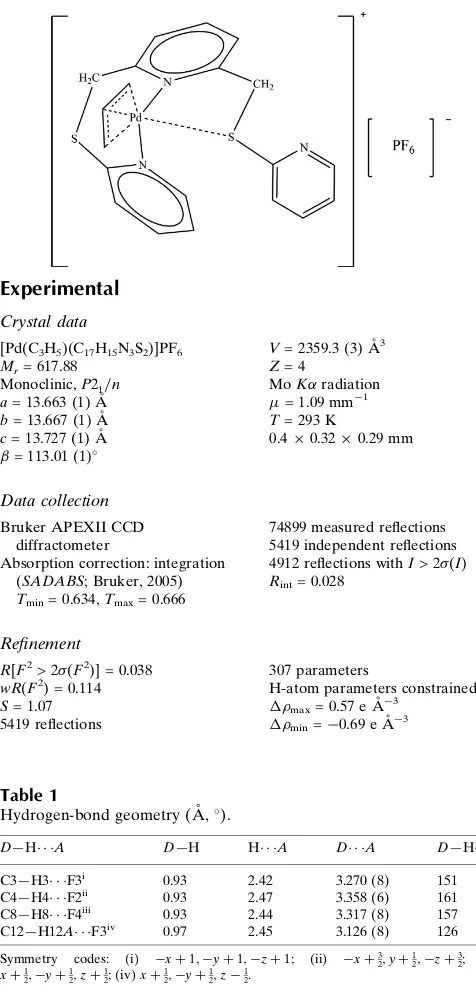
{2,6 Bis[(pyridin 2 yl)sulfanylmethyl]pyridine κ2N,N′}(η3 prop 2 enyl)palladium(II) hexafluorophosphate
Full text
Figure

Related documents
Natural Occurring Radioactive Materials and man-made Cs-137 as well as the derived radiological hazards have being investigated in soil used for agriculture and build- ing
Abstract: Every organization wants to achieve higher position in the market and higher position is achieved by increasing revenue and gaining reputation in the
The variables studied were the FDI, the human capital, the literacy ratio, the trade openness, the natural resources, the market size, the democratic institutions,
Of the physicians surveyed, 85% reported that they took adequate time to answer patient questions and 83% felt that they spent adequate time reviewing the entire discharge plan
Amino acid identity/similarity analysis between fish IL-4/13 molecules, tetrapod IL-4 and IL-13, and other γ-chain cytokines revealed the salmonid IL-4/13A and B isoforms to
Table 1 shows how the PSNR values increased on average by more than 3% for both reconstruction algorithms (FBP and OSEM) compared to the bicubic interpolation method.. For the
There are other examples of malformative disorders being re- lated to local mechanical factors, for example, arachnoid cysts with surrounding brain hypoplasia and Chiari I
Compared with a baseline of reports finalized by junior neuroradiologists and adjusting for the pres- ence of a CF flag, missed finding, examination time, imaging tech-