerebral Intravascular Oxygenation Correlates With Mean Arterial Pressure in
Services
Updated Information &
http://pediatrics.aappublications.org/content/106/4/625
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/106/4/625#BIBL
This article cites 39 articles, 5 of which you can access for free at:
Subspecialty Collections
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_ Fetus/Newborn Infant
http://www.aappublications.org/cgi/collection/agency_abcs Agency ABC's
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
2000;106;625
Pediatrics
Crocker and Joseph J. Volpe
Miles Tsuji, J. Philip Saul, Adre du Plessis, Eric Eichenwald, Jamil Sobh, Robert
Critically Ill Premature Infants
Cerebral Intravascular Oxygenation Correlates With Mean Arterial Pressure in
http://pediatrics.aappublications.org/content/106/4/625
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.