metal-organic papers
m648
Wuet al. [Zn(C14H8Br2N2O3)(C5H5N)2] doi:10.1107/S160053680600657X Acta Cryst.(2006). E62, m648–m649
Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
[3,5-Dibromosalicylaldehyde
(2-hydroxybenzoyl)-hydrazonato-j
3O
,
N
,
O
000]bis(pyridine-j
N
)zinc(II)
Yu Wu,a,bShao-Min Shi,aBing Jiaaand Zong-Qiu Hua*
a
Department of Chemistry, Central China Normal University, Wuhan, Hubei 430079, People’s Republic of China, andbDepartment of Chemistry, Sichuan University of Science and Engineering, Zigong, Sichuan 643000, People’s Republic of China
Correspondence e-mail: zqhu@mail.ccnu.edu.cn
Key indicators
Single-crystal X-ray study T= 292 K
Mean(C–C) = 0.012 A˚ Rfactor = 0.052 wRfactor = 0.157
Data-to-parameter ratio = 15.4
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 20 February 2006 Accepted 22 February 2006
#2006 International Union of Crystallography All rights reserved
In the title compound, [Zn(C14H8Br2N2O3)(C5H5N)2], the
ZnII ion is coordinated by one N and two O atoms from a Schiff base ligand and by the N atoms of two pyridine molecules to form a distorted trigonal–bipyramidal geometry.
Comment
Previously, we have reported the crystal structure and prop-erties of a 3,5-dibromosalicylaldehyde salicylhydrazone zinc(II) complex (Huet al., 2005). We now report the synthesis and crystal structure of the title compound, (I).
[image:1.610.234.435.506.724.2]The ZnIIion is coordinated by one N and two O atoms from the 3,5-dibromosalicylaldehyde salicylhydrazone ligand, and
Figure 1
by two N atoms of two pyridine molecules (Fig. 1). This ZnN3O2coordination forms a distorted trigonal–bipyramidal
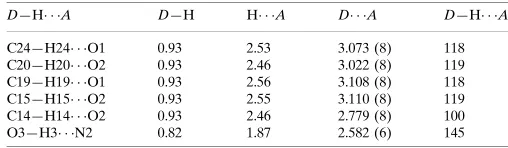
[image:2.610.311.565.232.306.2]geometry (Table 1). Intramolecular O—H N and C—H O hydrogen bonds are observed in the Schiff base ligand (Table 2).
Experimental
To an ethanol solution (100 ml) of salicylhydrazine (6 g), one molar equivalent of 3,5-dibromosalicylaldehyde in ethanol (50 ml) was added slowly with continuous stirring and 3,5-dibromo-salicylaldehyde salicylhydrazone precipitated immediately. 3,5-Dibromosalicylaldehyde salicylhydrazone (1 mmol), Zn(OAc)2
(1 mmol), dimethylformamide (30 ml) and pyridine (10 ml) were refluxed for 1 h. The hot solution was filtered and allowed to stand at room temperature for 21 d, whereupon green crystals of (I) were obtained.
Crystal data
[Zn(C14H8Br2N2O3)(C5H5N)2]
Mr= 635.61
Triclinic,P1
a= 8.9000 (11) A˚
b= 12.2172 (14) A˚
c= 13.1342 (16) A˚
= 101.696 (2) = 103.519 (2) = 110.744 (2)
V= 1232.9 (3) A˚3
Z= 2
Dx= 1.712 Mg m 3 MoKradiation Cell parameters from 1568
reflections
= 2.5–21.0 = 4.27 mm1
T= 292 (2) K Block, green
0.200.200.20 mm
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: none 6745 measured reflections 4740 independent reflections
2763 reflections withI> 2(I)
Rint= 0.021
max= 26.0
h=10!9
k=12!15
l=15!16
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.052
wR(F2) = 0.157
S= 1.01 4740 reflections 308 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0716P)2 + 0.5653P]
whereP= (Fo2+ 2Fc2)/3 (/)max= 0.001
max= 0.62 e A˚
3 min=0.33 e A˚
3
Table 1
Selected geometric parameters (A˚ ,).
Zn1—O1 1.976 (4)
Zn1—N1 2.032 (4)
Zn1—N3 2.057 (5)
Zn1—N4 2.074 (5)
Zn1—O2 2.077 (4)
O1—Zn1—N1 89.04 (17) O1—Zn1—N3 96.67 (18) N1—Zn1—N3 123.88 (17) O1—Zn1—N4 94.43 (19) N1—Zn1—N4 132.84 (17)
N3—Zn1—N4 102.42 (17) O1—Zn1—O2 165.38 (15) N1—Zn1—O2 77.01 (17) N3—Zn1—O2 94.78 (18) N4—Zn1—O2 91.95 (19)
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
C24—H24 O1 0.93 2.53 3.073 (8) 118 C20—H20 O2 0.93 2.46 3.022 (8) 119 C19—H19 O1 0.93 2.56 3.108 (8) 118 C15—H15 O2 0.93 2.55 3.110 (8) 119 C14—H14 O2 0.93 2.46 2.779 (8) 100 O3—H3 N2 0.82 1.87 2.582 (6) 145
H atoms were placed in idealized positions and allowed to ride on their parent atoms, with O—H = 0.82 A˚ , C—H = 0.93 A˚ andUiso(H) =
1.2–1.5Ueq(C,O).
Data collection:SMART(Bruker, 2000); cell refinement:SAINT
(Bruker, 2000); data reduction:SAINT; program(s) used to solve structure:SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
SHELXTL (Bruker, 1997); software used to prepare material for publication:SHELXTL.
This work was supported by Hubei Education Government of China (grant No. 20040131).
References
Bruker (1997). SHELXTL. Version 5.10. Bruker AXS Inc., Madison, Wisconsin, USA.
Bruker (2000).SMARTandSAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
Hu, Z.-Q., Yu, W. & Jia, B. (2005).Chin. J. Inorg. Chem.21, 1715–1718. Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of
supporting information
sup-1 Acta Cryst. (2006). E62, m648–m649
supporting information
Acta Cryst. (2006). E62, m648–m649 [https://doi.org/10.1107/S160053680600657X]
[3,5-Dibromosalicylaldehyde (2-hydroxybenzoyl)hydrazonato-κ
3O
,
N
,
O
′
]bis-(pyridine-
κ
N
)zinc(II)
Yu Wu, Shao-Min Shi, Bing Jia and Zong-Qiu Hu
[3,5-Dibromosalicylaldehyde (2-hydroxybenzoyl)hydrazonato-κ3O,N,O′]bis(pyridine-κN)zinc(II)
Crystal data
[Zn(C14H8Br2N2O3)(C5H5N)2]
Mr = 635.61
Triclinic, P1 Hall symbol: -P 1
a = 8.9000 (11) Å
b = 12.2172 (14) Å
c = 13.1342 (16) Å
α = 101.696 (2)°
β = 103.519 (2)°
γ = 110.744 (2)°
V = 1232.9 (3) Å3
Z = 2
F(000) = 628
Dx = 1.712 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 1568 reflections
θ = 2.5–21.0°
µ = 4.27 mm−1
T = 292 K Block, green
0.20 × 0.20 × 0.20 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
6745 measured reflections 4740 independent reflections
2763 reflections with I > 2σ(I)
Rint = 0.021
θmax = 26.0°, θmin = 1.7°
h = −10→9
k = −12→15
l = −15→16
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.052
wR(F2) = 0.157
S = 1.01 4740 reflections 308 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0716P)2 + 0.5653P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.62 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Zn1 0.90729 (9) 0.80295 (6) 0.09455 (6) 0.0634 (2)
N1 0.8059 (6) 0.9078 (4) 0.0277 (4) 0.0575 (11)
N2 0.7951 (6) 1.0006 (4) 0.1014 (4) 0.0615 (12)
N3 0.7671 (6) 0.6478 (4) 0.1269 (4) 0.0629 (12)
N4 1.1558 (6) 0.8307 (4) 0.1705 (4) 0.0634 (12)
O1 0.8960 (5) 0.7176 (4) −0.0538 (3) 0.0702 (11)
O2 0.9122 (6) 0.9257 (4) 0.2316 (3) 0.0765 (12)
O3 0.7074 (8) 1.1752 (5) 0.1680 (4) 0.1100 (17)
H3 0.7013 1.1142 0.1242 0.165*
Br1 0.56036 (12) 0.72427 (9) −0.50503 (6) 0.1092 (3) Br2 0.88419 (11) 0.51459 (8) −0.23223 (6) 0.0993 (3)
C1 0.6678 (8) 0.7256 (7) −0.3608 (5) 0.0735 (17)
C2 0.7279 (8) 0.6335 (6) −0.3526 (5) 0.0739 (17)
H2 0.7170 0.5750 −0.4148 0.089*
C3 0.8027 (7) 0.6351 (6) −0.2480 (5) 0.0640 (15)
C4 0.8237 (7) 0.7221 (5) −0.1492 (5) 0.0554 (13)
C5 0.7518 (7) 0.8070 (5) −0.1654 (5) 0.0560 (13)
C6 0.6762 (7) 0.8066 (5) −0.2717 (5) 0.0633 (15)
H6 0.6312 0.8635 −0.2805 0.076*
C7 0.7511 (7) 0.8971 (5) −0.0747 (5) 0.0599 (14)
H7 0.7070 0.9520 −0.0926 0.072*
C8 0.8578 (7) 1.0033 (5) 0.2049 (5) 0.0619 (15)
C9 0.8605 (7) 1.1010 (5) 0.2936 (5) 0.0650 (15)
C10 0.7878 (9) 1.1828 (6) 0.2710 (6) 0.0816 (18)
C11 0.7928 (11) 1.2694 (8) 0.3564 (8) 0.115 (3)
H11 0.7376 1.3193 0.3419 0.137*
C12 0.8784 (12) 1.2853 (9) 0.4653 (8) 0.121 (3)
H12 0.8882 1.3489 0.5228 0.145*
C13 0.9489 (11) 1.2042 (8) 0.4860 (7) 0.102 (2)
H13 1.0013 1.2104 0.5583 0.123*
C14 0.9418 (8) 1.1170 (6) 0.4024 (6) 0.0783 (18)
H14 0.9933 1.0653 0.4181 0.094*
C15 0.7036 (9) 0.6512 (6) 0.2074 (6) 0.087 (2)
H15 0.7133 0.7258 0.2499 0.104*
supporting information
sup-3 Acta Cryst. (2006). E62, m648–m649
H16 0.5737 0.5437 0.2861 0.159*
C17 0.6060 (12) 0.4364 (8) 0.1747 (10) 0.112 (3)
H17 0.5520 0.3649 0.1908 0.135*
C18 0.6708 (11) 0.4340 (7) 0.0945 (9) 0.110 (3)
H18 0.6646 0.3594 0.0549 0.132*
C19 0.7450 (9) 0.5348 (6) 0.0677 (6) 0.084 (2)
H19 0.7824 0.5275 0.0072 0.101*
C20 1.2215 (9) 0.8699 (6) 0.2796 (6) 0.0822 (19)
H20 1.1549 0.8861 0.3203 0.099*
C21 1.3818 (10) 0.8873 (7) 0.3343 (7) 0.096 (2)
H21 1.4208 0.9102 0.4109 0.115*
C22 1.4841 (9) 0.8712 (7) 0.2773 (8) 0.095 (2)
H22 1.5962 0.8866 0.3131 0.113*
C23 1.4190 (10) 0.8330 (9) 0.1689 (8) 0.106 (3)
H23 1.4871 0.8211 0.1277 0.128*
C24 1.2535 (8) 0.8101 (7) 0.1146 (6) 0.0843 (19)
H24 1.2104 0.7800 0.0380 0.101*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C18 0.118 (7) 0.069 (5) 0.146 (9) 0.041 (5) 0.049 (6) 0.032 (5) C19 0.098 (5) 0.070 (5) 0.102 (6) 0.043 (4) 0.047 (4) 0.030 (4) C20 0.092 (5) 0.097 (5) 0.064 (4) 0.054 (4) 0.016 (4) 0.025 (4) C21 0.100 (6) 0.091 (5) 0.078 (5) 0.044 (5) 0.002 (5) 0.016 (4) C22 0.066 (5) 0.092 (5) 0.103 (7) 0.025 (4) 0.006 (5) 0.028 (5) C23 0.078 (5) 0.150 (8) 0.106 (7) 0.055 (5) 0.038 (5) 0.050 (6) C24 0.075 (5) 0.116 (6) 0.076 (5) 0.048 (4) 0.031 (4) 0.037 (4)
Geometric parameters (Å, º)
Zn1—O1 1.976 (4) C9—C14 1.381 (8)
Zn1—N1 2.032 (4) C9—C10 1.412 (9)
Zn1—N3 2.057 (5) C10—C11 1.358 (10)
Zn1—N4 2.074 (5) C11—C12 1.392 (11)
Zn1—O2 2.077 (4) C11—H11 0.93
N1—C7 1.281 (7) C12—C13 1.385 (11)
N1—N2 1.382 (6) C12—H12 0.93
N2—C8 1.332 (7) C13—C14 1.339 (10)
N3—C15 1.310 (7) C13—H13 0.93
N3—C19 1.360 (8) C14—H14 0.93
N4—C24 1.319 (7) C15—C16 1.423 (11)
N4—C20 1.325 (8) C15—H15 0.93
O1—C4 1.289 (6) C16—C17 1.273 (13)
O2—C8 1.279 (6) C16—H16 0.93
O3—C10 1.342 (8) C17—C18 1.314 (12)
O3—H3 0.82 C17—H17 0.93
Br1—C1 1.906 (6) C18—C19 1.336 (10)
Br2—C3 1.883 (6) C18—H18 0.93
C1—C6 1.338 (9) C19—H19 0.93
C1—C2 1.419 (8) C20—C21 1.357 (9)
C2—C3 1.372 (8) C20—H20 0.93
C2—H2 0.93 C21—C22 1.347 (10)
C3—C4 1.428 (8) C21—H21 0.93
C4—C5 1.423 (7) C22—C23 1.317 (11)
C5—C6 1.399 (7) C22—H22 0.93
C5—C7 1.452 (8) C23—C24 1.375 (9)
C6—H6 0.93 C23—H23 0.93
C7—H7 0.93 C24—H24 0.93
C8—C9 1.478 (8)
O1—Zn1—N1 89.04 (17) C14—C9—C8 119.6 (6)
O1—Zn1—N3 96.67 (18) C10—C9—C8 122.2 (6)
N1—Zn1—N3 123.88 (17) O3—C10—C11 118.4 (7)
O1—Zn1—N4 94.43 (19) O3—C10—C9 122.5 (6)
N1—Zn1—N4 132.84 (17) C11—C10—C9 119.1 (7)
N3—Zn1—N4 102.42 (17) C10—C11—C12 121.6 (8)
O1—Zn1—O2 165.38 (15) C10—C11—H11 119.2
supporting information
sup-5 Acta Cryst. (2006). E62, m648–m649
N3—Zn1—O2 94.78 (18) C13—C12—C11 118.4 (8)
N4—Zn1—O2 91.95 (19) C13—C12—H12 120.8
C7—N1—N2 116.7 (4) C11—C12—H12 120.8
C7—N1—Zn1 127.3 (4) C14—C13—C12 120.3 (8)
N2—N1—Zn1 116.0 (3) C14—C13—H13 119.8
C8—N2—N1 111.1 (4) C12—C13—H13 119.8
C15—N3—C19 116.5 (5) C13—C14—C9 122.2 (7)
C15—N3—Zn1 123.4 (4) C13—C14—H14 118.9
C19—N3—Zn1 120.1 (4) C9—C14—H14 118.9
C24—N4—C20 117.5 (6) N3—C15—C16 119.7 (7)
C24—N4—Zn1 122.7 (4) N3—C15—H15 120.2
C20—N4—Zn1 119.9 (4) C16—C15—H15 120.2
C4—O1—Zn1 130.3 (4) C17—C16—C15 122.3 (9)
C8—O2—Zn1 111.6 (4) C17—C16—H16 118.9
C10—O3—H3 109.5 C15—C16—H16 118.9
C6—C1—C2 122.1 (6) C16—C17—C18 117.6 (9)
C6—C1—Br1 120.4 (5) C16—C17—H17 121.2
C2—C1—Br1 117.4 (5) C18—C17—H17 121.2
C3—C2—C1 116.5 (6) C17—C18—C19 122.5 (8)
C3—C2—H2 121.8 C17—C18—H18 118.8
C1—C2—H2 121.8 C19—C18—H18 118.8
C2—C3—C4 124.7 (5) C18—C19—N3 121.4 (7)
C2—C3—Br2 118.3 (5) C18—C19—H19 119.3
C4—C3—Br2 117.0 (4) N3—C19—H19 119.3
O1—C4—C5 124.2 (5) N4—C20—C21 122.7 (7)
O1—C4—C3 120.9 (5) N4—C20—H20 118.6
C5—C4—C3 114.9 (5) C21—C20—H20 118.6
C6—C5—C4 120.8 (5) C22—C21—C20 119.8 (8)
C6—C5—C7 116.4 (5) C22—C21—H21 120.1
C4—C5—C7 122.8 (5) C20—C21—H21 120.1
C1—C6—C5 120.9 (6) C23—C22—C21 117.3 (7)
C1—C6—H6 119.5 C23—C22—H22 121.3
C5—C6—H6 119.5 C21—C22—H22 121.3
N1—C7—C5 125.7 (5) C22—C23—C24 122.2 (8)
N1—C7—H7 117.1 C22—C23—H23 118.9
C5—C7—H7 117.1 C24—C23—H23 118.9
O2—C8—N2 124.2 (5) N4—C24—C23 120.4 (7)
O2—C8—C9 118.6 (5) N4—C24—H24 119.8
N2—C8—C9 117.3 (5) C23—C24—H24 119.8
C14—C9—C10 118.2 (6)
O1—Zn1—N1—C7 5.3 (5) O1—C4—C5—C7 −0.8 (8)
N3—Zn1—N1—C7 −92.0 (5) C3—C4—C5—C7 176.1 (5)
N4—Zn1—N1—C7 100.5 (5) C2—C1—C6—C5 2.7 (9)
O2—Zn1—N1—C7 −179.1 (5) Br1—C1—C6—C5 178.8 (4)
O1—Zn1—N1—N2 −174.8 (4) C4—C5—C6—C1 0.8 (8)
N3—Zn1—N1—N2 87.8 (4) C7—C5—C6—C1 −178.8 (5)
O2—Zn1—N1—N2 0.8 (3) Zn1—N1—C7—C5 −0.6 (8)
C7—N1—N2—C8 −179.2 (5) C6—C5—C7—N1 176.2 (5)
Zn1—N1—N2—C8 0.9 (5) C4—C5—C7—N1 −3.3 (8)
O1—Zn1—N3—C15 −165.6 (5) Zn1—O2—C8—N2 4.3 (7)
N1—Zn1—N3—C15 −72.3 (6) Zn1—O2—C8—C9 −177.2 (4)
N4—Zn1—N3—C15 98.3 (5) N1—N2—C8—O2 −3.6 (8)
O2—Zn1—N3—C15 5.2 (5) N1—N2—C8—C9 177.9 (4)
O1—Zn1—N3—C19 18.4 (5) O2—C8—C9—C14 8.8 (8)
N1—Zn1—N3—C19 111.7 (5) N2—C8—C9—C14 −172.6 (5)
N4—Zn1—N3—C19 −77.7 (5) O2—C8—C9—C10 −173.7 (6)
O2—Zn1—N3—C19 −170.7 (5) N2—C8—C9—C10 4.9 (8)
O1—Zn1—N4—C24 10.3 (5) C14—C9—C10—O3 179.5 (6)
N1—Zn1—N4—C24 −82.5 (6) C8—C9—C10—O3 1.9 (10)
N3—Zn1—N4—C24 108.1 (5) C14—C9—C10—C11 −3.3 (10)
O2—Zn1—N4—C24 −156.5 (5) C8—C9—C10—C11 179.1 (6)
O1—Zn1—N4—C20 −169.2 (4) O3—C10—C11—C12 −177.9 (8)
N1—Zn1—N4—C20 98.0 (5) C9—C10—C11—C12 4.8 (13)
N3—Zn1—N4—C20 −71.3 (5) C10—C11—C12—C13 −4.8 (14)
O2—Zn1—N4—C20 24.0 (5) C11—C12—C13—C14 3.4 (13)
N1—Zn1—O1—C4 −9.6 (5) C12—C13—C14—C9 −2.2 (12)
N3—Zn1—O1—C4 114.4 (5) C10—C9—C14—C13 2.1 (10)
N4—Zn1—O1—C4 −142.5 (5) C8—C9—C14—C13 179.7 (6)
O2—Zn1—O1—C4 −26.9 (10) C19—N3—C15—C16 1.1 (10)
O1—Zn1—O2—C8 15.3 (9) Zn1—N3—C15—C16 −175.0 (6)
N1—Zn1—O2—C8 −2.5 (4) N3—C15—C16—C17 1.1 (14)
N3—Zn1—O2—C8 −126.2 (4) C15—C16—C17—C18 −0.8 (16)
N4—Zn1—O2—C8 131.2 (4) C16—C17—C18—C19 −1.8 (16)
C6—C1—C2—C3 −3.0 (9) C17—C18—C19—N3 4.1 (13)
Br1—C1—C2—C3 −179.2 (4) C15—N3—C19—C18 −3.6 (10)
C1—C2—C3—C4 −0.1 (9) Zn1—N3—C19—C18 172.6 (6)
C1—C2—C3—Br2 179.8 (4) C24—N4—C20—C21 −1.0 (10)
Zn1—O1—C4—C5 8.9 (8) Zn1—N4—C20—C21 178.5 (5)
Zn1—O1—C4—C3 −167.8 (4) N4—C20—C21—C22 3.8 (11)
C2—C3—C4—O1 −179.8 (5) C20—C21—C22—C23 −3.2 (12)
Br2—C3—C4—O1 0.3 (7) C21—C22—C23—C24 0.1 (13)
C2—C3—C4—C5 3.2 (8) C20—N4—C24—C23 −2.2 (10)
Br2—C3—C4—C5 −176.7 (4) Zn1—N4—C24—C23 178.3 (6)
O1—C4—C5—C6 179.6 (5) C22—C23—C24—N4 2.7 (13)
C3—C4—C5—C6 −3.5 (7)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C24—H24···O1 0.93 2.53 3.073 (8) 118
C20—H20···O2 0.93 2.46 3.022 (8) 119
C19—H19···O1 0.93 2.56 3.108 (8) 118
C15—H15···O2 0.93 2.55 3.110 (8) 119
supporting information
sup-7 Acta Cryst. (2006). E62, m648–m649