SIGLEC1 is a biomarker of disease activity and indicates extraglandular manifestation in primary Sjögren's syndrome
Full text
Figure
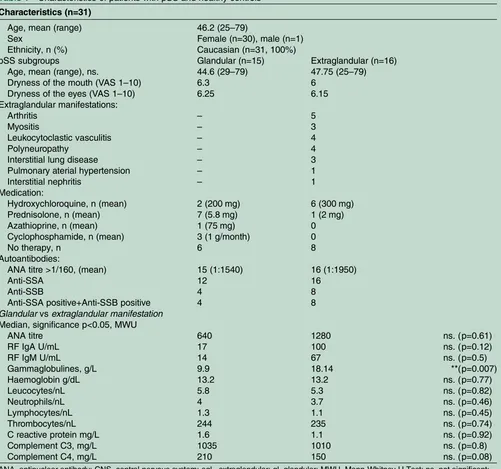
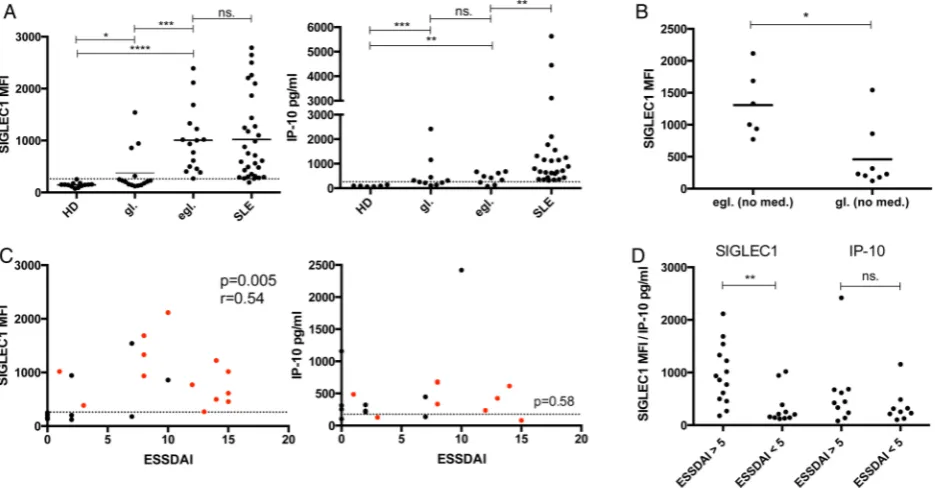
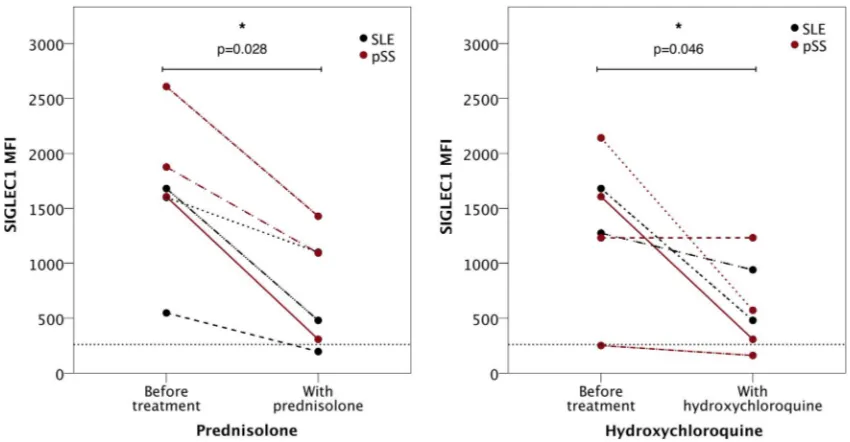
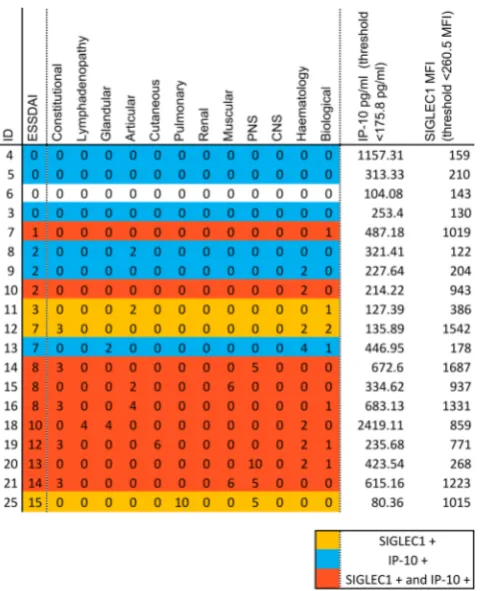
Related documents
The subsequent policy document (the Energy Strategy of Russia for the period until 2030) analysed and noted that “during the implementation of the Energy Strategy
What we can claim is that Mark’s Gospel is embedded with key terms that become part of later martyrdom language; that the author exhibits a preoccupation with persecution
The impact of three different drying methods (active solar, direct open sun and hot-air cabinet tray drying) and two different treatment methods (perforated and sliced) on
To confirm that ΔNp63 elevation results from an inactivated FANCD2, not from the off- target effect of FANCL silencing, we detected ΔNp63 expression in
IMIA plays a major global role in the application of information science and technology in the fields of healthcare and research in medical, health and bio informatics. The basic
Here, we present the burden of health care visits per reportable neurologic disease by country level from refugee camps supported by the United Nations High Commissioner for
This fi rst ever national survey of routine practice in Irish dialysis units seeks to identify routine care within the haemodialysis environment, with a particular focus on VA
One of the most intractable problems in the debate around maintaining the rule of law while combating the threat of terrorism is the question of secrecy