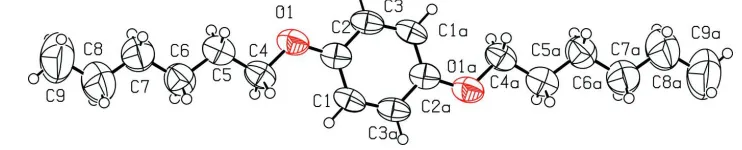
1,4 Bis(hexyloxy)benzene
Full text
Figure
Related documents
ABBREVIATIONS: ASPECTS ⫽ Alberta Stroke Program Early CT Score; CTO ⫽ carotid terminus occlusion; ECASS ⫽ European Cooperative Acute Stroke Study; IA ⫽ intra-arterial; EMS ⫽
It can be concluded that administration of curcumin, garlic, fenugreek, parsley, peppermint, pomegranate, propolis, olive leaves, rosemary, and sesame showed a remarkable
Watanabe, “A Phase II Trial of Neoadjuvant Preoperative Chemoradiotherapy with S-1 plus Irinotecan and Radiation in Patients with Locally Advanced Rectal Cancer: Clinical
DOI: 10.4236/ajps.2017.812216 3214 American Journal of Plant Sciences Table 6 shows that except for hilling alone at 6, 7, and 8 WAP, metribuzin alone at all tested rates, with
Ainsi, les importations de pétrole brut et de feedstocks (produits pétroliers semi-raffinés destinés à une distillation ultérieure dont sont exclues les importations de
The relationships between the application rate of the compost and the fresh and dry matter yield for grass grown in the Tertiary and Quaternary substrates after treatment with
Academic autonomy is a founding principle in higher schools governance, its essence being that the government recognizes the academic freedom, self-government and inviolability of
Foreign aid and sustainable inclusive human development in Africa.. Asongu, Simplice and