DRUG UTILIZATION STUDY IN PATIENTS OF NEONATAL INTENSIVE CARE UNIT OF TERTIARY
CARE HOSPITAL IN PUNJAB, INDIA
Dr. Jatinder Singh
1, Dr.
1Department of Pharmacology, GMC, Patiala 2Department of Community medicine, GMC, Patiala
3Department of Pharmacology, GMC, Patiala 4Department of Microbiology, PGIMER, Sangrur
A R T I C L E I N F O
INTRODUCTION
World health organization (WHO) defined drug utilization study (DUS) in 1977 as “the marketing, distribution, prescription and use of drugs in society, with special emphasis on the resulting medical, social and economic consequences” DUS is a method for drug evaluation in which it evaluates qualitative and quantitative aspect of the drugs. There are various methods for qualitative DUS but most commonly used are ‘the potential therapeutic value’ and ‘the expecte
of use’. The quantitative study can be of various types also but mainly used methods are ‘cost’, ‘number of units’, prescriptions’ and ‘sale of “top” products’.2
DUS generates data that can be further evaluated by various means like comparing the current guidelines/recommendations to the patterns observed in the hospital during the study to further improve the drug utilization. This data can also be used to compare the pattern and cost of drugs used in different regions over the time3.
International Journal of Current Advanced Research
ISSN: O: 2319-6475, ISSN: P: 2319-6505,
Available Online at www.journalijcar.org
Volume 8; Issue 05 (B); May 2019; Page No.
DOI: http://dx.doi.org/10.24327/ijcar.2019
Copyright©2019 Dr. Jatinder Singh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article History:
Received 15th February, 2019 Received in revised form 7th March, 2019
Accepted 13th April, 2019
Published online 28th May, 2019
Key words:
Drug Utilization Study, NICU, Respiratory Distress Syndrome (RDS)
*Corresponding author: Dr. Perbhat Kansal
Department of Pharmacology, GMC, Patiala
DRUG UTILIZATION STUDY IN PATIENTS OF NEONATAL INTENSIVE CARE UNIT OF TERTIARY
CARE HOSPITAL IN PUNJAB, INDIA
, Dr. Manhardeep Kaur
2, Dr. Perbhat Kansal
3*and
Department of Pharmacology, GMC, Patiala Department of Community medicine, GMC, Patiala
Department of Pharmacology, GMC, Patiala Department of Microbiology, PGIMER, Sangrur
A B S T R A C T
Introduction: Drug utilization study (DUS) is a method for drug
evaluates qualitative and quantitative aspect of the drug use. The main aim of DUS is to increase rational use of the drugs in population. In neonatal intensive care unit (NICU), premature infants are treated with many drugs. Hence, DUS in NICU was planned to study prescription pattern and pharmaco-economic analysis.
Methods: A cross-sectional observational study was carried out for six months in NICU in
Medical College and Hospital of Punjab after approval by Institutional Ethics Committee. Patients of both gender were enrolled after taking written informed consent and patients discharged/died within 24 hours of admission were excluded.
Results: In this study, 100 patient’s data was collected during the study period in which 67
patients were preterm, 32 were term and 1 was post term. Males were more frequently admitted than the females. Preterm babies had more duration of hospitalization (11.2 days) as compare to term and post term (7 days). Respiratory Distress Syndrome
major disease prevailing in preterm 63 out of 67 but only 22 from 32 term neonates were suffering from RDS. The average number of drugs prescribed in preterm (2.7) were much higher than the term (2.01) infants Amikacin, an antibiotic was the most commonly prescribed drug among all the groups, but the costliest drug prescribed in group A and group B was Cefoperazone. Among the antibiotic combinations most affordable were amikacin + cefotaxime to all the patients.
World health organization (WHO) defined drug utilization study (DUS) in 1977 as “the marketing, distribution, prescription and use of drugs in society, with special emphasis economic consequences”1 DUS is a method for drug evaluation in which it evaluates qualitative and quantitative aspect of the drugs. There are various methods for qualitative DUS but most commonly used are ‘the potential therapeutic value’ and ‘the expected degree of use’. The quantitative study can be of various types also but mainly used methods are ‘cost’, ‘number of units’,
DUS generates data that can be further evaluated by various current guidelines/recommendations to the patterns observed in the hospital during the study to further improve the drug utilization. This data can also be used to compare the pattern and cost of drugs used in different
The main aim of drug utilization study is to increase rational use of the drugs in population.
medical technology, there are variety of treatment modalit available but there is difference in cost of treatment and treatment related complications of these modalities. So, we need to find most efficacious, cost effective treatment modality.5 In current scenario, DUS plays vital role in mostvulnerable patients to issue an evidence prescription and helps us to evaluate risk
drugs, so that we can prescribe effective drugs without creating additional economic load on the society.
DUS can be conducted on any population type but some populations are more vulnerable than others like neonates. So rational use and appropriate prescribing is crucial in neonates, elderly etc.7Neonates, term and preterm are most venerable especially preterm because of organ immaturity and consequently difficulties adapting to extra
Prematurity due to above reason itself cause high mortality and risk further increase after their exposure to the high number of drugs8.
International Journal of Current Advanced Research
6505, Impact Factor: 6.614
www.journalijcar.org
; Page No.18607-18611
//dx.doi.org/10.24327/ijcar.2019.18611.3562
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Dr. Perbhat Kansal
DRUG UTILIZATION STUDY IN PATIENTS OF NEONATAL INTENSIVE CARE UNIT OF TERTIARY
and Dr. Baby
4(DUS) is a method for drug evaluation in which it evaluates qualitative and quantitative aspect of the drug use. The main aim of DUS is to In neonatal intensive care unit (NICU), DUS in NICU was planned to study
sectional observational study was carried out for six months in NICU in Medical College and Hospital of Punjab after approval by Institutional Ethics Committee. of both gender were enrolled after taking written informed consent and patients discharged/died within 24 hours of admission were excluded.
study, 100 patient’s data was collected during the study period in which 67 patients were preterm, 32 were term and 1 was post term. Males were more frequently admitted than the females. Preterm babies had more duration of hospitalization (11.2 days) mpare to term and post term (7 days). Respiratory Distress Syndrome (RDS) was major disease prevailing in preterm 63 out of 67 but only 22 from 32 term neonates were suffering from RDS. The average number of drugs prescribed in preterm (2.7) were much er than the term (2.01) infants Amikacin, an antibiotic was the most commonly prescribed drug among all the groups, but the costliest drug prescribed in group A and group B was Cefoperazone. Among the antibiotic combinations most affordable were
The main aim of drug utilization study is to increase rational use of the drugs in population. 4With advancement in the medical technology, there are variety of treatment modality available but there is difference in cost of treatment and treatment related complications of these modalities. So, we need to find most efficacious, cost effective treatment In current scenario, DUS plays vital role in to issue an evidence-based prescription and helps us to evaluate risk-benefit profile of drugs, so that we can prescribe effective drugs without creating additional economic load on the society.6
DUS can be conducted on any population type but some ations are more vulnerable than others like neonates. So rational use and appropriate prescribing is crucial in neonates, Neonates, term and preterm are most venerable especially preterm because of organ immaturity and s adapting to extra-maternal life. Prematurity due to above reason itself cause high mortality and risk further increase after their exposure to the high number of
Research Article
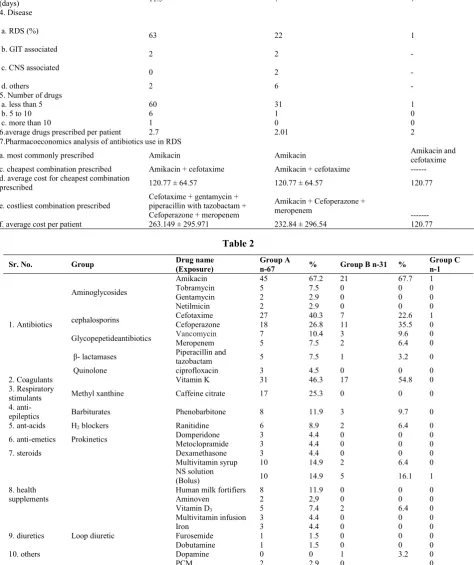
Table 1
Characters Group An= 67 Group Bn=32 Group Cn=1
M F M F M F
1. Gender 46 21 24 8 1 0
2. Gestational weight at hospitalization
(Average± SD) 1792 g± 752.07
1358
g±768.58 2780 g± 758.02 2467 g± 748.81 1820 g 0 3. Average duration of hospitalization
(days) 11.5 7 7
4. Disease
a. RDS (%)
63 22 1
b. GIT associated
2 2 -
c. CNS associated 0 2 -
d. others 2 6 -
5. Number of drugs
a. less than 5 60 31 1
b. 5 to 10 6 1 0
c. more than 10 1 0 0
6.average drugs prescribed per patient 2.7 2.01 2
7.Pharmacoeconomics analysis of antibiotics use in RDS
a. most commonly prescribed Amikacin Amikacin Amikacin and
cefotaxime c. cheapest combination prescribed Amikacin + cefotaxime Amikacin + cefotaxime --- d. average cost for cheapest combination
prescribed 120.77 ± 64.57 120.77 ± 64.57 120.77
e. costliest combination prescribed
Cefotaxime + gentamycin + piperacillin with tazobactam + Cefoperazone + meropenem
Amikacin + Cefoperazone + meropenem
--- f. average cost per patient 263.149 ± 295.971 232.84 ± 296.54 120.77
Table 2
Sr. No. Group Drug name
(Exposure)
Group A
n-67 % Group B n-31 %
Group C n-1
1. Antibiotics
Aminoglycosides
Amikacin 45 67.2 21 67.7 1
Tobramycin 5 7.5 0 0 0
Gentamycin 2 2.9 0 0 0
Netilmicin 2 2.9 0 0 0
cephalosporins Cefotaxime 27 40.3 7 22.6 1
Cefoperazone 18 26.8 11 35.5 0
Glycopepetideantibiotics Vancomycin 7 10.4 3 9.6 0
Meropenem 5 7.5 2 6.4 0
β- lactamases Piperacillin and
tazobactam 5 7.5 1 3.2 0
Quinolone ciprofloxacin 3 4.5 0 0 0
2. Coagulants Vitamin K 31 46.3 17 54.8 0
3. Respiratory
stimulants Methyl xanthine Caffeine citrate 17 25.3 0 0 0
4.
anti-epileptics Barbiturates Phenobarbitone 8 11.9 3 9.7 0
5. ant-acids H2 blockers Ranitidine 6 8.9 2 6.4 0
6. anti-emetics Prokinetics Domperidone 3 4.4 0 0 0
Metoclopramide 3 4.4 0 0 0
7. steroids Dexamethasone 3 4.4 0 0 0
8. health supplements
Multivitamin syrup 10 14.9 2 6.4 0
NS solution
(Bolus) 10 14.9 5 16.1 1
Human milk fortifiers 8 11.9 0 0 0
Aminoven 2 2,9 0 0 0
Vitamin D3 5 7.4 2 6.4 0
Multivitamin infusion 3 4.4 0 0 0
Iron 3 4.4 0 0 0
9. diuretics Loop diuretic Furosemide 1 1.5 0 0 0
10. others
Dobutamine 1 1.5 0 0 0
Dopamine 0 0 1 3.2 0
The general problem in Neonatal Intensive care unit (NICU) is that the efficacy and safety of drugs evaluated in adults usually applied to the neonates, such practices cause problems like off-label use of drugs, in-effective drug therapy, increase in mortality and morbidity, wastage of resources, with increase in cost treatment. Hence, drug utilization studies provide evidence-based data to be applied for improving their odds of survival even in premature infants.9
Results shown by some studies that, the anti-microbial agents are the most commonly used in NICU which are even not required and have inappropriate use up to 50% of times. Hence, rational drug use studies are most important part of pharmacological evaluation.10,11
Due to above stated reasons; we have planned DUS in NICU its prescription pattern and pharmaco-economic evaluation of drugs.
METHODS
A cross-sectional observational study was carried out for six months in NICU in Medical College and Hospital of Punjabafter approval by Institutional Ethics Committee. The data regarding patient demographics and drugs administered were collected daily in a structured performa. Patients were enrolled after taking written informed consent after informing the purpose of study. Patients of both gender who gave informed consent were included in the study. An exclusion criterion includes the patients who were discharged/died within 24 hours of admission and patients who did not gave the consent to be enrolled in study.
RESULTS
In this study, 100 patients datawas collected during the study period. Out of 100 patients, enrolled in study,67 patients were preterm (<37 weeks of gestation- Group A), 32 patients were of term age (37-42 weeks of gestation Group-B) and 1 was post term (>42 weeks of gestation Group-C). The characteristics of all the three groups are compared in table 1.
Among all the cases enrolled, the cases in group A showed that males were more frequently admitted than the females and the same trends were seen in group B and Group C. (Table1)
In comparison of groups, preterm babies had more duration of hospitalization (11.2 days) as compare to other 2 groups which showed (7 days) the similar results. (Table1)
Preterm had average weight around (1792 g ± 752.07 in males and 1358 g± 768.58 in females) and term pregnancies have (2780 g± 758.02 in males and 2467 g± 748.81 in females). (Table1)
The findings also suggest that RDS was major disease prevailing in preterm 63 out of 67 but only 22 from 32 term neonateswere suffering from RDS.So,focus of this study DUS was to find drugsutilized for treatment of RDS.
The average number of drugs prescribed in preterm (2.7) were much higher than the term (2.01) infants. In group C, there was one patient who was prescribed 2 drugs. (Table-1)
Amikacin, an antibiotic was the most commonly prescribed drug among all the groups, but the costliest drug prescribed in group A and group B was Cefoperazone. Among the antibiotic combinations most affordable were amikacin + cefotaxime to all the patients which only cost 120.77 ± 64.57per patient.
With addition of multi drugs the average treatment cost rose to 263.149 ± 295.971 in group A and 232.84 ± 296.54in group B as seen in table1.
Table 2 shows the Individual drug prescription in these 100 patients. Antibiotics were the most common prescribed drugs in which aminoglycosides were prescribed to almost every patient after that cephalosporins were second most commonly prescribed drugs.
As coagulants the vitamin k was used most commonly as compared to other drugs, which were used sparingly.Asmajority of patients were RDS patients so methyl xanthene was widely used as respiratory stimulants along with steroids in some patients.
Patients having CNS disorders were given phenobarbitone as antiepileptic and CNS depressant. Some of the patients were also given supplementsalong with the breast milk for proper development and growth which also have led to increased cost of treatment along with antibiotics.
DISCUSSION
Our study indicated male predominant pattern (71%), which is also consistent with the various DUS already conducted in India. In study conducted by Parkash J. et al12 in the Neonatal Unit of National Institute of Child Health, Karachi, Pakistan from 1st January 2001 to 31st December 2001showed 62.1%males. In another study conducted by Vaghela JP. et al in NICU of Guru Gobind Singh government Hospital, Gujrat from December 2013 to November 2014 showed 56% of the admissions were male 12Almost all DUS studies shows the same results of male predominance in the country mainly due to more access of medical services to male gender.
In our study patient predominance was maximum in preterm (67%) followed by term (32%) and post term (1%). Our results were in resemblance with other studies conducted in India. In a study conducted by Vaghela JP. et al in NICU of Guru Gobind Singh government Hospital, Gujrat from December 2013 to November 201413showed 56% admitted neonates were preterm. In another study conducted by Chaunakar SA. et al in NICU of a tertiary care hospital in Mumbai from July 2014 to March 2015 indicated 54.8% of admissions were preterms8. These indicating that preterm are more prone for admission in NICU than the term and post term neonates because preterm neonates require more medical care as compare to term.
Average length of stay of patients in our study in NICU is 11.2 days in preterm and 7 days of term patients. These results were comparable to the study conducted by Warrier at al. in Hutzel Women’s Hospital (HWH) in Detroit between January 1997 and June 2004, which was 15 days. Similarly, study conducted by Vaghela JP at Guru Gobind Singh Government Hospital, Gujarat showed the results of average stay is 15 days.12 It was slightly less in our study because of leaving against medical advice (LAMA) and discharge on request (DOR) or death of patients.
In drug prescribing pattern, average number of drugs prescribed to pre-term (2.7) were much higher than the term (2.01) patients. The antibiotics play a major role in this as most of the patients admitted were suffering from RDS and antibiotics play a major role in prophylaxis of infections in NICU. The most commonly used antibiotic was amikacin (aminoglycoside 67%) then followed by cefotaxime (cephalosporin 35%) and the most common combination prescribed among the antibiotics was of amikacin and cefotaxime which is also the cheapest among the antibiotics. These results were almostsimilar to the study of Amin AJ et al in the neonatal intensive care unit (NICU) at Sir Sayajirao Gaekwad Hospital, Vadodara from April 1st to September 31st, 2013 12
In European RDS treatment guidelines 2016recommend the antibiotics are often started in babies with RDS until sepsis has been ruled out, but policies should be in place to narrow the spectrum and minimize unnecessary exposure. A common regimen includes penicillin or ampicillin in combination with an aminoglycoside16. Antibiotics should be stopped as soon as possible once sepsis has been excluded, which matches our finding of the study. So, this study in our hospital shows that major of the RDS neonates were exposed to combination of two most preferred drugs for the treatment of RDS.
In 2003 the American Academy of Pediatrics recommended that vitamin K1 should be given to all neonates as a single, intramuscular dose of 0.5 to 1 mg, and this recommendation was recently reaffirmed in 200917,18 In our study second most common drug prescribed other than antibiotics is vitamin K (48%) which acts as predominant pro-coagulant. This is general practice to give vitamin-K as prophylaxis dose in new born. Some patients were also given respiratory stimulants.
This study also showed that only the 46% of patients were provided supportive treatment along with main treatment with anti-emetics, ant-acids, steroids and supplements, which also plays an important role in patient welfare.
A study conducted in Mumbai showed the average cost of hospitalization per neonate was 7383 INR. As preterm (<37 weeks) and low birth weight (<2.5 kg) neonates were exposed to significantly higher number of drugs, had longer hospital stay with overall cost of treatment compared to full term neonates (P <0.05), The average cost of antibiotics prescribed per patient in our hospital was263.149 ± 295.971 which is lower as per compared to another study 8. Main reason of this can be included to non-affordability of parents. But our study results8 were consistent to other studies that the average treatment cost is higher in preterm than term and then post term individuals.
The strength of our study is that it targets the drug utilization of three comparison groups especially in RDS with relevance
of antibiotics. Our study further explores the cost effectiveness of the treatment. The main limitation of our study was, it was short duration, conducted in 100 patients and in one center. More such studies should be conducted so that we can have drug utilization pattern in more patients which will generate more evidence in rational management of RDS.
CONCLUSION
In this study, we provide detailed and unique overview of drugs which are commonly used in patients admitted in NICU in northern hospitals. The data collected showed various similarities and important differences of drug usage and especially their pattern of usage in the hospital. As majority diseased population is RDS in preterm, so emphasis of our study was on the RDS group of patients and their drug use. Furthermore, as the antibiotic usage is more common and large number of antibiotics were prescribed, so pharmaco-economic analysis of antibiotics was done to find out cheapest and most effective therapy.
To summaries, there is lot of scope available for the further studies on this subject to improve and to rationalize the treatmenttopatients withspecialconsideration to the cost effectiveness as there is scarcity of resources in our developing country.
Funding/conflict of interests/ethical approval
Our study was not a sponsored study. There were no conflict of interests and the ethical approval was from the institutional ethical committee.
References
1. Introduction to Drug Utilization Research, WHO, 2013.
Available at
http://www.whocc.no/filearchive/publications/drug_util ization_research.pdf. Accessed 07 August 2018. 2. Capella D. Descriptive tools and analysis. In: Dukes
MNG. Editor. drug utilization studies methods and use. Copenhagen: WHO Regional Publications: 1993. p 55-78
3. Amin A., Shah P, Asari P, Malam P, Kalkoti, V, Behl, A. Drug utilization study of antimicrobial agents in patients of neonatal sepsis in neonatal intensive care unit at a tertiary care hospital in western part of India.International Journal of Basic & Clinical
Pharmacology, 2016:4(5), 895-902.
doi:http://dx.doi.org/10.18203/2319-2003.ijbcp20150862
4. World Health Organization. What is drug utilization research and why is it needed.Introduction to Drug Utilization Research. Oslo: World Health Organization. 2003:8-12.
5. Warrier I, Wei D, Girija N, Salari V, Aranda J. Patterns of Drug Utilization study in Neonatal Intensive care unit. J ClinPharmacol. 2006;46:449-55
6. European Medicines Agency decision - PDCO Meeting June 2015 86.European MedicinesAgency (EMA). http://www.ema.europa.eu/docs/en_GB/document_libra ry/ Other/2015/07/WC500190385.pdf Accessed on 12 Feb 2019
in a neonatalintensive care population. Am J Perinatol 23:279–85
8. Smyth RL,Weindling AM. Research in children: ethical and scientific aspects. Lancet1999; 354(2), SII21– 4. doi:10.1016/s0140-6736(99)90253-2
9. Chauthankar SA, Marathe PA, Potey AV, Nanavati RN, Drug utilization in neonatal intensive care unit of a tertiary-care hospital in Mumbai, India, Indian Pediatrics, 2017: 54 (11) 931-34
10. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit:date from a large national data set. Pediatrics 2006; 117: 1979-87.
11. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013 report. Available at:https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf#page=11 .(Accessed February 13, 2019).
12. Parkash J, Das N. Pattern of admissions to neonatal unit. J Coll Physicians Surg Park. 2005;15(6):341-4.
13. Vaghela JP, Sukhlecha A. Drugutilization study in neonatal intensive care unit of atertiary care teaching hospital. Int J Basic ClinPharmacol2017;6:2510-5. 14. Uppal R, Chhabra A, Narang A. Pattern of Drug Use in
neonatal intensive care unit. Indian Pediatr. 1998;35:647-9.
15. Choure, M. K., R. R. Jadhav, and S. L. Padwal. “drug utilization study in neonatal intensive care unit at rural tertiary care hospitaL.”Asian Journal of Pharmaceutical and Clinical Research, Vol. 10, no. 4, Apr. 2017, pp. 102-4, doi:10.22159/ajpcr.2017.v10i4.16111.
16. Sweet D G, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R et al: European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology 2017;111:107-125. doi: 10.1159/000448985
17. American Academy of Pediatrics Committee on Fetus and Newborn. Controversies concerning vitamin K and the newborn. Pediatrics. 2003;112:191–2.
18. American Academy of Pediatrics. Policy
Statement-AAP publications retired and
reaffirmed. Pediatrics. 2009;124:845.
How to cite this article:
Dr. Jatinder Singh et al (2019) 'Drug Utilization Study in Patients of Neonatal Intensive Care Unit of Tertiary Care Hospital in Punjab, India', International Journal of Current Advanced Research, 08(05), pp. 18607-18611.
DOI: http://dx.doi.org/10.24327/ijcar.2019.18611.3562