{9-Hexyl-2-[2-phenyl-6-(pyridin-2-yl)-pyridin-4-yl]-9
H
-carbazole}diiodidozinc
Hui Wang, Xue-Song Zhao, Jun-Shan Luo and Yu-Peng Tian*
Department of Chemistry, Anhui University, Hefei 230039, People’s Republic of China, and Key Laboratory of Functional Inorganic Materials, Chemistry, Hefei 230039, People’s Republic of China
Correspondence e-mail: yptian@ahu.edu.cn
Received 9 August 2013; accepted 13 September 2013
Key indicators: single-crystal X-ray study;T= 296 K; mean(C–C) = 0.009 A˚; Rfactor = 0.039;wRfactor = 0.122; data-to-parameter ratio = 17.2.
In the title compound, [ZnI2(C34H31N3)], the Zn II
atom is four-coordinated by two I atoms and the pyridine N atoms from the bidentate 60-phenyl-2,20-bipyridine ligand in a
distorted tetrahedral geometry.
Related literature
For the synthesis of the title compound and related structures, see: Alizadehet al.(2009); Gaoet al.(2009); Prokhorovet al.
(2011).
Experimental
Crystal data
[ZnI2(C34H31N3)]
Mr= 800.79
Monoclinic,P21=n
a= 15.3870 (14) A˚ b= 9.8771 (9) A˚ c= 21.3246 (19) A˚
= 99.306 (1)
V= 3198.2 (5) A˚3
Z= 4
MoKradiation
= 2.73 mm1
T= 296 K
0.220.220.21 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2002) Tmin= 0.586,Tmax= 0.598
23438 measured reflections 6226 independent reflections 5325 reflections withI> 2(I) Rint= 0.022
Refinement
R[F2> 2(F2)] = 0.039
wR(F2) = 0.122
S= 1.04 6226 reflections
362 parameters
H-atom parameters constrained
max= 1.10 e A˚
3
min=0.92 e A˚
3
Data collection:SMART(Bruker, 2002); cell refinement:SAINT
(Bruker, 2002); data reduction:SAINT; program(s) used to solve structure:SHELXS97(Sheldrick, 2008); program(s) used to refine
structure: SHELXL97 (Sheldrick, 2008); molecular graphics:
SHELXTL(Sheldrick, 2008); software used to prepare material for publication:SHELXTL.
This work was supported by the National Natural Science Foundation of China (21071001, 21271004).
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HP2061).
References
Alizadeh, R., Kalateh, K., Khoshtarkib, Z., Ahmadi, R. & Amani, V. (2009). Acta Cryst.E65, m1439–m1440.
Bruker (2002).SMARTandSAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
Gao, Y. H., Wu, J. Y., Li, Y. M., Sun, P. P., Zhou, H. P., Yang, J. X., Zhang, S. Y., Jin, B. K. & Tian, Y. P. (2009).J. Am. Chem. Soc.131, 5208–5213. Prokhorov, A. M., Slepukhin, P. A., Rusinov, V. L., Kalinin, V. N. &
Kozhevnikov, D. N. (2011).Chem. Commun.47, 7713–7715. Sheldrick, G. M. (2002).SADABS. University of Go¨ttingen, Germany. Sheldrick, G. M. (2008).Acta Cryst.A64, 112–122.
Acta Crystallographica Section E
Structure Reports Online
supporting information
Acta Cryst. (2013). E69, m553 [doi:10.1107/S1600536813025464]
{9-Hexyl-2-[2-phenyl-6-(pyridin-2-yl)pyridin-4-yl]-9
H
-carbazole}diiodidozinc
Hui Wang, Xue-Song Zhao, Jun-Shan Luo and Yu-Peng Tian
S1. Comment
In recent years, 6′-phenyl-2,2′-bipyridine based materials have attracted considerable interests because they have
significant applications in optoelectronic functional materials (Prokhorov et al., 2011). In addition, zinc complexes are particularly attractive and most studied for their biocompatibility (Gao et al., 2009). Herewith, in this study, we report the crystal structure of the title compound (I).
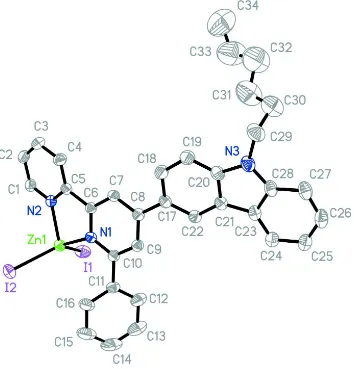
In (I) (Fig.1), the ZnII atom is four-coordinated by two I atoms and the N atoms from 6′-phenyl-2,2′-bipyridine rings in a
distorted tetrahedral geometry and with the coordinated pyridine moities oriented in an almost coplanar fashion with a
dihedral angle of 12.68 (1)°, which is larger than what is reported in the literature, with formula [ZnCl2(C12H12N2)] (II)
(7.57°) (Alizadeh et al., 2009), the reason is that the introduction of benzene increases steric hindrance. Zn—I bond distances are 2.5396 (6) and 2.5623 (6) Å, which are within normal range. Compared to (II),the distances of Zn—N are a
little larger. I—Zn—I and N—Zn—N bond angles are 118.56 (2)° and 80.1 (1)°, which is smaller than that of (II),
respectively.
S2. Experimental
A solution of 9-hexyl-2-(2-phenyl-6-(pyridin-2-yl)pyridin-4-yl)-9H-carbazole (0.48 g, 1 mmol) in methanol (20 ml) was mixed with a zinc iodide (0.32 g, 1 mmol) in methanol (5 ml) and the reaction mixture was reflux for 4 h. The reaction
mixture was cooled to room temperature and filtered into a large test tube. The light yellow crystals were obtained at
room temperature after a week. Yield: 85%. 1H NMR (400 MHz, DMSO-d6) 8.89(s, 1H), 8.78–8.80(t, 2H), 8.66–8.68(d,
1H), 8.38–8.44 (m, 4H), 8.03–8.10 (q, 2H), 7.76–7.78 (d, 1H), 7.49–7.66 (q, 6H), 7.25–7.29 (t, 1H), 4.39–4.42 (t, 2H),
1.76–1.82 (q, 2H), 1.19–1.32 (q, 6H), 0.79–0.82 (t, 3H).
S3. Refinement
All hydrogen atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C
Figure 1
The molecular structure of (I), with the atom-numbering scheme. Displacement ellipsoids are drawn at the 30%
probability level. H atoms have been omitted.
{9-Hexyl-2-[2-phenyl-6-(pyridin-2-yl)pyridin-4-yl]-9H-carbazole}diiodidozinc
Crystal data
[ZnI2(C34H31N3)]
Mr = 800.79 Monoclinic, P21/n
Hall symbol: -P 2yn
a = 15.3870 (14) Å
b = 9.8771 (9) Å
c = 21.3246 (19) Å
β = 99.306 (1)°
V = 3198.2 (5) Å3
Z = 4
F(000) = 1568
Dx = 1.663 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 9968 reflections
θ = 2.3–26.0°
µ = 2.73 mm−1
T = 296 K Block, yellow
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
phi and ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 2002)
Tmin = 0.586, Tmax = 0.598
23438 measured reflections 6226 independent reflections 5325 reflections with I > 2σ(I)
Rint = 0.022
θmax = 26.0°, θmin = 1.5°
h = −18→18
k = −12→11
l = −23→26
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.039
wR(F2) = 0.122
S = 1.04 6226 reflections 362 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0673P)2 + 4.6302P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.020 Δρmax = 1.10 e Å−3 Δρmin = −0.92 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full
covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
H34C −0.4687 1.1088 0.1061 0.324* I1 0.23267 (2) 0.40572 (4) −0.090212 (16) 0.07186 (13) I2 0.27913 (2) 0.09387 (3) 0.057852 (19) 0.07051 (13) N1 0.1767 (2) 0.4681 (3) 0.07773 (14) 0.0428 (7) N2 0.0721 (2) 0.2792 (3) 0.01525 (15) 0.0450 (7) N3 −0.0309 (3) 1.1340 (4) 0.2530 (2) 0.0645 (10) Zn1 0.20504 (3) 0.31593 (5) 0.01641 (2) 0.04805 (14)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
N2 0.0466 (17) 0.0403 (16) 0.0478 (17) 0.0002 (13) 0.0066 (14) −0.0011 (13) N3 0.073 (2) 0.058 (2) 0.067 (2) 0.010 (2) 0.026 (2) −0.0115 (19) Zn1 0.0455 (3) 0.0479 (3) 0.0521 (3) 0.00290 (19) 0.01186 (19) −0.0046 (2)
Geometric parameters (Å, º)
C1—N2 1.342 (5) C20—C21 1.416 (6) C1—C2 1.370 (6) C21—C22 1.382 (6) C1—H1 0.9300 C21—C23 1.447 (6) C2—C3 1.376 (7) C22—H22 0.9300 C2—H2 0.9300 C23—C24 1.392 (7) C3—C4 1.383 (6) C23—C28 1.407 (6) C3—H3 0.9300 C24—C25 1.379 (7) C4—C5 1.382 (6) C24—H24 0.9300 C4—H4 0.9300 C25—C26 1.390 (8) C5—N2 1.346 (5) C25—H25 0.9300 C5—C6 1.481 (5) C26—C27 1.371 (8) C6—N1 1.362 (5) C26—H26 0.9300 C6—C7 1.376 (5) C27—C28 1.408 (7) C7—C8 1.402 (6) C27—H27 0.9300 C7—H7 0.9300 C28—N3 1.390 (7) C8—C9 1.400 (6) C29—N3 1.454 (7) C8—C17 1.477 (5) C29—C30 1.499 (12) C9—C10 1.389 (5) C29—H29A 0.9700 C9—H9 0.9300 C29—H29B 0.9700 C10—N1 1.348 (5) C30—C31 1.429 (14) C10—C11 1.482 (6) C30—H30A 0.9700 C11—C16 1.382 (7) C30—H30B 0.9700 C11—C12 1.393 (7) C31—C32 1.520 (16) C12—C13 1.394 (8) C31—H31A 0.9700 C12—H12 0.9300 C31—H31B 0.9700 C13—C14 1.363 (11) C32—C33 1.439 (17) C13—H13 0.9300 C32—H32A 0.9700 C14—C15 1.357 (11) C32—H32B 0.9700 C14—H14 0.9300 C33—C34 1.416 (17) C15—C16 1.404 (8) C33—H33A 0.9700 C15—H15 0.9300 C33—H33B 0.9700 C16—H16 0.9300 C34—H34A 0.9600 C17—C22 1.399 (6) C34—H34B 0.9600 C17—C18 1.411 (6) C34—H34C 0.9600 C18—C19 1.382 (6) I1—Zn1 2.5396 (6) C18—H18 0.9300 I2—Zn1 2.5623 (6) C19—C20 1.389 (7) N1—Zn1 2.084 (3) C19—H19 0.9300 N2—Zn1 2.074 (3) C20—N3 1.380 (6)
C18—C17—C8 119.8 (4) H34A—C34—H34C 109.5 C19—C18—C17 121.7 (4) H34B—C34—H34C 109.5 C19—C18—H18 119.1 C10—N1—C6 118.1 (3) C17—C18—H18 119.1 C10—N1—Zn1 128.1 (3) C18—C19—C20 118.5 (4) C6—N1—Zn1 113.0 (2) C18—C19—H19 120.8 C1—N2—C5 119.2 (4) C20—C19—H19 120.8 C1—N2—Zn1 126.4 (3) N3—C20—C19 129.8 (4) C5—N2—Zn1 114.0 (2) N3—C20—C21 109.3 (4) C20—N3—C28 108.6 (4) C19—C20—C21 120.9 (4) C20—N3—C29 126.5 (5) C22—C21—C20 119.8 (4) C28—N3—C29 125.0 (5) C22—C21—C23 133.9 (4) N2—Zn1—N1 80.09 (12) C20—C21—C23 106.3 (4) N2—Zn1—I1 110.98 (9) C21—C22—C17 120.0 (4) N1—Zn1—I1 113.09 (9) C21—C22—H22 120.0 N2—Zn1—I2 103.73 (9) C17—C22—H22 120.0 N1—Zn1—I2 121.83 (9) C24—C23—C28 119.2 (4) I1—Zn1—I2 118.56 (2) C24—C23—C21 134.1 (4)