Beckman Coulter Gallios* Flow Cytometer and the
Detection of Micro-particles
Authors: T. Matt Holl, PhD; Karen Carr, PhD; John Mattachini; Frank Day Affiliation: Beckman Coulter, Inc. - Flow Cytometry
a Beckman Coulter Life Sciences:
Beckman Coulter Gallios* Flow Cytometer and the
Detection of Micro-particles
PRINCIPLE OF THE TECHNIQUE
Background:
Flow cytometry is routinely being used as a solution to detect small (~1-3 μm) particles and micro-particles (<1.0 μm). Using our newly innovated FSC detection system, the Gallios flow cytometer permits a wide dynamic range of small and micro-particle detection. The Gallios analyzer permits high sensitivity of fluorescence detection; therefore, sub-micron particle detection can be further enhanced using a fluorescence-based discriminator. Together, the Gallios is a superb platform for the identification and characterization of biological small and micro-particles. This technical publication is designed as a resource for investigators performing small and micro-particle research on the Gallios platform.
RESEARCH APPLICATIONS
Introduction:
Micro-particles (MP) are derived from outward protrusion and fission of plasma-membrane which is then released into the extracellular environment. Multiple signaling pathways (e.g. mobilization of intracellular calcium) must be activated and the coordinated reorganization of the cell’s cytoskeleton must occur for micro-particles to form. The formation and release of micro-particles often results in the exposure of phosphatidylserine (PS), a phospholipid normally located on the cytosolic side of cell membranes, to the surface of the vesicle by an enzyme called flippase [1]. This PS exposure has been well defined by studying cells as they undergo apoptosis [1] and is visualized by reactivity with fluorochrome-labeled AnnexinV reagents. The origin of micro-particle populations can be determined as the vesicle membrane is enriched with specific lipids and proteins reflecting their cellular progenitor [2].
Recently, biological micro-particles have been the focus of basic science and clinical research studies [3, 4]. Some areas of focus where active micro-particle investigation is currently ongoing include: thrombosis/ thromboembolism [5], Sickle cell anemia crisis [6], heart failure [7] and oncology [8].
However, a consensus on standardization by investigators is early in its development and heavily debated. These discussions to address standardization are often centered on i) flow cytometry instrumentation hardware/software, ii) particle size estimation protocols and iii) sample handling/preparation techniques. Fortunately, there have been strong attempts to establish and characterize standardization protocols that give investigators an initial reference point [10]. As with most early scientific efforts, these guidelines are sure to be refined as instrumentation and protocols become more sensitive and refined [9].
Until recently, many flow cytometry instruments lacked the sensitivity to detect particles below the 1μm threshold - a value commonly agreed upon to be the high end of micro-particle size. With an innovated Forward Angle Light Scatter detector, the Gallios flow cytometer has been optimized to detect micro-particles using strategically chosen FSC detector modes. The Narrow mode (N = 1-8°) is ideal for larger cells, the Wide mode (W = 1-19°) is optimized for smaller cells, and the Enhanced Wide mode (W2 = 1-19° with log amplification) allows the amplification of wide angle light scatter independent of low angle light scatter. As we will demonstrate here, using the Gallios light scatter W2 angle mode, researchers can greatly increase the resolution of micro-particle detection from background noise and similarly sized particles.
PROTOCOL
Standard Procedure:
Ensuring Instrumentation Alignment and Sensitivity
SPHERO Rainbow Calibration Particles (Spherotech, Inc. Cat# RCP-60-5) (6-peak) were used according to manufacturer’s recommended protocol. Gallios instrument voltages were set so that the “first peak” was fully visible on scale to accurately determine the “staining index” (SI). SI are useful measures of population discrimination [11, 12] as they provide intensity values normalized by the width of the background population
(MFI=Mean Fluorescence Intensity, SD = Standard Deviation): SI= (MFIpositive – MFIbackground) / (2 xSDbackground).
Flow-Check Pro beads (Cat. No: A69183) were used to ensure optimal system performance as per BCI standard quality control methods. The half-peak CV (HP-CV) of <2.0 for 488nm laser and <3.0 for the 638nm laser ensures that fluidics stability and laser alignment are optimal.
Establishing a “Size Reference Ladder”
Flow-Check Pro (10 μm, 6.0 μm and 3.0 μm beads) spiked with 1.0 μm and 0.5 μm latex beads (BCI Cat. No.s: A69183, 6602790 and 6602788 respectively) were used to characterize the dynamic range of particle detection under a single set of scatter and PMT voltages. Water only controls were used to identify “background” signals and identify instrument electronics noise.
All data was acquired using FSC parameter discrimination. For N and W FSC detection, 1.0 μm latex
beads and Flow-Check Pro fluorospheres were used to illustrate “small particle” identification. For W2 FSC
detection, 1.0 μm and 0.5 μm beads were used to estimate sub-micron particle detection sensitivity.
Using Fluorescence Discrimination to Increase Detection Sensitivity
Megamix beads (Biocytex, Cat. # 7801) and 0.21 μm Dragon Green beads (Bangs Laboratories, Inc., Cat.# CP01F/10772) were used according to manufacturer’s recommended protocol. Data was acquired
using FL1 (FITC) parameter discrimination and set for W2 FSC detection.
Characterization of Biological Microparticles
Human platelets were bulk isolated from normal donor peripheral blood samples [6] and treated in vitro with LPS to induce micro-particle formation . Micro-particles from culture supernatant were isolated by differential centrifugation [6]. Micro-particles were also isolated directly from human peripheral blood samples by centrifugation (low rcf) to remove intact cells and subsequently (at higher rcf) to enrich micro-particles for analysis [9]. All micro-particle enriched samples were labeled with AnnexinV-FITC (BCI Cat. No.: IM3546) and anti-human CD41-APC (BCI Cat. No.: B16894) to detect platelet-derived fractions. FMO controls were
performed to ensure specificity of reagent binding (data not shown). Gallios cytometer was setup using W2
FSC setting with FITC discriminator. AnnexinV-FITC+ events were analyzed by FSC/SSC to identify <1.0 μm
RESULTS
Figure 1: Proper maintenance of Gallios
flow cytometer permits high sensitivity of fluorescence detection.
(A) Multi-intensity beads were used demonstrate high sensitivity of FITC (SI=9.5) and APC (SI=6.4) channel detection.
(B) Flow-Check™ Pro beads were used to measure half-peak CVs (HP-CV) values for FITC and APC detection. Combined, these data demonstrate (i) high sensitivity of fluorescence detection, (ii) proper laser alignment and (iii) stability of system fluidics.
Figure 2: FSC detection angle controls
dynamic range of small particle detection.
The Gallios flow cytometer can detect forward light scatter using Narrow (1-8°), Wide (1-19°) and Enhanced Wide (1-19° with log amplification) settings. All data was generated using FSC parameter discrimination. For N and W FSC detection, 1.0 μm latex beads and Flow-Check Pro fluorospheres (10 μm (B), 6 μm (C) and 3 μm (D) beads) were used to illustrate “small particle” identification.
For W2 FSC detection, 1.0 μm and
0.5 μm beads were used to demonstrate sub-micron particle detection. Water only controls were used to identify “background” signals.
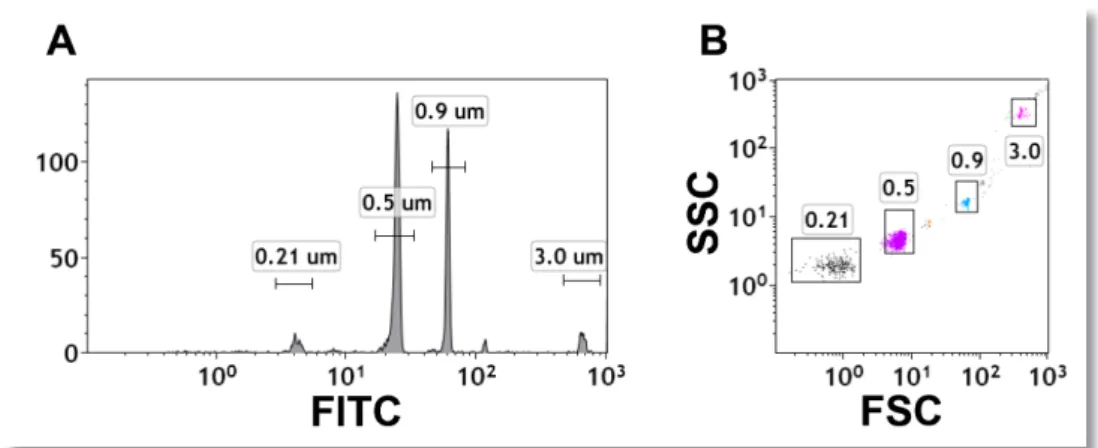
Figure 3: Enhanced sub-micron particle detection using fluorescence discriminator.
(A) Megamix beads were spiked with 0.21 μm fluorescent beads and acquired on the Gallios flow cytometer
(W2 FSC, FITC discriminator).
(B) FSC and SSC parameters were shown for all events to illustrate increased sensitivity to 200 nm detection.
Discussion
Here we present data that demonstrates the capability of the Gallios W2 FSC mode to detect sub-micron
particles. Our set-up included acquiring 6-Peak Spherotech Rainbow Calibration Particles to confirm the sensitivity and performance of the Gallios instrument (Figure 1). We set instrument PMT voltages so that the “first peak” of the beads was fully visible on scale and obtained accurate SI measurements for each fluorescent channel used. These values can be followed over time and used as an additional standardization tool to track instrument sensitivity/resolution. Flow-Check Pro beads were used to ensure optimal system performance as per BCI standard quality control methods. The half-peak CV (HP-CV) of <2.0 for 488 nm laser PMTs and <3.0 for the 638 nm laser PMTs ensures that fluidics stability and laser alignments are optimal. These HP-CV values represent the upper limit of permissible variation for system quality purposes; however, investigators should take all appropriate steps to reduce HP-CV values to their lowest reasonable levels for applications that require the greatest sensitivity and stability (e.g. micro-particle detection).
Flow-Check Pro beads were used with 1.0 μm Latex particles to create a Size Reference Ladder. The acquisition of Flow-Check Pro beads spiked with 1.0 μm Latex particles (acquired using N or W FSC modes) illustrates that the Gallios is highly sensitive to a dynamic range of small particle detection (Figure 2). To
focus on sub-micron particle detection, 0.5 μm and 1.0 μm Latex particles were detected with the W2 FSC
mode and were clearly visible above the instrument noise (Figure 2). These data demonstrate that the Gallios instrument can be easily configured to detect 0.5 μm to 10 μm particles solely based on light scatter discrimination.
In order to determine the ability of the Gallios to resolve particles less than 0.5 μm, a fluorescent discriminator was used to acquire Megamix beads spiked with 0.21 μm Dragon Green beads. The discriminator threshold was set to exclude instrument noise (diH20, data not shown) and these small, fluorescent particles were acquired. These data suggest that the Gallios instrument is highly capable of resolving 0.21 μm beads from background noise (Figure 3).
In order to confirm that these standardization processes were appropriate for the set-up of biological samples, we characterized human platelet micro-particles and platelet-free plasma. Prior to acquisition of the platelet micro-particles, Megamix beads and FMO controls were utilized to establish a gating strategy. The platelet micro-particles and platelet-free plasma samples were stained with CD41 and Annexin V (Figure 4). All biological samples were acquired using a FITC (FL-1) discriminator. The direct correlation of AnnexinV and CD41 (Figure 4A) seems logical based on the biological constraints of micro-particle formation. Furthermore, the staining pattern observed in plasma samples indicates that the micro-particles present in
REFERENCES
[1] Verhoven B, Schlegel R, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med 1995 Nov;182 (5): 1597-601.
[2] Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends in Cell Biology (2009).19 (2): 43-51.
[3] Piccin A, Murphy W, Smith O. Circulating microparticles: pathophysiology and clinical implications. Blood Reviews 2007; 21: 157-171.
[4] Morel O, Morel N, Jesel L, Freyssinet JM, Toti F. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin. ImmunoPath 2011; 33(5): 469-486. [5] Kasthuri R, Glover S, Boles J, Mackman N. Tissue Factor and Tissue Factor Pathway Inhibitor as key
regulators of global hemostasis: Measurement of their levels in coagulation assays. Semin Thromb Hemost 2010; Oct; 36(7): 764-771.
[6] Shet A, Aras O, Gupta K, Hass M, Rausch D, Saba N, Koopmeiners L, Key N, Hebbel R. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 2003; Oct 102 (7): 2678-2683.
[7] Garcia S, Chirinos J, Jimenez J, Del Carpio Munoz F, Canoniero M, Jy W, Jimenez J, Horstman L, Ahn Y. Phenotypic assessment of endothelial microparticles in patients with heart failure and after heart transplantation: switch from cell activation to apoptosis. J Heart Lung Transplant. 2005;24:2184–2189. [PubMed: 16364869].
[8] Toth B, Nieuwland R, Liebhardt S, Ditsch N, Steinig K, Stieber P, Rank A, Gohring P, Thaler CJ, Friese K. Circulating microparticles in breast cancer patients: a comparative analysis with established biomarkers. Anticancer Res. 2008; 28:1107–1112. [PubMed: 18507061].
[9] Orozoco A and Lewis D. Flow cytometric analysis of circulating micro-particles in plasma. Cytometry A. 2010 June; 77(6): 502-514.
[10] LaCroix R, Robert S, Poncelet P, Kasthuri R, Key N, Dignet-George F. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemo 2010; 8: 2571-2574. [11] Bigos M, Stovel R, Parks D. Evaluating multi-color fluorescence data quality among different instruments
and different laser powers-methods and results (abstract). Cytometry 2004; 59A: 42.
[12] Maecker HT, Frey T, Nomura LE, Trotter J. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A. 2004 Dec; 62(2): 169-73.
[13] Brown GT and McIntyre TM. Lipopolysaccharide signaling without a nucleus; Kinase cascades stimulate platelet shedding of proinflammatory IL-1β-rich microparticles. J Immunol 2011; 186: 5489-5496.
REAGENT DETAILS
Reagent Supplier Order Details
6-peak SPHERO Rainbow Calibration Particles Spherotech, Inc. RCP-60-5
Flow-Check™ Pro beads Beckman Coulter A69183
1.0 μm latex beads Beckman Coulter 6602790
0.5 μm latex beads Beckman Coulter 6602788
Megamix beads Biocytex 7801
0.21 μm Dragon Green beads Bangs Labs, Inc. CP01F/10772
AnnexinV-FITC Beckman Coulter IM3546
CD41-APC Beckman Coulter B16894
NOTES
The results demonstrated in this application sheet represent those generated on the Beckman Coulter Gallios Flow Cytometer. As differences exist in the performance between analyzers, the author cannot guarantee a similar appearance with the use of other flow cytometers.
Beckman Coulter, Inc. Gallios Flow Cytometer (3 laser, 10-color system using standard filter configuration) was used to perform these studies. Special Thanks to Nancy Fisher PhD (University of North Carolina) and Micah Mooberry MD (University of North Carolina) and Bob Zucker PhD (USEPA) for their input, time and samples.