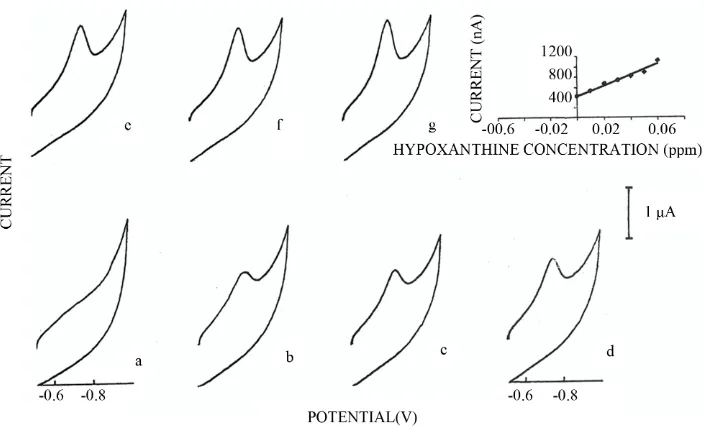
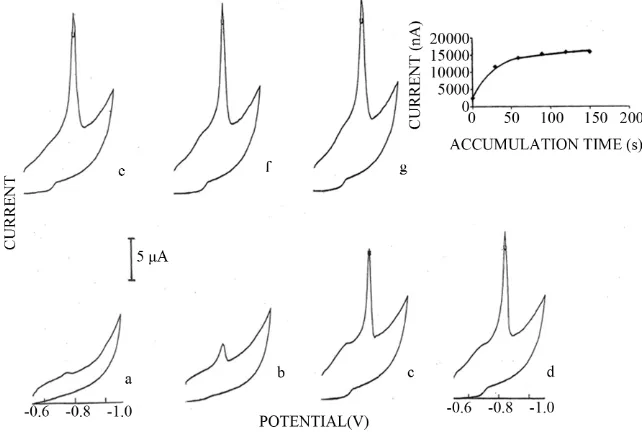
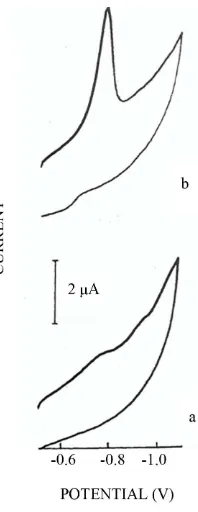
Determination of Hypoxanthine in the Presence of Copper by Adsorptive Stripping Voltammetry
Full text
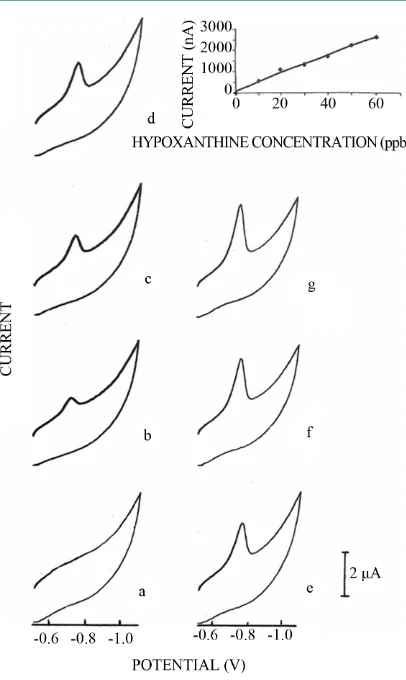
Figure
Related documents
"Insight therapies of all types address themselves to the rationality of the patient and behavioural therapies direct their operations to ths sensori- motor
In addition, this study embedded micro temperature sensors in the cathode flow field plate of fuel cell stack successfully, the temperature calibration curves of micro
The position to be adopted by the Community within the ACP-EC Committee of Ambassadors relating to the methods for calculating a reserve intended to finance decisions under
The cyclic voltammetry results of 2 M V(IV) in 3M H2SO4 electrolyte with various additive amounts showed the electrochemical activity of the electrolyte with 3% Tris
From the results obtained in this study, it can be suggested that iodide ions (I - ) were initially adsorbed on the metal surface and the components of leaf extracts of
The crystal planes are identified by comparing the peak positions with the standard data (JCPDS card no. Very broad peaks are obtained in the XRD pattern of
Cyclic voltammetric response for: (a) bare carbon paste electrode (b) carbon paste electrode modified with Ni(II) complex and (c) b in the presence of different
SnO2 exhibits a smaller size (below 10 nm) and a more homogeneous dispersion on graphene using Sn2+ as the precursor compared with the case using Sn4+ as the precursor..