ARTICLE
Safety and Immunogenicity of Concurrent
Administration of Live Attenuated Influenza Vaccine
With Measles-Mumps-Rubella and Varicella Vaccines
to Infants 12 to 15 Months of Age
Terry Nolan, MBBS, PhDa, David I. Bernstein, MDb, Stan L. Block, MDc, Milo Hilty, MDd, Harry L. Keyserling, MDe, Colin Marchant, MDf, Helen Marshall, MBBSg, Peter Richmond, MDh, Ram Yogev, MDi, Julie Cordova, BScj, Iksung Cho, MSj, Paul M. Mendelman, MDj, for the LAIV Study Group
aSchool of Population Health, University of Melbourne and Murdoch Children’s Research Institute, Victoria, Australia;bDepartment of Pediatrics, Cincinnati Children’s Hospital
Medical Center, Cincinnati, Ohio;cKentucky Pediatric Research, Bardstown, Kentucky;dPediatric Clinical Trials International, Columbus, Ohio;eDivision of Infectious
Diseases, Epidemiology, and Immunology, Emory University, Atlanta, Georgia;fSchool of Medicine, Boston University Medical Center, Boston, Massachusetts;gDiscipline
of Paediatrics, University of Adelaide and Children, Youth, and Women’s Health Service, Adelaide, Australia;hPrincess Margaret Hospital for Children, Subiaco, Australia; iInfectious Diseases, Children’s Memorial Hospital, Chicago, Illinois;jDepartments of Clinical Development and Biostatistics, MedImmune, Gaithersburg, Maryland
Financial Disclosure: Drs Nolan, Bernstein, Block, Hilty, Keyserling, Marchant, Marshall, Richmond, Yogev, and Mendelman and the LAIV Study Group participated in this research study, which was sponsored by MedImmune. The Murdoch Children’s Research Institute has received research grant support from MedImmune. Dr Bernstein is a consultant for MedImmune and has received honoraria for speaking engagements on behalf of MedImmune. Dr Block has served as a consultant and speaker for and has received research grant support from MedImmune. Dr Marchant has received research and grant support from GlaxoSmithKline, MedImmune, and Merck, has served on the speakers bureaus for GlaxoSmithKline and Sanofi Pasteur, and has served as a consultant for GlaxoSmithKline, MedImmune, Merck, and Sanofi Pasteur. Dr Marshall has been the principal investigator for several studies sponsored by the pharmaceutical industry, including MedImmune. Iksung Cho and Julie Cordova are current and former employees of MedImmune, respectively. Dr Mendelman is a former employee of and current consultant for MedImmune.
ABSTRACT
OBJECTIVE.This study evaluated the safety, tolerability, and immunogenicity of live attenuated influenza vaccine administered concurrently with measles-mumps-ru-bella vaccine and varicella vaccine to healthy children 12 to 15 months of age.
METHODS.Children were assigned randomly to receive (1) measles-mumps-rubella vac-cine, varicella vacvac-cine, and intranasal placebo on day 0, followed by 1 dose of live attenuated influenza vaccine on days 42 and 72; (2) measles-mumps-rubella, varicella, and live attenuated influenza vaccines on day 0, followed by a second dose of live attenuated influenza vaccine on day 42 and intranasally administered placebo on day 72; or (3) 1 dose of live attenuated influenza vaccine on days 0 and 42, followed by measles-mumps-rubella and varicella vaccines on day 72. Serum samples were collected before vaccination on days 0, 42, and 72. Reactogenicity events and adverse events were collected through day 41 after concurrent vaccinations and through day 10 after admin-istration of live attenuated influenza vaccine or placebo alone.
RESULTS.Among 1245 (99.5%) evaluable children, seroresponse rates and geometric mean titers for measles-mumps-rubella vaccine and varicella vaccine were similar with concurrent administration of live attenuated influenza vaccine or placebo (seroresponse rates ofⱖ96% for measles-mumps-rubella vaccine andⱖ82% for varicella vaccine in both groups). Hem-agglutinin-inhibiting antibody geometric mean titers and seroconversion rates to influenza strains in live attenuated influenza virus vaccine were similar after the vaccine was admin-istered alone (seroconversion rates of 98%, 92%, and 44% for H3, B, and H1 strains, respectively) or with measles-mumps-rubella and varicella vaccines (seroconversion rates of 98%, 96%, and 43%). The incidences of reactogenicity events and adverse events were similar among treatment groups.
CONCLUSIONS.Concurrent administration of live attenuated influenza vaccine with mea-sles-mumps-rubella vaccine and varicella vaccine provided equivalent immunogenicity, compared with separate administration, and was well tolerated.
What’s Known on This Subject
Childhood vaccines are often administered concurrently. There is a theoretical possibility that components of one vaccine may alter im-mune responses to another. The concurrent use of live attenuated influenza virus vaccine with other live vaccines for children has not been investigated.
What This Study Adds
Intranasally administered live attenuated influ-enza virus vaccine can be administered con-comitantly with measles-mumps-rubella and varicella vaccines to young children in routine clinical practice without reducing the immu-nogenicity or safety of any of the vaccines.
www.pediatrics.org/cgi/doi/10.1542/ peds.2007-1064
doi:10.1542/peds.2007-1064
This trial has been registered at www.clinicaltrials.gov (identifier NCT00192491).
Key Words
live attenuated influenza virus vaccine, varicella vaccine, measles-mumps-rubella vaccine, immunogenicity, concurrent vaccinations, children
Abbreviations
AE—adverse event CI— confidence interval GMT— geometric mean titer HAI— hemagglutination-inhibiting LAIV—live attenuated influenza vaccine MMR—measles-mumps-rubella RE—reactogenicity event
Accepted for publication Aug 15, 2007
Address correspondence to Terry Nolan, MBBS, PhD, School of Population Health, University of Melbourne, Level 2, 723 Swanston St, Carlton, Melbourne, Victoria 3010, Australia. E-mail: t.nolan@ unimelb.edu.au
T
HE ADVISORY COMMITTEEon Immunization Practices currently recommends that healthy children receive⬎25 vaccine doses during their first 2 years to prevent infections, including influenza.1This schedule frequently necessitates concurrent administration of vaccines.
Although seroresponse rates and adverse reactions after administration of combinations of live attenuated vaccines are similar to those observed with separate administration,2immunogenicity, safety, and lack of in-terference with concurrent administration of multiple live vaccines must be established. Because of this theo-retical risk, the Centers for Disease Control and Preven-tion recommends that live vaccines that are not admin-istered concurrently be adminadmin-istered at intervals of⬎4 weeks.2 Currently recommended vaccines for infants and young children that use live attenuated viruses as the immunogens include measles, mumps, rubella, varicella (frequently given as a combined product), and rotavirus vaccines.
Live attenuated influenza vaccine (LAIV, FluMist; MedImmune, Gaithersburg, MD) is currently approved for healthy children ⱖ2 years of age. Several studies have established that the frozen LAIV (and the recently licensed, refrigerator-stable formulation, referred to as cold-adapted influenza vaccine, trivalent) is immuno-genic, efficacious, and well tolerated in young chil-dren.3–9 It is important to demonstrate that concurrent administration of LAIV does not adversely affect the immune response to LAIV or other pediatric live vac-cines. The objective of this study was to evaluate the safety, tolerability, and immunogenicity of concurrent administration of LAIV with measles-mumps-rubella (MMR) and varicella vaccines in healthy infants 12 to 15 months of age.
METHODS
Study Design
This randomized, placebo-controlled trial was conducted over 2 study seasons at 44 sites in the United States and 3 sites in Australia. To avoid potentially confounding factors in the interpretation of immune responses, chil-dren were enrolled outside periods of peak influenza activity in their respective regions. Study seasons in the United States were May through October, 2001 and 2002, and those in Australia were November 2000 through May 2001 and November 2001 through May 2002.
Eligible children were prospectively assigned ran-domly, in a 1:1:1 ratio, to 1 of 3 groups, as follows: MMR/varicella group, concurrent administration of MMR vaccine, varicella vaccine, and intranasal placebo treatment on day 0, followed by a single dose of LAIV on days 42 and 72; MMR/varicella/LAIV group, concurrent administration of MMR, varicella, and LAIV vaccines on day 0, followed by a second dose of LAIV on day 42 and intranasal placebo treatment on day 72; LAIV group, a single dose of LAIV on days 0 and 42, followed by concurrent dosing with MMR and varicella vaccines on day 72. Randomization was stratified according to sea-son and site by using a block size of 6 and was achieved
with a predefined randomization schedule that assigned a treatment group to each unique participant number. Each participant was assigned a unique participant num-ber that was maintained throughout the trial. The MMR/ varicella and MMR/varicella/LAIV groups were double-blinded with respect to treatment for the duration of the study. Because no injectable placebo treatments were used, treatment assignment to the LAIV group was un-blinded after randomization.
The study was conducted in accordance with the Dec-laration of Helsinki, the US Code of Federal Regulations governing the protection of human subjects, and the International Conference on Harmonisation Guidance for Good Clinical Practice. The study protocol and in-formed consent documents were approved by the insti-tutional review board or independent ethics committee of each site. Written informed consent was obtained from each subject’s parent or legal guardian.
Study Participants
Eligible subjects were children 12 to 15 months of age who were in good health (determined by medical history and physical examination) and up to date with the pri-mary series of recommended vaccines (according to standard clinic practice and local vaccine availability). Exclusion criteria included previous vaccination against or diagnoses of measles, mumps, rubella, or varicella; hypersensitivity to egg, egg protein, or any component of the study vaccines or placebo treatment; known or suspected immunosuppression or immunosuppressed household member; acute febrile (⬎100.0°F oral) illness or clinically significant upper respiratory illness within 72 hours before enrollment; receipt of aspirin (acetylsali-cylic acid) or aspirin-containing products in the month before enrollment; receipt of any intranasally adminis-tered medication within 2 weeks before enrollment; re-ceipt of any live virus vaccine within 1 month before enrollment through 30 days after the final visit; receipt of any inactivated vaccine within 2 weeks before enroll-ment through 30 days after the final visit; receipt of any blood product within 3 months before vaccination; and history ofⱖ2 episodes of medically attended wheezing or medically attended wheezing illness or bronchodilator medication use within 4 weeks before enrollment.
Vaccines
LAIV vaccine (MedImmune Vaccines, Mountain View, CA) was supplied in intranasal sprayers with a total volume of 0.5 mL, containing allantoic fluid stabilized with sucrose/phosphate/glutamate and ⬃107 median
Station, NJ) and varicella virus vaccine (Varivax; Merck) were supplied as single-dose vials of lyophilized vac-cine.10,11
Study Evaluations
The primary objective was to compare the immune re-sponses to measles, mumps, rubella, and varicella anti-gens in the MMR/varicella/LAIV and MMR/varicella groups and to compare the immune responses to the 3 strains of influenza (A/H1N1, A/H3N2, and B) in the MMR/varicella/LAIV and LAIV groups. Serum samples were obtained before vaccination on day 0 and on day 42 (for MMR and varicella vaccine responses) and day 72 (for LAIV responses).
Immunogenicity to mumps, measles, rubella, and varicella antigens was assessed and validated by Merck Research Laboratories (West Point, PA), using antigen-specific enzyme-linked immunosorbent assays to detect serum antibody (immunoglobulin G), before and after vaccination with MMR and varicella vaccines. Serore-sponse criteria for measles, mumps, rubella, and vari-cella assays were predefined as ⱖ255 mIU/mL, ⱖ10 mumps antibody units per mL, ⱖ10 IU/mL, and ⱖ5 glycoprotein enzyme-linked immunosorbent assay units per mL, respectively.
Immunogenicity to influenza viruses was evaluated at MedImmune through measurement of serum hemag-glutination-inhibiting (HAI) titers to each of the strains contained in the vaccine, using standard assay proce-dures.12The HAI titer was defined as the reciprocal of the highest dilution of the test serum that inhibited hemag-glutination completely. A titer of ⬍4 was assigned to serum samples for which no inhibition could be de-tected, even at the lowest dilution tested (1:4 dilution). A fourfold or greater difference in titer between 2 serum samples was considered significant.13
A secondary objective of this study was to evaluate the safety and tolerability of concurrent administration of LAIV with MMR and varicella vaccines. An adverse event (AE) was defined as any unfavorable and unin-tended sign, symptom, disease, or worsening of a pre-existing condition associated temporally with vaccine administration. Reactogenicity events (REs) were pre-defined solicited AEs occurring after study vaccination, including injection site reactions and fever (⬎100.6°F rectal or aural,⬎100.0°F oral, or⬎99.6°F axillary). Se-rious AEs were defined as AEs that resulted in death, were life-threatening, required hospitalization or pro-longed existing hospitalization, resulted in a persistent or significant disability, or required medical interven-tion to prevent one of these outcomes. To conduct the key safety comparisons between the MMR/varicella and MMR/varicella/LAIV groups (whether LAIV vac-cine potentiated AEs associated with MMR/varicella vaccines), AEs and REs were recorded for 42 days after concomitant vaccination. To evaluate whether MMR/ varicella vaccines potentiated REs associated with LAIV vaccine (MMR/varicella/LAIV versus LAIV alone), REs were recorded for 10 days after intranasal vaccina-tion alone. Significant new medical condivaccina-tions and seri-ous AEs were recorded for 6 months after vaccination.
REs, AEs, concomitant medication use, and health care provider visits were recorded daily by parents or guardians on assessment worksheets. Parents or guardians of children in the MMR/varicella and MMR/varicella/LAIV groups also recorded the presence and size of injection site reac-tions. Study staff members contacted parents or guardians by telephone to collect information regarding AEs, REs, and significant new medical conditions,⬃3, 14, 28, and 42 days after concurrent administration of MMR/varicella vaccines and LAIV or placebo and 3 and 10 days after administration of LAIV or placebo alone.
Statistical Analyses
A sample size of 300 evaluable subjects per treatment group was required to provide overall power of 94% to demonstrate equivalent immune responses between the treatment groups and to provide ⱖ88% power to de-crease the probability of an inde-crease of ⬎10% in the incidence of REs. The immunogenicity population in-cluded children who received all scheduled treatments, and data were analyzed according to treatment received. The safety population included children with any RE or AE data for the visit/dose-specific safety evaluation pe-riod. Data for subjects in the safety population were analyzed according to treatment received. The primary end points were to demonstrate equivalent immunoge-nicity of MMR vaccine and varicella vaccine after con-current administration with intranasally administered LAIV or placebo and to demonstrate equivalent immu-nogenicity of intranasally administered LAIV adminis-tered alone or concurrently with MMR and varicella vaccines.
Equivalent immunogenicity of MMR and varicella vaccines with or without LAIV was evaluated by deter-mining postvaccination seroresponse rates for measles, mumps, rubella, and varicella antigens in baseline sero-negative children in the MMR/varicella/LAIV group, compared with those in the MMR/varicella group, and by comparing postvaccination geometric mean titers (GMTs) for measles, mumps, rubella, and varicella anti-gens in children in the MMR/varicella/LAIV group with GMTs for those in the MMR/varicella group regardless of baseline antibody titers. The definitions for seroresponse and for baseline seronegativity for measles, mumps, ru-bella, and varicella antigens are presented in Table 1. In the immunogenicity analysis, equivalent seroresponse was achieved if the lower limit of the 2-sided exact 95% con-fidence interval (CI) for the rate difference (MMR/ varicella/LAIV minus MMR/varicella) was greater than
⫺5 percentage points for measles, mumps, and rubella and greater than ⫺10 percentage points for varicella. Equivalence based on the ratio of GMTs (MMR/ varicella/LAIV GMT/MMR/varicella GMT) was achieved if the lower limit of the 2-sided 95% CI for the ratio was
⬎0.5.
MMR/varicella/LAIV vaccines, compared with those who received LAIV, and postvaccination (dose 2) GMTs for each of the vaccine strains among children who received MMR/varicella/LAIV vaccines, compared with those who received LAIV, regardless of baseline sero-status. The definitions for seroconversion and for base-line seronegativity for influenza strains are presented in Table 1. Equivalent seroconversion was achieved if the lower limit of the 2-sided 95% CI for the rate difference (MMR/varicella/LAIV minus LAIV) was greater than
⫺10 percentage points for all strains. GMTs were con-sidered equivalent if the lower limit of the 2-sided 95% CI for the ratio (MMR/varicella/LAIV GMT/LAIV GMT) was⬎0.5.
CIs for differences in seroresponse/seroconversion rates were constructed by using the method described by Miettinen and Nurminen.14CIs for all GMTs were based on the percentile-based bootstrap technique and in-cluded stratification according to season (1, 2) and con-tinent (Australia or North America), to control for po-tential previous exposure to the antigens under study that might vary according to these factors.
Incidence rates of REs were analyzed with 2-sided, exact, unconditional, 90% CIs for the rate difference.15 No formal statistical comparisons were performed for other AEs. All immunogenicity summaries and statistical analyses were performed with SAS 8 (SAS Institute, Cary, NC).
RESULTS
Study Participants
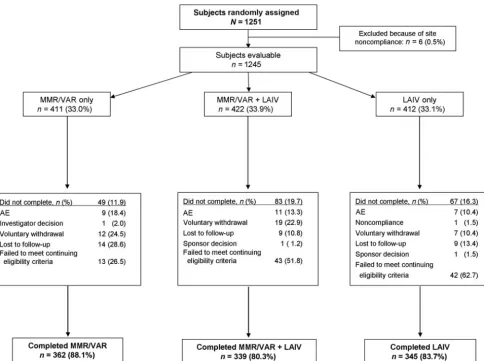
Of 1251 children assigned randomly, 1245 were evalu-able for safety and immunogenicity, including 411 in the MMR/varicella group, 422 in the MMR/varicella/LAIV group, and 412 in the LAIV group. All treatment groups were well matched with regard to age, gender, and ethnicity (Table 2). Data from 1 study site (n⫽6) were excluded from the analysis because the documentation of data did not meet good clinical practices standards.
A total of 1046 children (84.0%) completed the study. Subject disposition is presented in Fig 1. Ninety-eight subjects (7.9%) across the 3 treatment groups failed to meet continuing eligibility criteria and were withdrawn from the study. Of these, 49 children (50%; MMR/varicella/LAIV: 24; LAIV: 25) were withdrawn because of a local measles outbreak and subsequent unblinding of participants at 1 site; as specified in the study protocol, children who received MMR/varicella/
LAIV vaccines or LAIV alone were offered open-label, measles-containing vaccine. Other reasons for failure to meet continuing eligibility criteria included history of
ⱖ2 wheezing illnesses (n ⫽ 11), vaccine administered outside the dosing window (n⫽3), varicella infection (n
⫽ 3), receipt of other vaccine (n ⫽ 7), randomization error (n⫽8), egg allergy (n⫽2), wheezing or broncho-dilator use within 4 weeks (n⫽14), and use of a product containing salicylate (n⫽2).
For the evaluation of immunogenicity, 8 subjects as-signed randomly to the LAIV group received the treat-ment regimen for the MMR/varicella/LAIV group and were summarized as MMR/varicella/LAIV subjects. The immunogenicity population therefore consisted of 411 subjects in the MMR/varicella group, 430 subjects in the MMR/varicella/LAIV group, and 404 subjects in the LAIV group.
Immunogenicity
More than 90% of evaluated subjects in the MMR/ varicella/LAIV and MMR/varicella groups were seroneg-ative for measles, mumps, rubella, and varicella antigens at baseline. Equivalent seroresponse rates were demon-strated in baseline seronegative subjects for MMR and varicella vaccines, with and without concomitant LAIV administration (Table 3). Antigen-specific GMTs for MMR and varicella with and without concurrent LAIV administration and for the MMR/varicella and MMR/ varicella/LAIV groups were within the equivalence cri-teria. The postvaccination rubella GMT was higher in the MMR/varicella group than in the MMR/varicella/LAIV group, but both exceeded the seropositive threshold of
TABLE 2 Demographic Characteristics
MMR/Varicella (n⫽411)
MMR/Varicella/LAIV (n⫽422)
LAIV (n⫽412) Age, mo
Mean⫾SD 12.8⫾0.7 12.7⫾0.6 12.8⫾0.7
Median (range) 12.6 (12.0–15.9) 12.6 (12.0–15.7) 12.6 (12.0–16.0) Male gender,n(%) 214 (52.1) 214 (50.7) 194 (47.1) Race/ethnicity, n (%)
White 335 (81.5) 346 (82.0) 339 (82.3)
Black 19 (4.6) 24 (5.7) 16 (3.9)
Asian/Pacific Islander
7 (1.7) 3 (0.7) 4 (1.0)
Hispanic 33 (8.0) 32 (7.6) 34 (8.3)
Other 17 (4.1) 17 (4.0) 19 (4.6)
TABLE 1 Parameters for Measles, Mumps, Rubella, Varicella, and Influenza Assays
Antigen Units LoD Value Reported
If Below LoD
Value Used for GMT If Below LoD
Baseline Seronegative Definition
Seroresponse Criteria
Measles mIU/mL 120 ⬍120 60 ⬍255 ⱖ255
Mumps Mumps antibody units per mL 10 ⬍10 5 ⬍10 ⱖ10
Rubella IU/mL 10 ⬍10 5 ⬍10 ⱖ10
Varicella Glycoprotein ELISA units per mL OD cutoff point ⬍0.6 0.3 ⬍1.25 ⱖ5
All influenza strains Reciprocal of HAI titer 4 ⬍4 2 ⱕ4 ⱖ4-fold increase from baseline
10 IU. In contrast, the postvaccination measles titer was slightly higher in the MMR/varicella/LAIV group, com-pared with the MMR/varicella group (95% CI: 1.04 – 1.39).
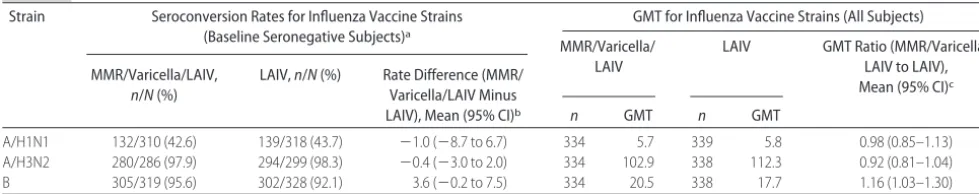
Equivalent immunogenicity was also demonstrated against the 3 influenza strains contained in the vaccine (A/H1N1, A/H3N2, and B) after 2 doses of LAIV with
and without concurrent administration of MMR and varicella vaccines in baseline seronegative subjects (Ta-ble 4). Strain-specific seroconversion rates (more than fourfold increase in HAI titer) in subjects who were seronegative at baseline were similar in the MMR/vari-cella/LAIV and LAIV groups for each of the influenza strains. Strain-specific GMTs after 2 doses of LAIV were
TABLE 3 Impact of Concurrent Administration of LAIV Vaccine on Immunogenicity of MMR and Varicella Vaccines
Antigen Seroresponse Rates (Baseline Seronegative Subjects)a GMT (All Subjects)
MMR/Varicella/LAIV,
n/N(%)
MMR/Varicella,
n/N(%)
Rate Difference (MMR/ Varicella/LAIV Minus MMR/Varicella), Mean
(95% CI)b
MMR/Varicella/ LAIV
MMR/Varicella GMT Ratio (MMR/Varicella/ LAIV to MMR/Varicella),
Mean (95% CI)c
n GMT n GMT
Measles 320/330 (97.0) 329/339 (97.1) ⫺0.1 (⫺2.9 to 2.7) 344 3388.4 350 2813.6 1.21 (1.04–1.39) Mumps 326/337 (96.7) 342/346 (98.8) ⫺2.1 (⫺4.7 to 0.1) 347 82.2 351 97.4 0.85 (0.74–0.96) Rubella 329/338 (97.3) 340/349 (97.4) ⫺0.1 (⫺2.8 to 2.6) 344 72.6 351 102.0 0.71 (0.63–0.81)
Varicella 279/316 (88.3) 263/318 (82.7) 5.6 (0.1–11.2) 347 9.8 352 9.3 1.06 (0.95–1.18)
aSeronegative criteria were as follows: measles,⬍255 mIU/mL; mumps,⬍10 mumps antibody units per mL; rubella,⬍10 IU/mL; varicella,⬍1.25 glycoprotein enzyme-linked immunosorbent assay
units per mL.
bWeighted average of the stratum-specific rate differences. Equivalence criteria were lower limit for the 95% CI ofⱖ5 percentage points for measles, mumps, and rubella andⱖ10 percentage points
for varicella; all results met the equivalence criteria.
cComputed with the percentile-based bootstrap technique. Equivalence criterion was lower limit for the 95% CI of⬎0.5; all results met the equivalence criterion.
FIGURE 1
also comparable between the MMR/varicella/LAIV and LAIV groups.
Safety
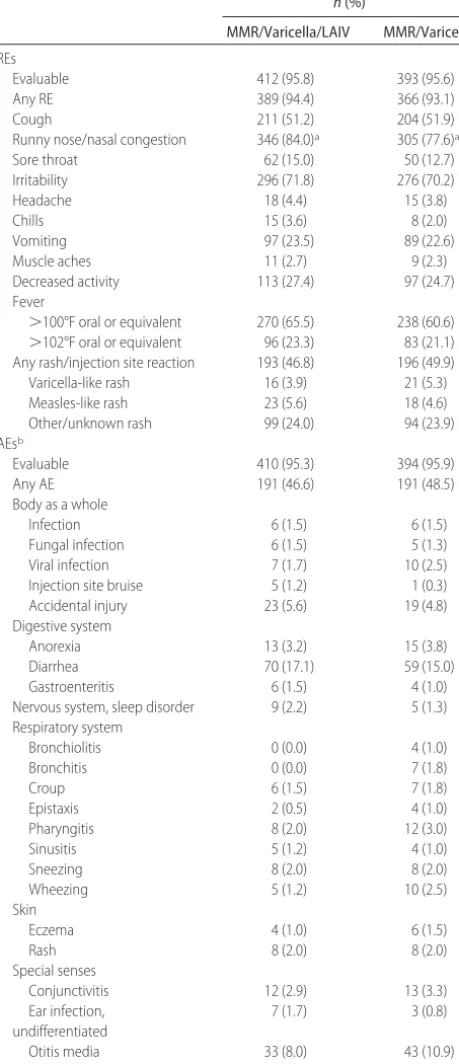
All vaccine regimens were generally well tolerated. Dur-ing the 42 days after the first dose of LAIV or placebo concurrent with MMR and varicella vaccines, only runny nose/nasal congestion occurred significantly more frequently among subjects who received LAIV, com-pared with placebo (Table 5). Nearly one half of all children in the MMR/varicella/LAIV and MMR/varicella groups experiencedⱖ1 AE (47% and 49%, respectively) (Table 5). The most frequently reported AEs during the 42 days after concurrent vaccination with MMR/varicel-la/LAIV vaccines or MMR/varicella vaccines were diar-rhea (17% vs 15%) and otitis media (8% vs 11%). Respiratory AEs occurred less frequently in the MMR/ varicella/LAIV group than in the MMR/varicella group, with more than twice as many children in the MMR/ varicella group reporting wheezing (2.5%), compared with the MMR/varicella/LAIV group (1.2%).
Irritability and fever were significantly more frequent within 10 days after vaccination in subjects who re-ceived LAIV concurrent with MMR and varicella vac-cines than in subjects who received LAIV alone (Table 6). There were no significant differences in REs within 10 days after the second dose of LAIV whether the first dose was administered concurrent with MMR and vari-cella vaccines or alone;ⱖ1 AE was reported by 25% and 32% of children, respectively, within 10 days after vac-cination (Table 6). Respiratory events occurred less fre-quently in the MMR/varicella/LAIV group than in the LAIV group (1.5% vs 4.1%, respectively). No AE was reported with a frequency of⬎10%.
No deaths were reported during the study. Nine ported serious AEs were considered to be possibly re-lated to study vaccine. In the MMR/varicella group, there were 2 cases of croup, 1 case of pneumonia, and 1 case of bronchiolitis. In the MMR/varicella/LAIV group, there was 1 case each of croup and bronchiolitis. In the LAIV group, there was 1 case each of a viral chest infec-tion, bronchiolitis, and bronchospasm. Nine children ex-perienced 9 significant new medical conditions, includ-ing asthma (1 in the MMR/varicella group and 3 in the LAIV group), speech delay (2 in the LAIV group), exces-sive language delay (1 in the MMR/varicella group),
cerebral palsy (1 in the MMR/varicella/LAIV group), and seizures (1 in the LAIV group).
DISCUSSION
Influenza is associated with a significant excess of out-patient visits, hospitalizations, and rare deaths among young children each year.16–20Routine annual influenza vaccination is now recommended for children between the ages of 6 months and 59 months, adding multiple vaccinations to the pediatric immunization schedule.21
The Advisory Committee on Immunization Practices recommends that injectable or nasally administered live vaccines not administered on the same day should be administered ⬎4 weeks apart whenever possible, to minimize the potential for interference.22 This recom-mendation is supported by the observation that immu-nization with a live measles vaccine can block the im-mune responses to a live smallpox vaccine if the measles vaccine is administered within 15 days after smallpox vaccine dosing but not if the vaccines are administered simultaneously, presumably because of the action of interferon induced in response to the initial live virus vaccine.23,24Similarly, administration of varicella vaccine within 28 to 30 days after receipt of MMR vaccine, but not simultaneous administration, has been associated with an increased risk of breakthrough varicella dis-ease.25,26 However, concomitant administration of live vaccines can produce interference; concomitant admin-istration of 2 live oral vaccines (polio vaccine and rota-virus vaccine) has been associated with a⬎40% reduc-tion in seroresponse rates to a live oral rotavirus vaccine.27,28Because it is common practice for children to receive several vaccines during the same office or clinic visit, it is important to establish the safety and immuno-genicity of concomitantly administered live virus vac-cines.
To date, limited data have been published on the impact on other vaccines of concurrent administration of either inactivated or live influenza virus vaccines, with respect to the immune responses of children or adults. The findings of the current study indicate equivalent immunogenicity with concurrent administration of MMR and varicella vaccines with LAIV, compared with separate administration. Seroresponse rates and ratios of antigen-specific antibody titers for measles, mumps, ru-bella, and varicella antigens present in the vaccines were
TABLE 4 Impact of Concurrent Administration of MMR and Varicella Vaccines on Immunogenicity of 2 Doses of LAIV
Strain Seroconversion Rates for Influenza Vaccine Strains (Baseline Seronegative Subjects)a
GMT for Influenza Vaccine Strains (All Subjects)
MMR/Varicella/ LAIV
LAIV GMT Ratio (MMR/Varicella/ LAIV to LAIV), Mean (95% CI)c MMR/Varicella/LAIV,
n/N(%)
LAIV,n/N(%) Rate Difference (MMR/ Varicella/LAIV Minus
LAIV), Mean (95% CI)b n GMT n GMT
A/H1N1 132/310 (42.6) 139/318 (43.7) ⫺1.0 (⫺8.7 to 6.7) 334 5.7 339 5.8 0.98 (0.85–1.13)
A/H3N2 280/286 (97.9) 294/299 (98.3) ⫺0.4 (⫺3.0 to 2.0) 334 102.9 338 112.3 0.92 (0.81–1.04)
B 305/319 (95.6) 302/328 (92.1) 3.6 (⫺0.2 to 7.5) 334 20.5 338 17.7 1.16 (1.03–1.30)
aSeronegativity was defined as baseline serum HAI titers ofⱕ4 for the given influenza strain.
similar, regardless of whether MMR and varicella vac-cines were administered concurrently with LAIV or con-currently with placebo. Similarly, responses elicited by 2 intranasal doses of LAIV were not affected by con-comitant administration of subcutaneously administered
MMR and varicella vaccines. The serum HAI responses to the 3 LAIV strains observed in this study are consis-tent with findings from other LAIV studies, including the lower immune response to the A/H1N1 strain.3,29,30 Overall, although the presence of antibody responses after the administration of LAIV is predictive of protec-tion, the lack of an antibody response is not indicative of the absence of protection.31
REs reported after vaccinations in this study were gen-erally typical of those observed in a young pediatric popu-lation after vaccination. The increased incidence of fever seen in children treated with MMR/varicella/LAIV vac-cines, compared with those treated with LAIV alone, can
TABLE 5 REs and AEs Reported Within 42 Days After Dose 1 of MMR/Varicella/LAIV or MMR/Varicella Vaccines
n(%)
MMR/Varicella/LAIV MMR/Varicella
REs
Evaluable 412 (95.8) 393 (95.6)
Any RE 389 (94.4) 366 (93.1)
Cough 211 (51.2) 204 (51.9)
Runny nose/nasal congestion 346 (84.0)a 305 (77.6)a
Sore throat 62 (15.0) 50 (12.7)
Irritability 296 (71.8) 276 (70.2)
Headache 18 (4.4) 15 (3.8)
Chills 15 (3.6) 8 (2.0)
Vomiting 97 (23.5) 89 (22.6)
Muscle aches 11 (2.7) 9 (2.3)
Decreased activity 113 (27.4) 97 (24.7)
Fever
⬎100°F oral or equivalent 270 (65.5) 238 (60.6) ⬎102°F oral or equivalent 96 (23.3) 83 (21.1) Any rash/injection site reaction 193 (46.8) 196 (49.9)
Varicella-like rash 16 (3.9) 21 (5.3)
Measles-like rash 23 (5.6) 18 (4.6)
Other/unknown rash 99 (24.0) 94 (23.9)
AEsb
Evaluable 410 (95.3) 394 (95.9)
Any AE 191 (46.6) 191 (48.5)
Body as a whole
Infection 6 (1.5) 6 (1.5)
Fungal infection 6 (1.5) 5 (1.3)
Viral infection 7 (1.7) 10 (2.5)
Injection site bruise 5 (1.2) 1 (0.3)
Accidental injury 23 (5.6) 19 (4.8)
Digestive system
Anorexia 13 (3.2) 15 (3.8)
Diarrhea 70 (17.1) 59 (15.0)
Gastroenteritis 6 (1.5) 4 (1.0)
Nervous system, sleep disorder 9 (2.2) 5 (1.3) Respiratory system
Bronchiolitis 0 (0.0) 4 (1.0)
Bronchitis 0 (0.0) 7 (1.8)
Croup 6 (1.5) 7 (1.8)
Epistaxis 2 (0.5) 4 (1.0)
Pharyngitis 8 (2.0) 12 (3.0)
Sinusitis 5 (1.2) 4 (1.0)
Sneezing 8 (2.0) 8 (2.0)
Wheezing 5 (1.2) 10 (2.5)
Skin
Eczema 4 (1.0) 6 (1.5)
Rash 8 (2.0) 8 (2.0)
Special senses
Conjunctivitis 12 (2.9) 13 (3.3)
Ear infection, undifferentiated
7 (1.7) 3 (0.8)
Otitis media 33 (8.0) 43 (10.9)
Pain ear 4 (1.0) 3 (0.8)
aDifference between treatment groups was significant, on the basis of the 2-sided 95% CI of the
difference (MMR/varicella/LAIV minus MMR/varicella).
bAEs reported forⱖ1% of children.
TABLE 6 REs and AEs Reported Within 10 Days After Dose 1 of MMR/Varicella/LAIV or LAIV Vaccines
n(%)
MMR/Varicella/LAIV LAIV
REs
Evaluable 412 (95.8) 388 (96.0)
Any RE 364 (88.3) 325 (83.8)
Cough 119 (28.9) 118 (30.4)
Runny nose/nasal congestion 293 (71.1) 271 (69.8)
Sore throat 19 (4.6) 24 (6.2)
Irritability 249 (60.4)a 200 (51.5)a
Headache 12 (2.9) 4 (1.0)
Chills 5 (1.2) 7 (1.8)
Vomiting 59 (14.3) 36 (9.3)
Muscle aches 7 (1.7) 3 (0.8)
Decreased activity 72 (17.5) 57 (14.7)
Fever
⬎100°F oral or equivalent 213 (51.7)a 113 (29.1)a ⬎101°F oral or equivalent 121 (29.4)a 54 (13.9)a ⬎102°F oral or equivalent 67 (16.3)a 30 (7.7)a ⬎103°F oral or equivalent 13 (3.2) 9 (2.3) ⬎104°F oral or equivalent 3 (0.7) 5 (1.3) AEsb
Evaluable 412 (95.8) 388 (96.0)
Any AE 103 (25.0) 125 (32.2)
Body as a whole
Injection site bruise 5 (1.2) 0 (0.0)
Accidental injury 6 (1.5) 9 (2.3)
Digestive system
Anorexia 9 (2.2) 13 (3.4)
Diarrhea 39 (9.5) 33 (8.5)
Nervous system
Insomnia 0 (0.0) 4 (1.0)
Sleep disorder 7 (1.7) 5 (1.3)
Respiratory system
Epistaxis 1 (0.2) 5 (1.3)
Sneezing 4 (1.0) 6 (1.5)
Wheezing 1 (0.2) 5 (1.3)
Skin, rash 4 (1.0)c 27 (7.0)
Special senses
Conjunctivitis 6 (1.5) 11 (2.8)
Ear infection, undifferentiated 4 (1.0) 2 (0.5)
Otitis media 8 (1.9) 11 (2.8)
aDifference between treatment groups was significant, on the basis of 2-sided 95% CIs of the
difference (MMR/varicella/LAIV minus LAIV).
bAEs reported forⱖ1% of children.
cRash was intended to be collected as a RE for the MMR/varicella/LAIV group (see Table 5), although
be attributed in large part to receipt of MMR and varicella vaccines, because an increased incidence of fever was re-ported in previous studies of concurrent immunization with MMR and varicella vaccines.32,33Of note, respiratory AEs (including wheeze) were less frequent in the MMR/ varicella/LAIV group than in the MMR/varicella group in the 42 days after vaccination.
CONCLUSIONS
Concomitant administration of live vaccines (MMR, varicella, and LAIV vaccines) to children 12 to 15 months of age did not affect significantly the serore-sponse rates for MMR and varicella vaccines with simul-taneous administration of LAIV. Strain-specific serocon-version rates for each of the 3 LAIV vaccine strains were not altered by concomitant administration of MMR and varicella vaccines. Concurrent administration of MMR vaccine, varicella vaccine, and intranasally administered LAIV was generally well tolerated. These findings sug-gest that LAIV can be administered concomitantly to young children with MMR and varicella vaccines in routine clinical practice with no diminution of immuno-genicity or safety. This is important because LAIV offers potential benefits to young children, such as a broad immune response that includes both systemic and mu-cosal antibody responses and cellular immune respons-es,34 protection against strains that are antigenically “drifted” from the vaccine strains,4,35–38 and needle-free, intranasal administration.
ACKNOWLEDGMENTS
This study was supported by MedImmune.
The LAIV Study Group included Parisa Arman, San Miguel Medical Clinic (Panorama City, CA); Russell T. Bain, Suncoast Clinical Research (New Port Richey, FL); Marshall Benbow, Southwest Children’s Research Asso-ciates (San Antonio, TX); David Bernstein, Cincinnati Children’s Hospital Medical Center (Cincinnati, OH); Mark Blatter, Primary Physicians Research (Pittsburgh, PA); Stan Block, Kentucky Pediatric/Adult Research (Bardstown, KY); William Borkowsky, Bellevue Hospital (New York, NY); Gerald Bottenfield, R/D Clinical Re-search (Lake Jackson, TX); Kevin Browngoehl, Drexel Hill Pediatrics (Drexel Hill, PA); Robert J. Carson, Pedi-atric Associates of Mt Carmel (Cincinnati, OH); Milo Hilty, Pediatric Clinical Trials International (Columbus, OH); Jeffrey A. Hirschfield (St Petersburg, FL); David Hudson, Tennessee Pediatrics (Hendersonville, TN); Louise Iwaishi, Hawaii Pacific Health (Honolulu, HI); Jerome Kaltman, Lake Forest Pediatric Associates (Lake Forest, IL); Harry Keyserling, Emory University School of Medicine (Atlanta, GA); James C. King, University of Maryland at Baltimore (Baltimore, MD); Leonard Krilov, Winthrop University Hospital (Mineola, NY); Erik Lam-berth, Pennridge Pediatrics (Sellersville, PA); Stephen Lambert, Murdoch Children’s Research Institute (Victo-ria, Australia); Thomas Latiolais, ARK-LA-TEX Chil-dren’s Clinic (Bossier City, LA); John Lauzon, Owens-boro Pediatrics (OwensOwens-boro, KY); Stephen Luber, Rockwood Clinic (Spokane, WA); Colin Marchant,
Bos-ton University Medical Center (BosBos-ton, MA); Eugenia Marcus, Pediatric Health Care at Newton-Wellesley (Newton, MA); Helen Marshall, University of Adelaide and Children, Youth, and Women’s Health Service (Ad-elaide, Australia); Douglas Mitchell, Center for Pediatric Research (Norfolk, VA); Sharon Nachman, Stony Brook University School of Medicine (Stony Brook, NY); Cora-zon Oca, Southland Clinical Research (Fountain Valley, CA); Terry Nolan, University of Melbourne and Mur-doch Children’s Research Institute (Victoria, Australia); William Parker, Willis Knighton Medical Center (Shreveport, LA); Michael E. Pichichero, University of Rochester Medical Center (Rochester, NY); Bernard Pol-lara, University of South Florida (Tampa, FL); Keith Reisinger, Primary Physicians Research (Pittsburgh, PA); Peter Richmond, Princess Margaret Hospital for Children (Subiaco, Australia); Jose Romero, Creighton University (Omaha, NE); Mark Sawyer, University of California, San Diego, Pediatric Pharmacology Research Unit (La Jolla, CA); Mark Schane, Longmont Medical Research Network at Longmont Clinic (Longmont, CO); Richard Schwartz, Advanced Pediatrics (Vienna, VA); Shelly Senders, Senders & Associates Pediatrics (University Heights, OH); Peter Short, East Coast Clinical (Salisbury, MA); Rafael Solis, San Miguel Medical Clinic (North Hollywood, CA); Malcom J. Sperling, Edinger Medical Group (Fountain Valley, CA); Craig A. Spiegel, Radiant Research (Bridgeton, MO); Tibisay Villalobos, Affinity Health Group (Tifton, GA); Emmanuel B. Walter, Duke Children’s Primary Care (Durham, NC); Peter Wright, Vanderbilt Medical Center (Nashville, TN); Ram Yogev, Children’s Memorial Hospital (Chicago, IL).
We thank the participating children and their parents, the study nurses and coordinators, and the clinical test-ing laboratory staff members. We thank Dr John Boslego and Dr Florian Schoedel of Merck Research Laboratories for their assistance with the analysis and interpretation of serologic responses to the MMR and varicella antigens and CSL Ltd for assistance in the conduct of this study. We also thank David P. Greenberg, MD, who was the central safety monitor for this study, Colin Hessel, MS, and Stephen Chan, BS, who conducted the statistical analysis and programming, and Catherine Grillo, MS, Gerard P. Johnson, PhD, and Janet Stead, BM, BS, who provided medical writing and editorial assistance on be-half of MedImmune.
REFERENCES
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 –18 years: United States, 2007. MMWR Morb Mortal Wkly Rep.2007;55(51–52): Q1–Q4
2. Atkinson WL, Pickering LK, Schwartz B, et al. General recom-mendations on immunization: recomrecom-mendations of the Advi-sory Committee on Immunization Practices (ACIP) and the
American Academy of Family Physicians (AAFP). MMWR
Recomm Rep.2002;51(RR-2):1–35
3. Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children.N Engl J Med.1998;338(20):1405–1412 4. Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of
intra-nasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine.J Pediatr.2000;136(2):168 –175 5. Piedra PA, Yan L, Kotloff K, et al. Safety of the trivalent,
cold-adapted influenza vaccine in preschool-aged children. Pe-diatrics.2002;110(4):662– 672
6. Vesikari T, Karvonen A, Korhonen T, et al. A randomized, double-blind study of the safety, transmissibility and pheno-typic and genopheno-typic stability of cold-adapted influenza virus vaccine.Pediatr Infect Dis J.2006;25(7):590 –595
7. Ashkenazi S, Vertruyen A, Arı´stegui J, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J.2006;25(10): 870 – 879
8. Belshe RB, Edwards KM, Vesikari T, et al. Comparative safety and efficacy of live attenuated and inactivated influenza vac-cines in infants and young children.N Engl J Med.2007;356(7): 685– 696
9. Vesikari T, Fleming DM, Aristegui JF, et al. Safety, efficacy, and effectiveness of cold-adapted influenza vaccine-trivalent against community-acquired, culture-confirmed influenza in young children attending day care. Pediatrics. 2006;118(6): 2298 –2312
10. Merck.MMR II (Measles, Mumps, and Rubella Virus Vaccine Live): Full Prescribing Information. Whitehouse Station, NJ: Merck; 2003
11. Merck. Varivax (Varicella Virus Vaccine Live [Oka/Merck]): Full Prescribing Information. Whitehouse Station, NJ: Merck; 2005 12. Centers for Disease Control and Prevention.The 1995–96 WHO
Influenza Reagent Kit for the Identification of Influenza Isolates. Atlanta, GA: Centers for Disease Control and Prevention; 1995 13. Brammer L, Fukuda K, Arden N. Influenza surveillance: United States, 1992–93 and 1993–94. MMWR Survill Summ. 1997;46(SS-1):1–12
14. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med.1985;4(2):213–226
15. Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions.Biometrics.1999;55(4): 1202–1209
16. Loughlin J, Poulios N, Napalkov P, Wegmuller Y, Monto AS. A study of influenza and influenza-related complications among children in a large US health insurance plan database. Phar-macoeconomics.2003;21(4):273–283
17. O’Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children.Pediatrics.2004;113(3):585–593
18. Grijalva CG, Craig AS, Dupont WD, et al. Estimating influenza hospitalizations among children.Emerg Infect Dis.2006;12(1): 103–109
19. Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004.N Engl J Med.2005;353(24):2559 –2567
20. Thompson WW, Shay DK, Weintraub E, et al. Mortality asso-ciated with influenza and respiratory syncytial virus in the United States.JAMA.2003;289(2):179 –186
21. Centers for Disease Control and Prevention. Recommended childhood and adolescent immunization schedule: United States, 2006.MMWR.2006;54(52):Q1–Q4
22. Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep.2006;55(RR-15):1– 48
23. Petralli JK, Merigan TC, Wilbur JR. Action of endogenous interferon against vaccinia infection in children.Lancet.1965; 2:401– 405
24. Petralli JK, Merigan TC, Wilbur JR. Circulating interferon after measles vaccination.N Engl J Med.1965;273(4):198 –201 25. Centers for Disease Control and Prevention. Simultaneous
ad-ministration of varicella vaccine and other recommended childhood vaccines: United States, 1995–1999. MMWR Morb Mortal Wkly Rep.2001;50(47):1058 –1061
26. Verstraeten T, Jumaan AO, Mullooly JP, et al. A retrospective cohort study of the association of varicella vaccine failure with asthma, steroid use, age at vaccination, and measles-mumps-rubella vaccination. Pediatrics. 2003;112(2). Available at: www.pediatrics.org/cgi/content/full/112/2/e98
27. Giammanco G, De Grandi V, Lupo L, et al. Interference of oral poliovirus vaccine on RIT 4237 oral rotavirus vaccine.Eur J Epidemiol.1988;4(1):121–123
28. Vodopija I, Baklaic Z, Vlatkovic R, et al. Combined vaccination with live oral polio vaccine and the bovine rotavirus RIT 4237 strain.Vaccine.1986;4(4):233–236
29. Zangwill KM, Droge J, Mendelman P, et al. Prospective, ran-domized, placebo-controlled evaluation of the safety and im-munogenicity of three lots of intranasal trivalent influenza vaccine among young children.Pediatr Infect Dis J.2001;20(8): 740 –746
30. Nolan T, Lee MS, Cordova JM, et al. Safety and immunoge-nicity of a live-attenuated influenza vaccine blended and filled at two manufacturing facilities. Vaccine. 2003;21(11–12): 1224 –1231
31. Ambrose CS, Walker RE, Connor EM. Live attenuated influ-enza vaccine in children.Semin Pediatr Infect Dis.2006;17(4): 206 –212
32. Shinefield HR, Black SB, Staehle BO, et al. Safety, tolerabil-ity and immunogenictolerabil-ity of concomitant injections in sepa-rate locations of M-M-R II, VARIVAX and TETRAMUNE in healthy children vs. concomitant injections of M-M-R II and TETRAMUNE followed six weeks later by VARIVAX.Pediatr Infect Dis J.1998;17(11):980 –985
33. White CJ, Stinson D, Staehle B, et al. Measles, mumps, rubella, and varicella combination vaccine: safety and immunogenicity alone and in combination with other vaccines given to children: Measles, Mumps, Rubella, Varicella Vaccine Study Group.Clin Infect Dis.1997;24(5):925–931
34. Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus.Curr Top Microbiol Immunol.1989;146:107–116
35. Kemble G, August M, Dawson D, et al. FluMist elicits crossre-active antibodies to Fujian-like A/H3N2 strains in ferrets (Ab-stract 1930). Presented at the Pediatric Academic Societies’ 2004 Annual Meeting; May 1– 4, 2004; San Francisco, CA 36. Nichol KL, Mendelman PM, Mallon KP, et al. Effectiveness of
live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999; 282(2):137–144
37. Belshe RB, Nichol KL, Black SB, et al. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vac-cine in an indicated population aged 5– 49 years.Clin Infect Dis. 2004;39(7):920 –927
DOI: 10.1542/peds.2007-1064
2008;121;508
Pediatrics
Cho and Paul M. Mendelman
Iksung
Colin Marchant, Helen Marshall, Peter Richmond, Ram Yogev, Julie Cordova,
Terry Nolan, David I. Bernstein, Stan L. Block, Milo Hilty, Harry L. Keyserling,
Infants 12 to 15 Months of Age
Influenza Vaccine With Measles-Mumps-Rubella and Varicella Vaccines to
Safety and Immunogenicity of Concurrent Administration of Live Attenuated
Services
Updated Information &
http://pediatrics.aappublications.org/content/121/3/508
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/121/3/508#BIBL
This article cites 33 articles, 3 of which you can access for free at:
Subspecialty Collections
_sub
http://www.aappublications.org/cgi/collection/vaccine:immunization
Vaccine/Immunization
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su
Infectious Disease following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2007-1064
2008;121;508
Pediatrics
Cho and Paul M. Mendelman
Iksung
Colin Marchant, Helen Marshall, Peter Richmond, Ram Yogev, Julie Cordova,
Terry Nolan, David I. Bernstein, Stan L. Block, Milo Hilty, Harry L. Keyserling,
Infants 12 to 15 Months of Age
Influenza Vaccine With Measles-Mumps-Rubella and Varicella Vaccines to
Safety and Immunogenicity of Concurrent Administration of Live Attenuated
http://pediatrics.aappublications.org/content/121/3/508
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.