Buccal Cell DNA Mutation Analysis for Diagnosis of Cystic Fibrosis in
Newborns and Infants Inaccessible to Sweat Chloride Measurement
Richard B. Parad, MD, MPH
ABSTRACT. Objectives. To assess the application of DNA-based cystic fibrosis transmembrane conductance regulator (CFTR) gene mutation analysis as a primary cystic fibrosis (CF) diagnostic test in preterm and term newborns and infants for whom the quantitative pilo-carpine iontophoresis test (QPIT) cannot be used.
Design. Retrospective survey.
Setting. DNA Diagnostic Laboratory, Children’s Hos-pital, Boston, Massachusetts. Buccal cell DNA samples were received from inpatients, outpatients, and three neonatal intensive care units.
Outcome Measure. Detection of at least 1 of 12 CFTR mutations.
Patients. Between November 1, 1992, and April 30, 1994, 28 newborns and infants under 12 months of age at risk for CF had CFTR DNA mutation analysis performed because a sweat chloride (SC) value could not be ob-tained. QPIT was either not performed (infant weight<2 kg, QPIT not available at site of hospitalization, or infant not accessible to QPIT laboratory) or was inconclusive (sweat volume <75 mg or indeterminate SC [>40, <60 mEq/L]). The postnatal age at time of testing ranged from 1 day to 11 months, and gestational age at birth from 25 to 40 weeks.
Results. Six (21%) of 28 infants with unobtainable or indeterminate QPIT had 1 or 2 CFTR mutations detected. Immediate CF diagnosis by direct detection of 2 CFTR mutations was made in 5 of these 6 patients. Definitive CF diagnosis in the infant with 1 CFTR mutation was delayed until an elevation in SC could be documented. The patients with no CFTR mutations detected had a low likelihood of CF.
Conclusions. For infants in whom CF is suspected but QPIT cannot be obtained, buccal cell DNA-based CFTR mutation analysis can be used as a rapid, noninvasive primary diagnostic test. This simple mode of DNA col-lection may aid in the diagnosis of other inherited dis-orders in newborns. Pediatrics 1998;101:851– 855; cystic fibrosis, CFTR, sweat chloride, pilocarpine iontophoresis, DNA mutation analysis, premature newborn.
ABBREVIATIONS. SC, sweat chloride; QPIT, quantitative pilo-carpine iontophoresis test; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; CLD, chronic lung dis-ease.
F
or.35 years, a sweat chloride (SC) concentra-tion of $60 mEq/L measured by quantitative pilocarpine iontophoresis testing (QPIT) has been the gold standard for the diagnosis of cystic fibrosis (CF). QPIT is optimally performed on sweat samples weighing 75 mg obtained at a sweat rate of at least 1 g/m2/min.1QPIT has several limitations in newborns. Adequate quantities of sweat are difficult to obtain in premature infants2and newborns who weigh ,2 kg. In any infant, if the amount of sweat collected is inadequate, the test must be repeated at a later time and diagnosis is delayed. Also, because SC values are normally high at birth and fall toward baseline after 48 hours of life, testing in the immedi-ate newborn period may yield false positive or inde-terminate (SC $40, ,60 mEq/L) results.3 As with inadequate sweat collection, indeterminate SC re-sults necessitate repeat testing and cause diagnostic delay. In addition, a newborn or infant may not have access to a laboratory where qualified QPIT is per-formed.Potential problems related to delay of CF diagno-sis include late initiation of appropriate therapeutic and preventive nutritional and pulmonary interven-tions, prolonged parental anxiety awaiting definitive diagnosis, and delay in counseling of family mem-bers. Premature or ill infants are likely to have the longest delay in definitive diagnosis when the pri-mary diagnostic test is QPIT.
As a result of the identification of the cystic fibrosis transmembrane conductance regulator (CFTR) gene4 and many of the mutations that cause CF, molecular diagnosis based on DNA evaluation has become in-creasingly available. DNA mutation analysis is cur-rently used for prenatal testing, family counseling, and diagnosis in cases of atypical CF presentation. Although the number of mutations evaluated in commercially available tests has been increasing (eg, 70 mutation test, Genzyme Genetics, Framingham, MA), the per mutation cost has steadily fallen. Mu-tation detection rates among affected mixed North American populations are reported up to 80% to 90% depending on the population tested and the muta-tions evaluated.5
Early DNA-based tests required blood sample vol-umes (5 to 20 mL) that were relatively large for newborns. Although microquantities of blood, in-cluding dried blood spots such as those obtained for newborn screening,6 can now provide adequate amounts and quality of genomic DNA for mutation analysis, many laboratories do not currently offer From the Divisions of Newborn Medicine (Joint Program in Neonatology),
Respiratory Diseases and Genetics, Children’s Hospital, Harvard Medical School, Boston, Massachusetts.
Received for publication Jun 21, 1996; accepted Sep 17, 1997.
testing on such microsamples. The use of buccal cell DNA collected by a noninvasive cheekbrushing tech-nique is now accepted for use in older children and adults.7To the best of our knowledge, this is the first reported assessment of buccal cell DNA use for pri-mary diagnosis of CF in premature and young in-fants who would not otherwise have had immediate access to definitive CF diagnosis by QPIT.
METHODS Population
Cheekbrush collection methods were made available for infants born at Brigham and Women’s and Beth Israel Hospitals, and to newborns and infants admitted to Children’s Hospital, Boston, Massachusetts. Charts were retrospectively reviewed from all in-fants under 12 months of age on whom cheekbrushes were sent for CFTR mutation analysis to the DNA Diagnostic Laboratory at Children’s Hospital between November 1, 1992 and April 30, 1994 (n541). Preterm and term infants who were: 1) too small to be tested (,2 kg), 2) unable to generate an adequate sweat volume, 3) tested by QPIT with indeterminate SC values ($40,,60 mEq/L), 4) critically ill and unable to be transported for QPIT, or 5) born at an institution where QPIT was not available, were included in the study population. Gestational age at birth, age at time of DNA and SC (if performed) testing, reason for testing, and SC results were documented.
Buccal cells were obtained from each child by vigorously twirl-ing and brushtwirl-ing a cheekbrush (Cyto-pak, Medical Packagtwirl-ing Corp, Camarillo, CA) up and down against the right and left buccal mucosa of each infant for 30 seconds. Brushes were care-fully repackaged in their protective sheaths, taking care not to touch the brush against any surface. Samples were transported at room temperature and then stored at 4°C until DNA extraction could be performed.
DNA was prepared by cutting the brush off into 600mL of 50 mM NaOH in a 1.5 mL polypropylene microcentrifuge tube. The tubes were vortexed and heated to 95°C for 5 minutes. The brushes were then discarded and 60mL 1 M Tris, pH 8.0, was added to the tubes.7
CFTR mutation analysis was performed for 12 mutations (DF508, G551D, G542X, 62111G3T,DI507, 1717–1G3A, R117H, N1303K, W1282X, R560T, R553X, and 3849110kb C3T). Geno-typing for all CFTR mutations, except for 3849110kb C3T, were evaluated by the multiplex amplification refractory mutation sys-tem using the primers and methods reported by Ferrie modified for the use of 20mL of buccal cell DNA solution per reaction tube.8
CFTR mutation 3849110kb C3T was assessed by restriction en-zyme digestion withHph I.9
QPIT was performed by standard methods.1,10The minimum
acceptable sweat weight, per National Committee for Clinical Laboratory Standards guidelines, was 75 mg.
Positive predictive values for detection of 1 or 2 CFTR muta-tions were calculated using Bayes theorem and the Hardy-Wein-berg equation.
RESULTS Study Population Characteristics
Twenty-eight of the 41 infants genotyped in this age group met inclusion criteria (Table 1). Fifteen (54%) of the 28 had been born preterm (,37 weeks). The smallest infant studied weighed 700 g and was mechanically ventilated. The oldest patient was 11 months of age at the time of testing. Samples were collected within the first week of life in 12 (42%) infants. Four (14%) infants had a positive family history for CF.
Genotype and SC Status by Diagnostic Category Patients were characterized as affected with CF (2 CFTR mutations or 1 CFTR mutation with a confir-matory SC$60 mEq/L), unaffected (no CFTR
muta-tions detected), or indeterminate (SC $40, ,60 mEq/L) (Table 2).
Inconclusive QPIT had preceded genotyping in 5 patients. Two of these patients had produced an inadequate volume of sweat (1 subsequently found to have CF and 1 found to be normal), and 3 had indeterminate SC (none had mutations detected). QPIT was used to confirm genotype results in 11 patients (2 with 2 mutations and 9 with no muta-tions) and all results were concordant.
Of the 6 patients ultimately characterized as af-fected, 2 had confirmed elevated SC ($60 mEq/L), and 4 did not undergo postgenotyping QPIT. Of the 19 infants with no mutation detected, 9 (47%) had follow-up QPIT, and all were normal (SC,40 mEq/ L). For 12 of the 28 patients, QPIT was not performed and genotyping was the sole diagnostic test.
The genotype of each patient is presented in Table 2. Based on detection of either 2 CFTR mutations or 1 CFTR mutation and a subsequent SC$60 mEq/L, 6 patients (21%) were ultimately determined to be affected with CF. Five of 28 (18%) had a definitive diagnosis with DNA testing alone. In the absence of a detected mutation, patients were considered unaf-fected. The 3 patients (11%) with indeterminate SC levels had no mutations detected by genotyping, remained classified as diagnosis unknown, and con-tinued in clinical follow-up.
Based on an 80% abnormal allele detection rate in the Children’s Hospital, Boston CF clinic population, a carrier rate of 1/25 and a CF risk of 21% in the study population, a positive predictive value was estimated both for the detection of 2 mutations (100%) and for 1 mutation (67%). The chance of CF given no detected mutations is 1% under these as-sumptions. Alternatively, if the prior risk estimate is based on our sweat test laboratory positive SC rate among similar age infants, the positive predictive value for 1 mutation drops to 33%, and the chance of disease when no mutations are detected is 0.3%.
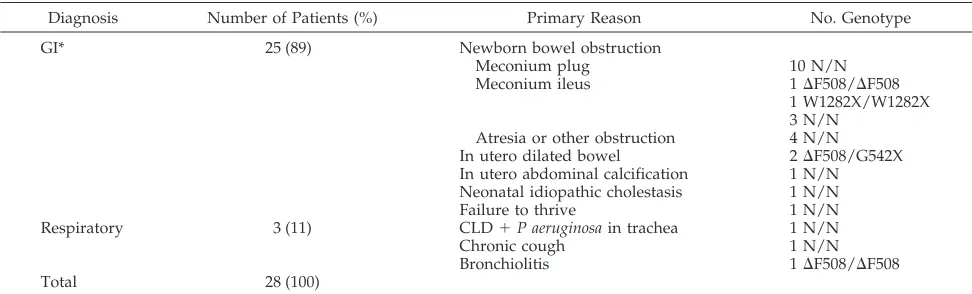
To provide a sense of why a diagnosis of CF was entertained in this cohort, patients were character-ized by the clinical history leading to consideration of a diagnosis of CF (Table 3). Eighty-nine percent of
TABLE 1. Study Population Characteristics
Characteristic Number of Patients (%)
Gestational age at birth
Preterm 15 (54)
24–28 wk (n55) 29–33 wk (n54) 34–37 wk (n56)
Term 13 (46)
Total 28 (100)
Age at time of brushing
,1 d 2 (7)
1 d–1 wk 10 (36)
1–4 wk 6 (21)
2–11 mo 10 (36)
Total 28 (100)
Family history
Positive* 4 (14)
Negative 24 (86)
Total 28 (100)
patients (25/28) had testing prompted by an abnor-mality of the gastrointestinal tract or nutritional sta-tus. Of these 25 patients, 89% (22/25) presented in the newborn period with partial or complete bowel obstruction. Ten of these 22 infants passed a meco-nium plug, and none of those 10 carried any of the 12 screened mutations. Of the 5 newborns with meco-nium ileus, 2 had genotypes confirming CF. Of the 5 newborns with intestinal atresia or other surgically corrected obstruction, 1 had an abnormal genotype. One infant with prolonged idiopathic cholestasis and 1 infant with prenatally identified intraabdominal calcifications had no mutations detected.
Three infants were tested because of respiratory symptomatology. A 4-month old with hyponatremia and bronchiolitis who was mechanically ventilated for several weeks, and was thus inaccessible to QPIT, was found to be a DF508 homozygote. No CFTR mutations were identified in the infant with chronic cough or in the preterm infant on mechanical venti-lation with chronic lung disease (CLD) andP aerugi-nosa growing from tracheal secretions.
The primary justifications for performing CFTR genotyping are summarized in Table 4. Fourteen of the 28 infants (50%) were genotyped, rather than undergoing QPIT, because of low weight. Most of these infants were premature infants with either bowel obstruction or a family history of CF.
Sample Efficacy
Extreme prematurity, low infant weight and sever-ity of illness did not limit abilsever-ity to obtain adequate cheekbrush samples. In,5% of samples submitted, a
repeat sample was requested because of an inade-quate DNA sample on the cheekbrush. In all cases, results were ultimately obtained after repeat cheek-brush sampling.
DISCUSSION
Richards7 recently showed that DNA extracted from traditional large blood draws could be substi-tuted with buccal cell DNA collected by cheekbrush for polymerase chain reaction-based CFTR mutation analysis. We have demonstrated that the buccal cell DNA acquired from newborns and infants via cheek-brush is also of adequate quantity and quality to carry out this analysis, and obviates the need to draw blood from such patients. No limitations to sample collection because of birth weight, gestational age, or severity of illness were observed.
Using the nonradioactive allele specific polymer-ase chain reaction-bpolymer-ased amplification refractory mutation system approach, DNA was extracted, am-plified, and visualized on an agarose gel within 24 hours of sample collection and provided a rapid noninvasive diagnostic test for CF in newborns.
Although small amounts of buccal cell DNA pro-vide adequate substrate to carry out mutation anal-ysis, several significant limitations are inherent to a primary DNA-based diagnostic approach. Only 12 of the.600 known CFTR mutations were tested in this study. Thus, a genotype evaluation that reveals no CFTR mutations could be misleading by providing a false-negative result. In our local CF population, 4% of patients with SC $60 mEq/L have no detectable CFTR mutations. Based on this value, approximately
TABLE 2. Genotypes and Sweat Chloride Confirmation by Diagnostic Category
Diagnostic Category Number of Patients (%) Primary SC Before Genotype Genotype Confirmatory SC After Genotyping (mEq/L)
Affected 6 (21) 1 inadequate volume 2DF508/DF508 2 ND*
2 G542X/W1282X 2 ND*
1DF508/N $60
1 W1282X/W1282X $60
Unaffected 19 (68)
Confirmed (SC,40) 1 inadequate volume 9 N/N 9,40
Presumed (no SC*) 10 N/N 10 ND*
Unknown (indeterminate) 3 (11) 3$40,,60 mEq/L 3 N/N 3 ND*
Total 28 (100)
* ND, not done.
TABLE 3. Reason for Considering Diagnosis of Cystic Fibrosis
Diagnosis Number of Patients (%) Primary Reason No. Genotype
GI* 25 (89) Newborn bowel obstruction
Meconium plug 10 N/N
Meconium ileus 1DF508/DF508
1 W1282X/W1282X 3 N/N
Atresia or other obstruction 4 N/N In utero dilated bowel 2DF508/G542X In utero abdominal calcification 1 N/N Neonatal idiopathic cholestasis 1 N/N
Failure to thrive 1 N/N
Respiratory 3 (11) CLD1P aeruginosain trachea 1 N/N
Chronic cough 1 N/N
Bronchiolitis 1DF508/DF508
Total 28 (100)
1% of the cohort studied could still have CF in the absence of detected mutations. The small chance of a missed CFTR mutation remained in the 10 infants who did not undergo follow-up QPIT.
The QPIT can also generate false-negative results. CF has been reported in patients with SC,40 mEq/ L.9One mutation associated with this clinical variant, 3849110kb C3T, was included in our 12-mutation screen. Additional mutations are likely to be associ-ated with mild phenotypes and low SC.11
In the clinical setting suspicious of CF when either QPIT was indeterminate or DNA analysis was neg-ative, we considered that both tests should be per-formed in concert. Follow-up QPIT was recom-mended in the 23 (82%) patients with 1 or no mutations detected. The indication for QPIT is clear when DNA analysis reveals 1 mutation, as genotype cannot distinguish a CF carrier from an affected com-pound heterozygote with 1 identified mutation. For the other 22 patients, although QPIT may seem re-dundant, the estimated 1% false-negative rate justi-fied QPIT confirmation. None of the follow-up QPIT values contradicted the original assessment based on genotype.
For infants who are either too small or too ill to undergo QPIT, or when attempted QPIT is incon-clusive, an appropriate alternative for CF diagno-sis is CFTR mutation analydiagno-sis using buccal cell DNA collected by cheekbrush. Eighteen percent of the cohort studied were confirmed to have CF based on the identification of 2 CFTR mutations. In 1 patient, a diagnosis of CF was strongly suspected because of the identification of 1 CFTR mutation, and subsequently confirmed when SC could be obtained. These patients would have had a delay in diagnosis while waiting to undergo initial or repeat QPIT. Diagnosis by DNA mutation analysis allowed for earlier initiation of therapy, minimiza-tion of parental uncertainty, and an ability to pro-vide more accurate counseling to family members. For the latter, timing may be of particular impor-tance if there is a family member who is either
pregnant or considering having children. Con-versely, 79% of the patients had CF ruled out by the negative mutation screen.
In older or heavier infants, diagnostic delays be-cause of indeterminate SC or inadequate sweat vol-ume might also be alleviated by DNA testing. Previ-ously, for those infants generating inadequate sweat samples, repeating QPIT until an adequate sweat volume could be collected had been the only means available to confirm a CF diagnosis. Similarly, infants with adequate sweat volumes but persistently inde-terminate SC had no diagnostic alternatives to repet-itive QPIT. Repeating the QPIT throughout weeks to months did not guarantee success in clarifying a diagnosis.
Although a 14% to 25% incidence of CF has been reported among symptomatic newborns who passed meconium plugs,12,13none of the 10 patients with bowel obstruction attributable to meconium plug had CFTR mutations detected. All 10 infants were preterm and weighed ,2 kg. Because of the technical difficulties in obtaining adequate sweat samples, QPIT has not previously been reported in a series of low birth weight infants passing meco-nium plugs. Extremely low birth weight infants pass meconium plugs more commonly than term newborns, possibly because of immaturity of bowel motility. Thus, passing a meconium plug in a preterm infant may be a less specific sign for CF than in a term infant. In addition, passage of a meconium plug does not necessarily constitute the meconium plug syndrome (including microcolon) as defined by fluoroscopy. The high proportion of low birth weight infants in this cohort could ac-count for findings discrepant with prior reports of meconium plug associated with CF. DNA-based CFTR mutation analysis can rule out a CF diagno-sis in this clinical setting with reasonable certainty. Patients most likely to have abnormal CFTR geno-types were those presenting either with bowel ob-struction not related to meconium plug, or with a positive family history for CF. Because one third of
TABLE 4. Primary Justification for Performing Cheekbrush CFTR Genotyping
Reason for Cheekbrush Genotyping
Number of Patients (%)
Clinical Reason for Considering CF (No.) Genotype
,2 kg
Premature or intrauterine growth restriction
14 (50) 2 Transient in utero dilated bowel (twins), affected sibling 2 G542X/W1282X* 1 In utero abdominal calcifications 1 N/N
10 Meconium plug (3 with CLD, 1 in utero dilated bowel) 10 N/N 1 CLD/P aeruginosain tracheal aspirate 1 N/N
.2 kg
Inadequate sweat amount or inconclusive SC
5 (18) 1 Meconium ileus, affected sibling 1DF508/DF508* 1 Scrotal swelling, in utero abdominal calcifications 1 N/N
1 Chronic cough 1 N/N
1 Affected relative 1 N/N
1 Failure to thrive, affected uncle 1 N/N
Sweat lab inaccessible: 9 (32) 1 Bronchiolitis 1DF508/DF508*
Patient intensive care
unit-bound or no laboratory in hospital
8 Bowel obstruction
5 Meconium ileus 1DF508/N*
1 W1282X/W1282X* 3 N/N
3 Small bowel atresia 3 N/N
Total 28 (100)
newborns with bowel obstruction have CF,14 this finding should be a strong indication for testing. Although only 15% of newly diagnosed CF patients have a family history of CF, testing should not be excluded when the history is negative. Several of the infants we tested presented with symptoms that are occasionally associated with CF (eg, newborn cho-lestasis). DNA testing was also of value in excluding a CF diagnosis in this group.
At our institution, the cost of the CFTR 12 mu-tation DNA screen is approximately half that of the QPIT. DNA testing could be criticized for being redundant, resulting in requests for two tests rather than one. However, as advances in DNA testing technology lead to higher mutation detec-tion rates, the need to rule out false negatives should diminish.
Buccal cell DNA can be rapidly and noninva-sively collected from infants of any gestational age and size and is effective for analysis of CFTR mu-tations. CFTR mutation detection allowed for the diagnosis of CF in 20% of a high-risk cohort of infants who lacked definitive QPIT. Conversely, absence of CFTR mutation detection made CF highly unlikely in 80% of the cohort. However, because of the possibility of false-negative results, confirmatory QPIT should still be performed on symptomatic patients with negative mutation screens. Genetic testing using newborn buccal cell DNA will more readily allow for primary diagno-sis of inherited disorders as the human genome project links more genes and their mutations to diseases that affect children.
ACKNOWLEDGMENTS
Supported in part by National Institutes of Health grants DK2273 and by an American Lung Association Trudeau Scholar-ship.
REFERENCES
1. LeGrys VA, Barlow WK, Bracey A, et al.Sweat Testing: Sample Collection and Quantitative Analysis. Wayne, PA: National Committee for Clinical Laboratory Standards. Document C34-A. 1994
2. Harpin VA, Rutter N. Sweating in preterm babies.J Pediatr. 1982;100: 614 – 619
3. Hardy JD, Davison SH, Higgins MU, Polycarpou PN. Sweat tests in the newborn period.Arch Dis Child. 1973;48:316 –318
4. Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis.Science. 1989;245:1073–1080
5. The Cystic Fibrosis Genetic Analysis Consortium. Population variation of common cystic fibrosis mutations.Hum Mut. 1994;4:167–177 6. Dauphinais RM. A cost analysis of blood-spot screening newborns for
cystic fibrosis.J Clin Immunoassay. 1992;15:121–125
7. Richards B, Skoletsky J, Shuber AP, et al. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum Mol Genet. 1993;2:159 –163
8. Ferrie RM, Schwarz MJ, Robertson NH, et al. Development, multiplex-ing, and application of ARMS tests for common mutations in the CFTR gene.Am J Hum Genet. 1992;51:251–262
9. Highsmith WE, Burch LH, Zhou Z, et al. A novel mutation in the cystic fibrosis gene in patients with pulmonary disease but normal sweat chloride concentrations.N Engl J Med. 1994;331:974 –980
10. Shwachman H, Antonowicz J. The sweat test in cystic fibrosis.Ann N Y Acad Sci. 1962;93:600
11. Chillon M, Casals T, Mercier B, et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens.N Engl J Med. 1995;332:1475–1480
12. Olsen MM, Luck SR, Lloyd-Still J, Raffensperger JG. The spectrum of meconium disease in infancy.J Pediatr Surg. 1982;17:479 – 481 13. Rosenstein BJ, Langbaum. TS. Incidence of meconium abnormalities in
newborn infants with cystic fibrosis.Am J Dis Child. 1980;134:72–73 14. Lloyd-Still JD. Meconium. In: Lloyd-Still JD, ed.Cystic Fibrosis. Boston,
DOI: 10.1542/peds.101.5.851
1998;101;851
Pediatrics
Richard B. Parad
and Infants Inaccessible to Sweat Chloride Measurement
Buccal Cell DNA Mutation Analysis for Diagnosis of Cystic Fibrosis in Newborns
Services
Updated Information &
http://pediatrics.aappublications.org/content/101/5/851 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/101/5/851#BIBL This article cites 12 articles, 2 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/genetics_sub Genetics
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_ Fetus/Newborn Infant
http://www.aappublications.org/cgi/collection/endocrinology_sub Endocrinology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.101.5.851
1998;101;851
Pediatrics
Richard B. Parad
and Infants Inaccessible to Sweat Chloride Measurement
Buccal Cell DNA Mutation Analysis for Diagnosis of Cystic Fibrosis in Newborns
http://pediatrics.aappublications.org/content/101/5/851
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.