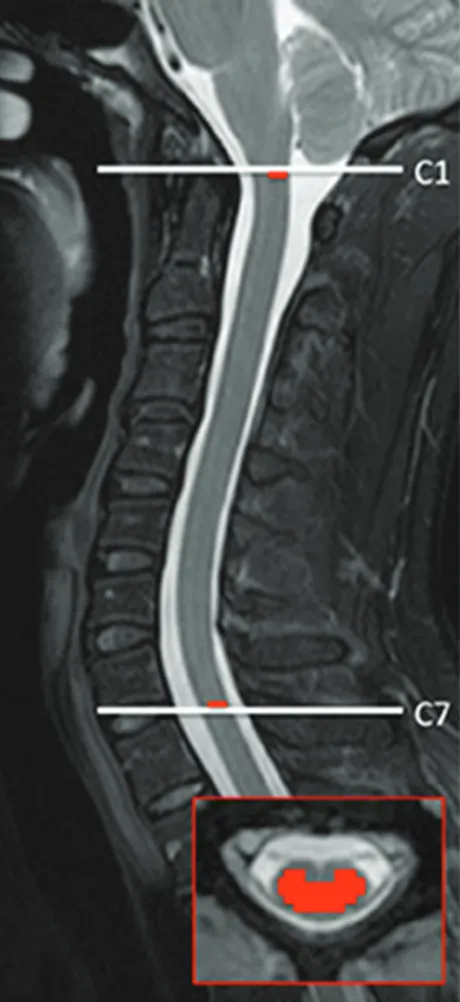
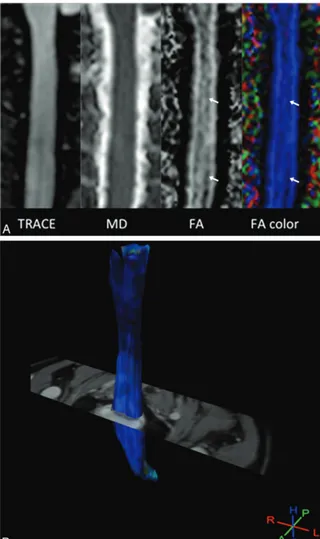
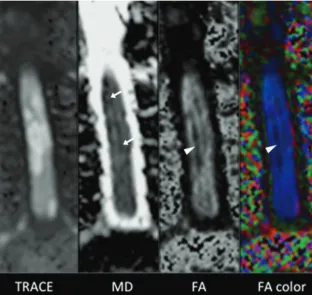
Pulse Triggered DTI Sequence with Reduced FOV and Coronal Acquisition at 3T for the Assessment of the Cervical Spinal Cord in Patients with Myelitis
Full text
Figure

Related documents
Cells in the support practice factor grid were modified to reflect the estimated success of each rehabilitation site and thus could be used to generate an overall estimate of
In the molecule, all the H atoms of the three hydroxyl groups are oriented in the same direction around the ring, forming two intramolecular hydrogen bonds between a pair of
Sequence analysis of the entire mito- chondrial genome showed the presence of the well-known T4363C mutation in tRNA Gln , as well as the ND1 T3394C mutation, and a set
Each research analyst responsible for the content of this research report certifies that the views expressed in the research report accurately reflect the research analyst’s
For the sake of convenience to show the rapid prototype modeling method and prepare for the next section to testify the coordination and fidelity of our
The correlation between TOWL and weight or thickness of nail samples has been investigated in four different states during the characterization process of the
The aggregate Nominal Amount of all Notes Outstanding (including Notes issued under the Previous Programme Memoranda) and the aggregate Calculation Amount of all
a) The Bidder shall provide the services and carry out its other obligations under the agreement with due diligence, efficiency, economy, confidentiality, promptness and