Acta Cryst.(2003). E59, m759±m761 DOI: 10.1107/S1600536803017835 Shuhua Renet al. Na8[CoMo4O12(C2H3O7P2)2]18H2O
m759
metal-organic papers
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
A new tetramolybdenumcobaltdiphosphonate,
Na
8[CoMo
4O
12{MeC(O)(PO
3)
2}
2]
18H
2O
Shuhua Ren,aGanglin Xue,a* Jun
Li,aQizhen Shi,aDaqi Wangb
and Jiwu Wangc
aDepartment of Chemistry/Shaanxi Key
Laboratory of Physico-Inorganic Chemistry, Northwest University, Xian, Shaanxi 710069, People's Republic of China,bDepartment of Chemistry, Liaochen University, Liaochen, Sandong 252059, People's Republic of China, andcDepartment of Chemistry, Yanan University, Yanan, Shaanxi 716000, People's Republic of China
Correspondence e-mail: xglin707@163.com
Key indicators Single-crystal X-ray study T= 298 K
Mean(C±C) = 0.007 AÊ H-atom completeness 15% Rfactor = 0.036 wRfactor = 0.069
Data-to-parameter ratio = 15.0
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2003 International Union of Crystallography Printed in Great Britain ± all rights reserved
A new tetramolybdate-diphosphate compound with the formula Na8[CoMo4O12{MeC(O)(PO3)2}2]18H2O,
octa-sodium(I) cobalt(II) tetramolybdenum(VI) bis(1-hydroxy-ethylidenediphosphate) octadecahydrate, has been synthesized under weakly acidic conditions (pH = 5.5±6.0). The [CoMo4O12{MeC(O)(PO3)2}2]8ÿ anion comprises two
[Mo2O6{MeC(O)(PO3)2}]5ÿ fragments linked by one
six-coordinate CoII atom. 1±Hydroxyethylidenediphosphonate
as a pentadentate ligand bonds to two pairs of face-sharing MoO6octahedra. The Co atom in the anion is situated on a
centre of inversion.
Comment
In recent years, metal organophosphonate systems have received considerable attention because of their unusual structural chemistry and their applications as sorbents, cata-lysts and catalyst supports, and ion exchangers (Rodrigues, 1986; Rosenthal & Caruso, 1992; Burwellet al., 1992; Cao & Mallouk, 1993). Furthermore, the organophosphonate group has also been shown to form molecular anion clusters with molybdenum (Kwaket al., 1975; Stalick & Quicksall, 1976). In this kind of compound, it is normal to form Mo5(Yayasakiet
al., 1987; Finn & Zubieta, 2001), Mo6(Yayasaki et al., 1987;
Kortz & Pope, 1995; Caoet al., 1993; Khanet al., 1993), Mo7
(Yayasakiet al., 1987; Dumaset al., 2002) polyoxomolybdates, the MoO6 octahedra being linked by edge-sharing or
angle-sharing.
The H+/MoO
42ÿ/hedp (hedp =
1-hydroxyethylidenedi-phosphonate) acid system has been investigated previously (Tolkacheva et al., 1992). The complexes are formed with Mo:L (L = hedp) ratios of 1:2, 2:2, 2:1, 3:1 and 6:1, mainly depending on pH. A few transition metal±hedp complexes have been structurally characterized, including M(hedpH2)2
(M= Ni, Fe),M2(hedpH2)2(M= Cu, Fe) andM3(hedp)2(M=
Cu) (Zheng et al., 2002). A number of one-dimensional lanthanide±hedpHn(n= 1±3; Nash et al., 1998) compounds
have also been structurally characterized. However, the transition metal/MoO42ÿ/hedp system has not been reported.
metal-organic papers
m760
Shuhua Renet al. Na8[CoMo4O12(C2H3O7P2)2]18H2O Acta Cryst.(2003). E59, m759±m761In this paper, we describe the synthesis and structure of Na8[CoMo4O12{MeC(O)(PO3)2}2]18H2O.
Selected geometric parameters of the title compound are listed in Table 1. The anion [CoMo4O12{MeC(O)(PO3)2}2]8ÿ
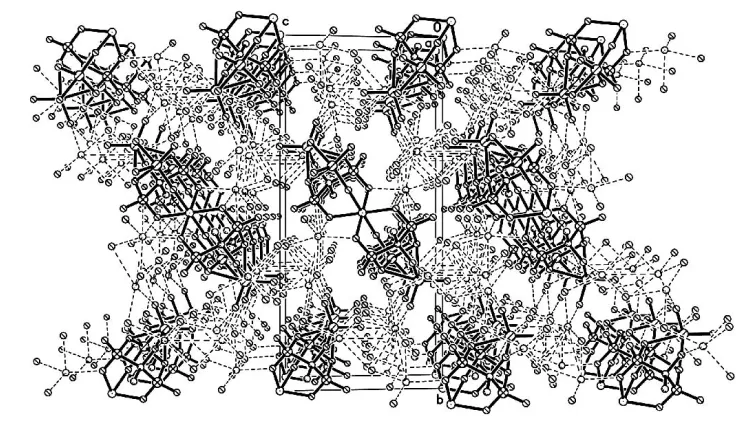
and crystal packing of the compound are illustrated in Figs. 1 and 2. The tetramolybdocobalt diphosphonate polyanion, [CoMo4O12{MeC(O)(PO3)2}2]8ÿ, comprises a CoII atom
connecting two [Mo2O6{MeC(O)(PO3)2}]5ÿ fragments. Each
of the fragments donates three oxo groups, two of them come from {CPO3} groups of the chelating hedp ligand and the third
from the oxo group bridging to a molybdenum site. The anion is situated on a centre of inversion. Each of Mo6+cation in the
title compound has a distorted octahedal con®guration with two apical Mo O bonds of length 1.717 (4) and 1.731 (3) AÊ and four other Mo O bonds with lengths ranging from 1.745 (3) to 2.419 (3) AÊ. Based on the valence-bond calcula-tions, the bond values for the two molybdenum are 5.985 and 5.967, respectively, indicating that Mo atoms have oxidation state +6. Another structural feature in [CoMo4O12
-{MeC(O)(PO3)2}2]8ÿis the presence of a pair of face-sharing
MoO6 octahedra. Edge- and angle-sharing octahedra the
normal connecting fashions in polyoxomolybdate anions, but face-sharing MoO6octahedra are rare. The two Mo atoms in
the asymmetric unit of the title compound are non-bonded to each other, according to the average Mo Mo distance of 3.24 (1) AÊ.
In contrast with the majority of diphosphonates, the short length of the MeC(O)Ð tether in hedp permits formation of stable six-membered rings with the metal ions. In Fig. 1, one can see that there are three types of six-membered rings in the
anion, viz. CoÐOÐMoÐOÐPÐO, CoÐOÐPÐCÐPÐO
and MoÐOÐPÐCÐPÐO.
Experimental
The title compound was synthesized by reacting Na2MoO4, hedp and
CoCl2in the ratio 12:1:1 at pH 5.5. The mixed solution was kept at
333 K for 2 h and was then ®ltrated. The clear ®ltrate was kept at room temperature. Within a few days, red block-shaped crystals formed in a very good yield. Elemental analysis results are in complete agreement with the structural composition of the title compound.
Crystal data
Na8[CoMo4O12(C2H3O7P2)2]
-18H2O
Mr= 1544.87
Monoclinic,P21=n
a= 9.660 (3) AÊ
b= 22.381 (8) AÊ
c= 10.441 (4) AÊ
= 99.049 (5) V= 2229.3 (14) AÊ3
Z= 2
Dx= 2.301 Mg mÿ3
MoKradiation Cell parameters from 2446
re¯ections
= 2.3±25.8 = 1.80 mmÿ1
T= 298 (2) K Block, red
0.280.130.08 mm
Data collection
Bruker SMART CCD area-detector diffractometer
'and!scans
Absorption correction: multi-scan (SADABS; Bruker, 1999)
Tmin= 0.632,Tmax= 0.869
12 461 measured re¯ections
4434 independent re¯ections 2784 re¯ections withI> 2(I)
Rint= 0.058
max= 26.4
h=ÿ12!11
k=ÿ27!12
l=ÿ13!13
Re®nement
Re®nement onF2
R[F2> 2(F2)] = 0.036
wR(F2) = 0.069
S= 0.85 4434 re¯ections 295 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0182P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.005 max= 0.61 e AÊÿ3 min=ÿ0.67 e AÊÿ3 Table 1
Selected geometric parameters (AÊ,).
Mo1ÐO11 1.729 (3) Mo1ÐO10 1.730 (3) Mo1ÐO9 1.746 (4) Mo1ÐO8 2.068 (3) Mo1ÐO4 2.339 (3) Mo1ÐO1 2.418 (3) Mo2ÐO12 1.717 (4) Mo2ÐO13 1.719 (4) Mo2ÐO8 1.841 (3) Mo2ÐO7 1.984 (3) Mo2ÐO4 2.335 (3) Mo2ÐO1 2.345 (3) Co1ÐO9 2.038 (3) Co1ÐO9i 2.038 (3)
Co1ÐO5 2.064 (3) Co1ÐO5i 2.064 (3)
Co1ÐO2 2.160 (3) Co1ÐO2i 2.160 (3)
P1ÐO3 1.515 (4) P1ÐO2 1.516 (4) P1ÐO1 1.568 (4) P1ÐC1 1.824 (5) P2ÐO5 1.507 (3) P2ÐO6 1.512 (4) P2ÐO4 1.562 (3) P2ÐC1 1.830 (5) O7ÐC1 1.446 (6) O11ÐMo1ÐO10 105.17 (16)
O11ÐMo1ÐO9 103.01 (17) O10ÐMo1ÐO9 103.78 (17) O11ÐMo1ÐO8 96.33 (15) O10ÐMo1ÐO8 92.92 (15) O9ÐMo1ÐO8 149.92 (14) O11ÐMo1ÐO4 90.49 (14) O10ÐMo1ÐO4 158.27 (14) O9ÐMo1ÐO4 86.80 (13) O8ÐMo1ÐO4 70.05 (12) O11ÐMo1ÐO1 157.41 (14) O10ÐMo1ÐO1 92.64 (14) O9ÐMo1ÐO1 85.68 (14) O8ÐMo1ÐO1 68.49 (12) O4ÐMo1ÐO1 68.96 (11) O12ÐMo2ÐO13 104.74 (18) O12ÐMo2ÐO8 103.33 (16) O13ÐMo2ÐO8 103.17 (16) O12ÐMo2ÐO7 97.90 (16) O13ÐMo2ÐO7 97.40 (15) O8ÐMo2ÐO7 145.36 (14) O12ÐMo2ÐO4 165.20 (15) O13ÐMo2ÐO4 90.01 (15) O8ÐMo2ÐO4 73.83 (13) O7ÐMo2ÐO4 78.65 (12)
O12ÐMo2ÐO1 94.95 (15) O13ÐMo2ÐO1 160.21 (14) O8ÐMo2ÐO1 73.65 (13) O7ÐMo2ÐO1 77.55 (13) O4ÐMo2ÐO1 70.27 (11) O9ÐCo1ÐO9i 180.0 (2)
O9ÐCo1ÐO5 88.69 (13) O9iÐCo1ÐO5 91.31 (13)
O9ÐCo1ÐO5i 91.31 (13)
O9iÐCo1ÐO5i 88.69 (13)
O5ÐCo1ÐO5i 180.0 (2)
O9ÐCo1ÐO2 84.68 (13) O9iÐCo1ÐO2 95.32 (13)
O5ÐCo1ÐO2 92.80 (12) O5iÐCo1ÐO2 87.20 (12)
O9ÐCo1ÐO2i 95.32 (13)
O9iÐCo1ÐO2i 84.68 (13)
O5ÐCo1ÐO2i 87.20 (12)
O5iÐCo1ÐO2i 92.80 (12)
O2ÐCo1ÐO2i 180.0 (2)
P1ÐO1ÐMo2 112.84 (17) P1ÐO1ÐMo1 127.57 (18) Mo2ÐO1ÐMo1 85.74 (11) P1ÐO2ÐCo1 127.0 (2)
Symmetry code: (i)ÿx;ÿy;ÿz. Figure 1
The H atoms on C atoms were treated as riding, with CÐH = 0.96 AÊ andUiso(H) = 1.5Ueqof the parent atom. The positions of the
water H atoms could not be located.
Data collection:SMART(Bruker, 1999); cell re®nement:SMART; data reduction:SHELXTL(Bruker, 1999); program(s) used to solve structure:SHELXS97 (Sheldrick, 1997); program(s) used to re®ne structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
SHELXTL; software used to prepare material for publication:
SHELXTL.
We sincerely acknowledge the support of the Visiting Scholar Foundation of Key Laboratories in Universities of the People's Republic of China.
References
Bruker (1999).SMART, SAINT, SADABSandSHELXTL.Bruker AXS Inc., Madison, Wisconsin, USA.
Burwell, D. A., Valentine, K. G., Timmermans, J. H. & Thompson, M. E. (1992).J. Am. Chem. Soc.114, 4144±4150.
Cao, G., Haushalter, R. C. & Strohmaier, K. G. (1993).Inorg. Chem.32, 127± 128.
Cao, G. & Mallouk, T. E. (1991).Inorg. Chem.30, 1434±1438.
Dumas, E., Sassoye, C., Smith, K. D. & Sevov, S. C. (2002).Inorg. Chem.41, 4029±4032.
Finn, R. C. & Zubieta, J. (2001).Inorg. Chem.40, 2466±2467. Khan, M. I. & Zubieta, J. (1993).Inorg. Chem. Acta,206, 131±133. Kortz, U. & Pope, M. T. (1995).Inorg. Chem.34, 2160±2163.
Kwak, W., Pope, M. T. & Scully, T. F. (1975).J. Am. Chem. Soc.97, 5735±5738. Nash, K. L., Rogers, R. D., Ferraro, J. & Zhang, J. (1998).Inorg. Chim. Acta,
269, 211±223.
Rodrigues, A. E. (1986).Ion Exchange Science and Technology. Dordrecht: Martinus Nijhoff.
Rosenthal, G. L. & Caruso, J. (1992).Inorg. Chem.31, 3104±3106.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of GoÈttingen, Germany.
Stalick, J. K. & Quicksall, C. O. (1976).Inorg. Chem.15, 1577±1584. Tolkacheva, E. O., Popov, K. I., Larchenko, V. E. & Dyatlova, N. M. (1992).
Russ. J. Inorg. Chem.37, 1618±1625.
Yayasaki, A., Andersson, I. & Pettersson, L. (1987).Inorg. Chem.26, 3926± 3933.
Zheng, L.-M., Song, H.-H. & Xin, X.-Q. (2002).Comments Inorg. Chem.22, 129±149.
Acta Cryst.(2003). E59, m759±m761 Shuhua Renet al. Na8[CoMo4O12(C2H3O7P2)2]18H2O
m761
metal-organic papers
Figure 2
supporting information
sup-1
Acta Cryst. (2003). E59, m759–m761
supporting information
Acta Cryst. (2003). E59, m759–m761 [doi:10.1107/S1600536803017835]
A new tetramolybdenumcobaltdiphosphonate, Na
8[CoMo
4O
12{MeC(O)
(PO
3)
2}
2]
·
18H
2O
Shuhua Ren, Ganglin Xue, Jun Li, Qizhen Shi, Daqi Wang and Jiwu Wang
S1. Comment
In recent years, metal organophosphonate systems have received considerable attention because of their unusual
structural chemistry and their applications as sorbents, catalysts and catalyst supports, and ion exchangers (Rodrigues et
al., 1986; Rosenthal et al., 1992; Burwell et al., 1992; Cao et al., 1991). Furthermore, the organophosphonate group has also been shown to form molecular anion clusters with molybdenum (Kwak et al., 1975; Stalick et al., 1976). In this kind
of compound, it is ordinary to form Mo5 (Yayasaki et al., 1987; Finn et al., 2001), Mo6 (Yayasaki et al., 1987; Kortz et
al., 1995; Cao et al., 1993; Khan et al., 1993), Mo7 (Yayasaki et al., 1987; Dumas et al., 2002) polyoxomolybdate, the
MoO6 octahedra being linked by sharing edge or angle.
The H+/MoO
42−/hedp (hedp = 1-hydroxyethylidenediphosphonate) acid system has been investigated (Tolkacheva et al.,
1992). The complexes are formed with Mo:L (L = hedp) ratios of 1:2, 2:2, 2:1, 3:1 and 6:1, mainly depending on pH. A
few transition metal–hedp complexes have been structurally characterized, including M(hedpH2)2 (M = Ni, Fe),
M2(hedpH2)2 (M = Cu, Fe) and M3(hedp)2 (M = Cu) (Zheng et al., 2002). A number of one-dimensional lanthanide–
hedpHn (n = 1–3; Nash et al., 1998) compounds have also been structurally characterized. However, the transition
metal/MoO42−/hedp system has not been reported. In this paper, we describe the synthesis and structure of
Na8[CoMo4O12{MeC(O)(PO3)2}2].18H2O.
The geometric parameters of the title compound are listed in Table 1. The anion [CoMo4O12{MeC(O)(PO3)2}2]8− and
crystal packing of the compound are illustrated in Figs. 1 and 2, respectively. The tetramolybdocobalt diphosphonate
polyanion, [CoMo4O12{MeC(O)(PO3)2}2]8−, consists of a CoII atom connecting two [Mo2O6{MeC(O)(PO3)2}]5− fragments.
Each of the fragments donates three oxo groups, two of them come from {CPO3} groups of the chelating hedp ligand and
the third from the oxo group bridging to a molybdenum site. The anion possesses of C2 h symmetry, the six-bonded CoII
atom lies on the center of symmetry. Each of Mo6+ cation in the title compound has a distorted octahedal configuration
with two apical Mo···O bonds ranging from 1.717 to 1.731 Å and four other Mo···O bond at length ranging from 1.745 to
2.419 Å. Based on the valence-bond calculations, the bond values for the two molybdenum are 5.985 and 5.967,
respectively, indicating that Mo atoms have oxidation state +6. Another structural feature in [CoMo4O12{MeC(O)
(PO3)2}2]8− is the presence of a pair of face-sharing octahedral of MoO6. The edge- and angle-sharing octahedral are
ordinary as the fundamental octahedral connecting fashions in the polyoxomolybdate anions, but the face-sharing
octahedralof MoO6 is rare. The two Mo atoms within a pair in the title compound are nonbonded to each other according
to the average Mo—Mo distance of 3.24 Å.
In contrast with the most of diphosphonates the short length of the MeC(O)– tether in hedp permits formation of stable
six-membered rings with the metal ions. In Fig. 1, one can see that there exist three types of six-membered rings in the
supporting information
sup-2
Acta Cryst. (2003). E59, m759–m761 S2. Experimental
The synthesis of the title anion was accomplished by reacting Na2MoO4, hedp and CoCl2 according to the ratio of 12:1:1
at pH 5.5. The mixed solution was kept at 333 K for 2 h and was then filtrated. The clear filtrate was kept at room
temperature. Within a few days, red block-shaped crystals formed in a very good yield. The elemental analysis results are
completely in agreement with the structural composition of the title compound.
S3. Refinement
The H atoms on C atoms were treated as riding, with C—H = 0.96 Å and Uiso(H) = 1.5Ueq of the parent atom. The
[image:5.610.127.484.211.404.2]positions of the water H atoms were not found.
Figure 1
The anion of the title compound, with the atom-numbering scheme and 50%-probability displacement ellipsoids.
Figure 2
[image:5.610.114.486.438.662.2]supporting information
sup-3
Acta Cryst. (2003). E59, m759–m761
octasodium(I) cobalt(II) tetramolybdenum(VI) bis(1-hydroxyethylidenediphosphate) octadecahydrate
Crystal data
Na8[CoMo4O12(C2H3O7P2)2]·18H2O
Mr = 1544.87
Monoclinic, P21/n
a = 9.660 (3) Å
b = 22.381 (8) Å
c = 10.441 (4) Å
β = 99.049 (5)°
V = 2229.3 (14) Å3
Z = 2
F(000) = 1522
Dx = 2.301 Mg m−3
Melting point: not measured K Mo Kα radiation, λ = 0.71073 Å Cell parameters from 2446 reflections
θ = 2.3–25.8°
µ = 1.80 mm−1
T = 298 K Block, red
0.28 × 0.13 × 0.08 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan
(SADABS; Bruker, 1999)
Tmin = 0.632, Tmax = 0.869
12461 measured reflections 4434 independent reflections 2784 reflections with I > 2σ(I)
Rint = 0.058
θmax = 26.4°, θmin = 2.7°
h = −12→11
k = −27→12
l = −13→13
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.036
wR(F2) = 0.069
S = 0.85 4434 reflections 295 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0182P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.005
Δρmax = 0.61 e Å−3
Δρmin = −0.67 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-4
Acta Cryst. (2003). E59, m759–m761
Na1 0.0824 (2) 0.06291 (11) −0.2582 (2) 0.0378 (6) Na2 0.5771 (2) 0.16266 (10) 0.3648 (2) 0.0329 (6) Na3 0.0831 (2) 0.33871 (12) 0.1922 (2) 0.0485 (7) Na4 0.4156 (3) 0.30286 (12) 0.1834 (3) 0.0570 (8) P1 0.18111 (14) 0.03598 (6) 0.28463 (13) 0.0191 (3) P2 0.19697 (13) 0.11605 (6) 0.05250 (12) 0.0176 (3) O1 0.0746 (3) 0.08418 (15) 0.3183 (3) 0.0199 (8) O2 0.1104 (3) −0.01204 (15) 0.1948 (3) 0.0223 (8) O3 0.2689 (4) 0.01161 (17) 0.4065 (3) 0.0302 (9) O4 0.0874 (3) 0.15448 (15) 0.1112 (3) 0.0174 (8) O5 0.1314 (3) 0.06809 (15) −0.0387 (3) 0.0198 (8) O6 0.2951 (3) 0.15678 (16) −0.0061 (3) 0.0256 (9) O7 0.3258 (3) 0.13260 (15) 0.2865 (3) 0.0202 (8) O8 −0.0256 (3) 0.18937 (15) 0.3018 (3) 0.0237 (8) O9 −0.1147 (3) 0.06137 (16) 0.0824 (3) 0.0247 (9) O10 −0.2394 (3) 0.10619 (16) 0.2796 (3) 0.0262 (9) O11 −0.2148 (3) 0.17514 (16) 0.0667 (3) 0.0259 (9) O12 0.2117 (4) 0.18257 (17) 0.4921 (3) 0.0314 (9) O13 0.2220 (4) 0.24900 (16) 0.2755 (3) 0.0294 (9) O14 0.2670 (4) 0.01666 (19) −0.3326 (4) 0.0393 (11) O15 0.1455 (4) 0.16576 (19) −0.2635 (4) 0.0398 (11) O16 −0.0628 (5) 0.0895 (2) −0.4498 (4) 0.0553 (13) O17 0.5490 (4) 0.08430 (18) 0.5262 (4) 0.0380 (10) O18 0.5278 (4) 0.20830 (18) 0.1525 (4) 0.0358 (10) O19 0.4917 (4) 0.2284 (2) 0.5209 (4) 0.0423 (11) O20 0.7857 (4) 0.21692 (19) 0.4718 (4) 0.0412 (11) O21 0.3029 (4) 0.37110 (17) 0.3058 (4) 0.0350 (10) O22 −0.0500 (5) 0.3941 (2) 0.3119 (4) 0.0599 (14) C1 0.2929 (5) 0.0824 (2) 0.1999 (5) 0.0199 (12) C2 0.4256 (5) 0.0499 (3) 0.1756 (5) 0.0267 (13) H2A 0.4004 0.0168 0.1181 0.040* H2B 0.4757 0.0355 0.2564 0.040* H2C 0.4841 0.0771 0.1369 0.040*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-5
Acta Cryst. (2003). E59, m759–m761
O3 0.038 (2) 0.029 (2) 0.023 (2) 0.0077 (19) 0.0016 (17) 0.0027 (18) O4 0.0206 (18) 0.0146 (19) 0.0183 (18) 0.0015 (15) 0.0069 (14) −0.0013 (15) O5 0.027 (2) 0.0133 (19) 0.0201 (18) −0.0036 (16) 0.0074 (15) −0.0029 (16) O6 0.027 (2) 0.025 (2) 0.026 (2) −0.0049 (17) 0.0072 (16) 0.0004 (17) O7 0.0203 (18) 0.0164 (19) 0.0238 (19) 0.0009 (16) 0.0029 (15) −0.0039 (16) O8 0.027 (2) 0.017 (2) 0.027 (2) 0.0007 (17) 0.0057 (16) −0.0064 (17) O9 0.0233 (19) 0.021 (2) 0.030 (2) 0.0000 (17) 0.0059 (16) −0.0066 (18) O10 0.0253 (19) 0.020 (2) 0.035 (2) −0.0019 (17) 0.0108 (17) −0.0029 (18) O11 0.0255 (19) 0.022 (2) 0.030 (2) 0.0051 (17) 0.0037 (16) 0.0030 (18) O12 0.040 (2) 0.028 (2) 0.026 (2) −0.003 (2) 0.0061 (17) −0.0038 (19) O13 0.035 (2) 0.016 (2) 0.038 (2) −0.0046 (17) 0.0079 (18) −0.0035 (18) O14 0.043 (2) 0.040 (3) 0.035 (2) 0.003 (2) 0.0058 (19) 0.004 (2) O15 0.040 (2) 0.042 (3) 0.037 (2) −0.005 (2) 0.0049 (19) 0.006 (2) O16 0.061 (3) 0.065 (3) 0.037 (3) −0.014 (3) −0.002 (2) 0.005 (2) O17 0.045 (2) 0.028 (2) 0.043 (3) 0.002 (2) 0.011 (2) 0.002 (2) O18 0.036 (2) 0.031 (2) 0.042 (2) 0.0024 (19) 0.0095 (19) 0.002 (2) O19 0.034 (2) 0.041 (3) 0.054 (3) 0.002 (2) 0.012 (2) −0.019 (2) O20 0.037 (2) 0.042 (3) 0.045 (3) 0.000 (2) 0.008 (2) −0.015 (2) O21 0.044 (2) 0.024 (2) 0.037 (2) −0.002 (2) 0.0038 (19) −0.004 (2) O22 0.057 (3) 0.067 (4) 0.062 (3) 0.006 (3) 0.028 (2) 0.014 (3) C1 0.020 (3) 0.021 (3) 0.019 (3) 0.005 (2) 0.002 (2) 0.003 (2) C2 0.023 (3) 0.026 (3) 0.032 (3) 0.003 (3) 0.004 (2) 0.000 (3)
Geometric parameters (Å, º)
Mo1—O11 1.729 (3) Na3—O22 2.293 (5) Mo1—O10 1.730 (3) Na3—O21 2.377 (4) Mo1—O9 1.746 (4) Na3—O19ii 2.396 (5)
Mo1—O8 2.068 (3) Na3—O17ii 2.429 (5)
Mo1—O4 2.339 (3) Na3—O13 2.494 (5) Mo1—O1 2.418 (3) Na3—Na4 3.326 (4) Mo2—O12 1.717 (4) Na3—Na2ii 3.410 (4)
Mo2—O13 1.719 (4) Na4—O15iv 2.312 (5)
Mo2—O8 1.841 (3) Na4—O21 2.364 (4) Mo2—O7 1.984 (3) Na4—O20ii 2.404 (5)
Mo2—O4 2.335 (3) Na4—O18 2.422 (5) Mo2—O1 2.345 (3) Na4—O13 2.538 (4) Co1—O9 2.038 (3) Na4—O16iv 2.806 (6)
Co1—O9i 2.038 (3) Na4—Na1iv 3.418 (4)
Co1—O5 2.064 (3) P1—O3 1.515 (4) Co1—O5i 2.064 (3) P1—O2 1.516 (4)
Co1—O2 2.160 (3) P1—O1 1.568 (4) Co1—O2i 2.160 (3) P1—C1 1.824 (5)
Co1—Na1 3.249 (2) P1—Na1i 3.351 (3)
Co1—Na1i 3.249 (2) P2—O5 1.507 (3)
supporting information
sup-6
Acta Cryst. (2003). E59, m759–m761
Na1—O2i 2.364 (4) O2—Na1i 2.364 (4)
Na1—O15 2.384 (5) O7—C1 1.446 (6) Na1—P1i 3.351 (3) O10—Na2v 2.455 (4)
Na1—Na4ii 3.418 (4) O15—Na4ii 2.312 (5)
Na2—O18 2.419 (5) O16—Na4ii 2.806 (6)
Na2—O19 2.434 (4) O17—Na3iv 2.429 (5)
Na2—O10iii 2.455 (4) O19—Na3iv 2.396 (5)
Na2—O20 2.465 (4) O20—Na4iv 2.404 (5)
Na2—O17 2.476 (4) C1—C2 1.529 (7) Na2—O7 2.527 (4) C2—H2A 0.9600 Na2—Na3iv 3.410 (4) C2—H2B 0.9600
Na2—Na4 3.865 (4) C2—H2C 0.9600
O11—Mo1—O10 105.17 (16) O22—Na3—O21 95.52 (18) O11—Mo1—O9 103.01 (17) O22—Na3—O19ii 125.03 (18)
O10—Mo1—O9 103.78 (17) O21—Na3—O19ii 139.39 (17)
O11—Mo1—O8 96.33 (15) O22—Na3—O17ii 88.93 (17)
O10—Mo1—O8 92.92 (15) O21—Na3—O17ii 98.62 (16)
O9—Mo1—O8 149.92 (14) O19ii—Na3—O17ii 85.39 (15)
O11—Mo1—O4 90.49 (14) O22—Na3—O13 124.16 (18) O10—Mo1—O4 158.27 (14) O21—Na3—O13 71.46 (14) O9—Mo1—O4 86.80 (13) O19ii—Na3—O13 82.55 (15)
O8—Mo1—O4 70.05 (12) O17ii—Na3—O13 145.58 (17)
O11—Mo1—O1 157.41 (14) O22—Na3—Na4 140.53 (16) O10—Mo1—O1 92.64 (14) O21—Na3—Na4 45.29 (11) O9—Mo1—O1 85.68 (14) O19ii—Na3—Na4 94.13 (13)
O8—Mo1—O1 68.49 (12) O17ii—Na3—Na4 100.05 (13)
O4—Mo1—O1 68.96 (11) O13—Na3—Na4 49.20 (10) O12—Mo2—O13 104.74 (18) O22—Na3—Na2ii 128.99 (15)
O12—Mo2—O8 103.33 (16) O21—Na3—Na2ii 112.10 (13)
O13—Mo2—O8 103.17 (16) O19ii—Na3—Na2ii 45.55 (11)
O12—Mo2—O7 97.90 (16) O17ii—Na3—Na2ii 46.54 (11)
O13—Mo2—O7 97.40 (15) O13—Na3—Na2ii 105.43 (12)
O8—Mo2—O7 145.36 (14) Na4—Na3—Na2ii 80.47 (8)
O12—Mo2—O4 165.20 (15) O15iv—Na4—O21 100.41 (17)
O13—Mo2—O4 90.01 (15) O15iv—Na4—O20ii 128.27 (18)
O8—Mo2—O4 73.83 (13) O21—Na4—O20ii 113.42 (17)
O7—Mo2—O4 78.65 (12) O15iv—Na4—O18 82.21 (15)
O12—Mo2—O1 94.95 (15) O21—Na4—O18 152.90 (18) O13—Mo2—O1 160.21 (14) O20ii—Na4—O18 84.06 (16)
O8—Mo2—O1 73.65 (13) O15iv—Na4—O13 142.23 (19)
O7—Mo2—O1 77.55 (13) O21—Na4—O13 70.88 (14) O4—Mo2—O1 70.27 (11) O20ii—Na4—O13 87.23 (15)
O9—Co1—O9i 180.0 (2) O18—Na4—O13 90.43 (15)
O9—Co1—O5 88.69 (13) O15iv—Na4—O16iv 73.42 (16)
O9i—Co1—O5 91.31 (13) O21—Na4—O16iv 77.85 (15)
O9—Co1—O5i 91.31 (13) O20ii—Na4—O16iv 76.78 (15)
supporting information
sup-7
Acta Cryst. (2003). E59, m759–m761
O5—Co1—O5i 180.0 (2) O13—Na4—O16iv 135.42 (16)
O9—Co1—O2 84.68 (13) O15iv—Na4—Na3 144.63 (15)
O9i—Co1—O2 95.32 (13) O21—Na4—Na3 45.60 (11)
O5—Co1—O2 92.80 (12) O20ii—Na4—Na3 72.72 (12)
O5i—Co1—O2 87.20 (12) O18—Na4—Na3 131.97 (14)
O9—Co1—O2i 95.32 (13) O13—Na4—Na3 48.07 (10)
O9i—Co1—O2i 84.68 (13) O16iv—Na4—Na3 87.35 (12)
O5—Co1—O2i 87.20 (12) O15iv—Na4—Na1iv 44.12 (12)
O5i—Co1—O2i 92.80 (12) O21—Na4—Na1iv 65.17 (12)
O2—Co1—O2i 180.0 (2) O20ii—Na4—Na1iv 119.27 (15)
O9—Co1—Na1 106.17 (11) O18—Na4—Na1iv 125.69 (13)
O9i—Co1—Na1 73.83 (11) O13—Na4—Na1iv 134.79 (13)
O5—Co1—Na1 43.87 (10) O16iv—Na4—Na1iv 42.61 (10)
O5i—Co1—Na1 136.13 (10) Na3—Na4—Na1iv 102.31 (9)
O2—Co1—Na1 133.35 (10) O15iv—Na4—Na2 80.25 (13)
O2i—Co1—Na1 46.65 (10) O21—Na4—Na2 116.38 (14)
O9—Co1—Na1i 73.83 (11) O20ii—Na4—Na2 114.39 (14)
O9i—Co1—Na1i 106.17 (11) O18—Na4—Na2 37.00 (11)
O5—Co1—Na1i 136.13 (10) O13—Na4—Na2 72.14 (11)
O5i—Co1—Na1i 43.87 (10) O16iv—Na4—Na2 152.16 (13)
O2—Co1—Na1i 46.65 (10) Na3—Na4—Na2 119.97 (10)
O2i—Co1—Na1i 133.35 (10) Na1iv—Na4—Na2 118.92 (9)
Na1—Co1—Na1i 180.00 (10) O3—P1—O2 113.7 (2)
O5—Na1—O14 108.32 (16) O3—P1—O1 111.1 (2) O5—Na1—O16 148.76 (19) O2—P1—O1 112.22 (19) O14—Na1—O16 102.64 (18) O3—P1—C1 108.4 (2) O5—Na1—O2i 77.91 (13) O2—P1—C1 110.3 (2)
O14—Na1—O2i 124.36 (17) O1—P1—C1 100.2 (2)
O16—Na1—O2i 88.22 (15) O3—P1—Na1i 98.56 (16)
O5—Na1—O15 87.73 (15) O2—P1—Na1i 38.60 (14)
O14—Na1—O15 102.25 (16) O1—P1—Na1i 87.48 (14)
O16—Na1—O15 81.69 (17) C1—P1—Na1i 146.71 (17)
O2i—Na1—O15 133.39 (16) O5—P2—O6 113.68 (19)
O5—Na1—Co1 39.07 (9) O5—P2—O4 113.39 (18) O14—Na1—Co1 112.91 (13) O6—P2—O4 109.5 (2) O16—Na1—Co1 129.00 (14) O5—P2—C1 110.2 (2) O2i—Na1—Co1 41.64 (9) O6—P2—C1 108.8 (2)
O15—Na1—Co1 122.30 (13) O4—P2—C1 100.5 (2) O5—Na1—P1i 98.75 (11) P1—O1—Mo2 112.84 (17)
O14—Na1—P1i 107.11 (14) P1—O1—Mo1 127.57 (18)
O16—Na1—P1i 75.60 (13) Mo2—O1—Mo1 85.74 (11)
O2i—Na1—P1i 23.59 (9) P1—O2—Co1 127.0 (2)
O15—Na1—P1i 146.03 (13) P1—O2—Na1i 117.8 (2)
Co1—Na1—P1i 60.02 (5) Co1—O2—Na1i 91.71 (13)
O5—Na1—Na4ii 98.80 (13) P2—O4—Mo2 112.35 (17)
O14—Na1—Na4ii 134.74 (14) P2—O4—Mo1 129.50 (19)
O16—Na1—Na4ii 54.53 (14) Mo2—O4—Mo1 87.78 (11)
supporting information
sup-8
Acta Cryst. (2003). E59, m759–m761
O15—Na1—Na4ii 42.46 (11) P2—O5—Na1 132.5 (2)
Co1—Na1—Na4ii 111.02 (8) Co1—O5—Na1 97.06 (14)
P1i—Na1—Na4ii 103.60 (8) C1—O7—Mo2 117.2 (3)
O18—Na2—O19 108.64 (16) C1—O7—Na2 121.0 (3) O18—Na2—O10iii 86.26 (14) Mo2—O7—Na2 121.56 (16)
O19—Na2—O10iii 153.34 (15) Mo2—O8—Mo1 111.85 (16)
O18—Na2—O20 103.66 (16) Mo1—O9—Co1 150.26 (19) O19—Na2—O20 74.78 (14) Mo1—O10—Na2v 132.27 (19)
O10iii—Na2—O20 80.34 (14) Mo2—O13—Na3 129.78 (19)
O18—Na2—O17 153.62 (16) Mo2—O13—Na4 145.1 (2) O19—Na2—O17 83.55 (16) Na3—O13—Na4 82.74 (14) O10iii—Na2—O17 92.55 (14) Na4ii—O15—Na1 93.41 (16)
O20—Na2—O17 102.10 (15) Na1—O16—Na4ii 82.85 (15)
O18—Na2—O7 77.02 (13) Na3iv—O17—Na2 88.07 (16)
O19—Na2—O7 88.20 (13) Na2—O18—Na4 105.93 (17) O10iii—Na2—O7 117.21 (14) Na3iv—O19—Na2 89.82 (16)
O20—Na2—O7 162.33 (16) Na4iv—O20—Na2 126.7 (2)
O17—Na2—O7 80.18 (14) Na4—O21—Na3 89.10 (15) O18—Na2—Na3iv 153.16 (13) O7—C1—C2 111.4 (4)
O19—Na2—Na3iv 44.63 (12) O7—C1—P1 103.2 (3)
O10iii—Na2—Na3iv 117.32 (12) C2—C1—P1 112.7 (4)
O20—Na2—Na3iv 70.51 (12) O7—C1—P2 103.8 (3)
O17—Na2—Na3iv 45.40 (11) C2—C1—P2 112.5 (3)
O7—Na2—Na3iv 100.72 (11) P1—C1—P2 112.5 (3)
O18—Na2—Na4 37.07 (10) C1—C2—H2A 109.5 O19—Na2—Na4 71.58 (13) C1—C2—H2B 109.5 O10iii—Na2—Na4 119.95 (12) H2A—C2—H2B 109.5
O20—Na2—Na4 93.45 (13) C1—C2—H2C 109.5 O17—Na2—Na4 146.05 (12) H2A—C2—H2C 109.5 O7—Na2—Na4 76.38 (10) H2B—C2—H2C 109.5 Na3iv—Na2—Na4 116.13 (8)