The deposition of pure aluminium via cold spray for the corrosion protection of steel
Ildiko Peter1a, Barry Aldwell2b, Rocco Lupoi2c, Mario Rosso1d1 Department of Applied Science and Technology, Politecnico di Torino, Italy
2 Department of Mechanical and Manufacturing Engineering, Trinity College Dublin, Ireland aildiko.peter@polito.it, baldwellb@tcd.ie, clupoir@tcd.ie, dmario.rosso@polito.it
In this paper, development and characterization of cold sprayed protective pure Al coatings on a non-regular steel surface will be proposed. The research is oriented to the optimization of the deposition route selecting the most appropriate process parameters and the really applicable layer thickness, and on the study of how the defects and the imperfections of the substrate influence the coating performance and the corrosion resistance of the coated material.
1 Introduction
Due to their outstanding mechanical strength and relatively low cost, steel is widely used in our modern society. It is considered a strategic material for many industrial applications. However, the tendency to oxidize in environment containing halide ions or aggressive species can limit its applications [1-3], that can be reduced by ani corrosion coating. Cr (VI) compounds are the most commonly used anticorrosion layers, but due to their high toxicity makes this solution less attractive [4-6] and a strong effort is taking on to substitute them with other non-toxic elements [7-9]. Actually, various technological solutions are available to deposit protective layers onto steel substrate. Some of them are related to nitriding processes, physical vapour deposition, chemical vapour deposition or atmosphere plasma jet metallization [10, 11]. High-velocity oxy fuel process has attracted much attention for coating realization, due to its ability of creating coatings with lower porosity, higher hardness, superior bond strength and less decarburization compared to many of the other thermal spraying methods [12, 13].
Aluminium and its alloys are remarkable materials for different industrial applications and the driving force for their use, apart from their performance, is principally coming from the need to moderate the CO2 gas emission, which is considered one of the most important product responsible for the generation of the greenhouse effect. Full or partial replacement of a heavier steel counterparts with lighter elements (Al, Mg, Ti based alloys) or the combined use of steel and light alloys is a growing topic [14]. The corrosion resistance of aluminium comes from its facility to develop a natural oxide film on the surface reducing the corrosion rate. Due to the hydrogen discharge, the electrodeposition of Al from aqueous solution is difficult, while high vacuum techniques, i.e. chemical vapour deposition and physical vapour deposition are time-consuming and very expensive too. Electrodeposition from molten salts constitutes a reliable solution, but the high temperature involved makes this route inadequate for some applications. Additionally, it reveals high energy consumption, pollutant emission and difficult realization of the procedures. The development of a molten salts called “room temperature molten salts”, gives different occasion to the development of this process. The
main difficulty using this kind of materials is related to their water and air stability [15] and the development of ionic liquids in order to reach higher stability is still now limited. The room temperature ionic liquid, namely AlCl3-[EMIm]Cl has a number of attractive features such as very low vapour pressure, relatively high electrical conductivity and a wide electrochemical potential window and they can be considered as potential electrolytes for the electrolytic extraction, recycling, refining and deposition of aluminium. Anyway, carefully managing the ionic liquids, the deposition can be realized [16, 17], but it appears difficult to do it in a totally safely manner.
On the other hand, cold spray (CS) as low-temperature coating process is developed and is still to be more and more employed for the quick realization of a compact coating layer onto different substrate materials. Several material systems have already been coated using CS and generally speaking the suitability of the material used in this process is mostly governed by their deformation properties. Materials with relatively low melting point and lower mechanical strength satisfy this condition and Zn, Al, Cu and their alloys are ideal materials for this purpose [18- 20]; however the deposition of strong metals such as Ti is also possible [21]. Recently, the deposition of diamond was also demonstrated possible using powder that are cladded with a ductile phase [23]. In this context, the aim of the present paper concerns to the development and characterization of cold sprayed protective Al coatings on a non-regular steel surface. The research is carryed out with a twofold purpose: one of them is oriented on the optimization of the deposition route selecting the most appropriate process parameters (i.e. velocity orprotective environment.) and the really applicable layer thickness, while the second one is targeted to investigate how the defects and the imperfections of the substrate influences the coating performance and the corrosion resistance of the material.
2 Materials and methods
2.1 Coated samples: substrate, coating material and deposition technique
subsequently sectioned and prepared according to the specific application. Commercially pure Al (99.5%) was used for the deposition.
The nozzle used was a de-laval deisgn, with 6mm exit diameter. Samples were generated using nitrogen and helium as processing gas, with a level of temperature pre-heating.
2.2 Morphological analysis and corrosion resistance evaluation
Coated samples have been carefully extracted by a cutting procedure from the macro samples and then have been prepared by a typical metallographic technique involving mounting and polishing procedures. The microstructure of the samples has been investigated using an optical microscope, (OM, MeF4 Reichart-Jung) and Scanning Electron Microscopy (SEM, Leo 1450VP) equipped with Energy X-rays Dispersive Spectroscopy unit (EDS, Oxford microprobe) used for compositional analysis. X-ray technique (X-ray, PANanalytical tool with Cu Kα wavelength of 1.5418 Å) has been employed to verify the deposition, to identify the different phases present and to give a qualitative and comparative information about the presence of the residual stress in the interested regions.
The corrosion resistance of the samples has been investigated by the accelerated salt spray chamber test. The corrosion resistance has been monitored on the whole sample surface inserted in the test chamber containing the corrosive media.The solution used was NaCl 5wt% and the test has been carried out for 168 hours. The chamber was maintained at a temperature of 35°C for the entire test period of the test. At pre-set intervals the samples have been extracted from the chamber, they have been inspected and introduced again in the chamber till the end of the test. Following the complete immersion, the samples have been cleaned by water and ethanol washing and then have been dried. Following the visual inspection of the whole configuration, some significant areas have been observed by SEM and EDS analysis and comparison of different zones has been carried out.
3 Results and discussion
The Al powders used for the research has been analysed by SEM, EDS and X-Ray. The morphology of the powders is reported in Fig.1. Globular grain shape and a relatively regular variation of the grains size (20÷40 µm) can be evidenced. The Xray pattern will be illustrated later on together with the diffraction patterns of the substrate and the coating layer. Some preliminary studied have been carried out using an AISI 1045 steel sheets and performing some thin layer deposition in order to identify the starting parameters for the deposition (Fig.1 right side). . In this case, N2 was used at 30bar inlet pressure, using 78, 155, 224 and 268 C (top to bottom) as nozzle inlet temperature.
Fig. 1. SEM micrograph for the powders used for coating (left) and photos oft he preliminary coatings (right).
Using N2 as carrier gas a porous and irregular coating layer was realized (Fig.2a) with N2 at 30bar, 250C, and with a nozzle Transverse Speed (TS) of 15mm/s.The coating geometry is in the form of “tracks”; thus, to cover the full surface tracks were overlapped (4mm) Generally, porosity can act as crack initiation site during service compromising the real lifetime of the coated surface. To overcome this step the further coating have been realized on some steel disks using helium as carrier gas, with 10bar inlet pressure, room temperature, and 60mm/s for TS. The same tracks overlap as for the nitrogen case was used.
These conditions generate a more homogeneous coating layer with no presence of macro-porosity (Fig.2b). The samples coated with N2 have been abandoned, while those obtained using He have been submitted to further investigations in order to assess the coating performance, the adhesion of the coating to the steel substrate and its corrosion resistance.
[image:2.595.310.534.51.161.2]
Fig. 2. Photographs of the coating realized with N2 (a) and with He (b) as particle carrier gas. .
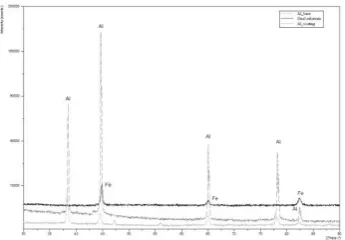
After cutting and standard metallographic preparation, X-Ray diffraction measurement (Fig. 3) reveals that the coating perfectly covers the steel substrate. It can also be observed that during the deposition process no phase change arise.
[image:2.595.310.538.448.646.2]Fig. 3. X-ray pattern showing showing the diffraction signs fort he substrate, starting coating material and the coated steel.
Similar to the initial powder the coating diffraction pattern shown the presence of Al peaks, matching the JCPDS 01-089-2837 data card. There are no important oxidation and the absence of any chemical reactions during the deposition process has been detected.
[image:3.595.69.242.48.168.2]SEM analysis has been carried out to evaluate the coating adhesion to the substrate, the thickness of the coating produced, the presence of internal porosity and to identify the possible defects. Fig. 4 reports the SEM microstructures for two realized thicknesses: Fig.4a, b are related to a coating thickness of about 480 µm and Fig.4 c, d with a coating thickness of about 1.2 mm, respectively. At higher magnification, it is evidenced that a continuous coating layer with good adhesion has been obtained spraying a thinner layer (Fig.4c) compared to the thicker one (Fig.4d). The interface between the substrate and the coating layer in this case is more or less defect free (Fig.4b). Contrarily, in the case of denser layer the development of some cracks and the presence of porosities have been revealed, highlighted with yellow arrows in Fig.4d.
Fig. 4. SEM micrographs of the coated samples, showing: a general overview (a, c), detail on the cross sections (b, d)
Preliminary results concerning the residual stress present in the coating and on the subsequent layer are in good agreement with the data reported in [19],
increasing the coating layer thickness a decrease of the shear adhesive bond strength occurs due to the accumulated residual stress in the coating layer.
The coating layer with thickness of about 1.2 mm did not provide an adequate protection to the substrate. Within a few hours of its exposure to the corrosive media the steel substrate has been highly corroded and the surface is totally covered by corrosion products principaly made of iron oxide (rust). In such situations no significative differences between the un-coated and un-coated substrate appear. The porosity of the coating and the large cracks growth illustrated in Fig.4d determine the corrosion performance and in this case it cause the rapid failure of the substrate with very high corrosion rate.
On the other hand, the samples with thinner thickness, as expected, has very promising corrosion resistance up to 168 h. Generally, the disposition of the samples in the salt chamber is like that reported in Fig.5, while the photos of the samples extracted and examined periodically have been illustrated in Fig.6, indicating also their performance in the corrosion media in hour. The un-coated sample shows after a short exposure, the first sign of the corrosion as in the early experiment and it proceed very quickly. After 168 h the surface is completely covered by the corrosion product. On the contrary, even if the coated steel show some differences compared to the initial state after its contact with the salt solution, the coating survives the entire period of the test. Formation of some sediment coming principally from the salt solution and partially from the oxidation of the Al has been detected. The coating layer loss its original shininess as reported in the photos on the right side in Fig.6.
The most relevant origin of these weaknesses is related to the fact that only one side of the sample has been coated and the other sides are exposed to the corrosion in a same way as the un-coated substrate.
Fig. 5 Disposition of the samples in during accelerated salt chamber test
[image:3.595.43.279.499.674.2] [image:3.595.313.540.519.688.2]reveal only the presence of Al2O3 and NaCl as major compunds as reported in Fig.7.
Fig. 6. Photographs of the un-coated and coated
samples, taken at prefixed time, after their permanence in a salt spray chamber.
Fig. 7. Results of EDS analysis showing the area
where the analysis was performed and the compositional analysis results.
As introduced, the sample shows some imperfections as indicated by the yellow arrows in Fig.8b which are
[image:4.595.320.541.79.592.2]more accentuated after corrosion test as reported in Fig.8c.
.
Fig. 8. SEM micrographs of the coated samples showing the zone with the presence of defect (b) and some details of the coating in the defect free area (c) and in the area with no macro defect (a).
[image:4.595.53.275.80.429.2] [image:4.595.57.289.503.688.2]during the time cause a rapid corrosion of the whole surfaces.
The adhesion of the coating layer is compromised in the region close to the defect and the coating layer is detached from the substrate as reported in the highlighted area of Fig.8 a. In other regions, with no significant imperfections the coating layer adher properly to the substrate (Fig.4b) and the interface is continuous. In such areas on the top of the coating only the sign of the compounds described in the previous section has been detected. The research pointed out that the covering all the sides of the sample, if achieved with no significative defects, can lead to a good protection against corrosion
4 Conclusions
In the present paper development and characterization of cold sprayed protective Al coatings on a non-regular steel surface were performed. The research was focalized, firstly, one the optimization of the deposition route choosing the most suitable process parameters and the most appropriate layer thickness and furthermore to investigate how the defects of the substrate influence the coating performances and the corrosion resistance of the coated material.
Cold spray method is appropriate technique for Al deposition because thank to the low process temperature do not involve any phase transformation during deposition.
A coating of about 480 µm thickness was deposited successfully onto AISI 1045 steel substrate, using helium as carrier gas with 10 bar nozzle inlet pressure. The research demonstrates that defects are dangerous as corrosion resistance concerns and compromise the adhesion of the coating layer to the substrate, negatively influencing the device lifetime. The feasibility of the cold spray deposition in case of non-conformal substrates was demonstrated. Some on-going research is related to the realization of coating with no significant defects and including all the walls of the sample by creating a barrier against corrosion.
Acknowledgements
The authors wish to thank eng. E. Pallavicini and D. Pezzini for their contribution to some measurements.
5 Literature
[1] S. Fajardo, D.M. Bastidas, M. Criado, J.M. Bastidas: Electrochemical study on the corrosion behaviour of a new low-nickel stainless steel in carbonated alkaline solution in the presence of chlorides, Electrochimica Acta 129 (2014) pp.160– 170.
[2] S.M. Abd El Haleem, E.E. Abd El Aal, S. Abd El Wanees, A. Diab: Environmental factors affecting the corrosion behaviour of reinforcing steel: I. The early stage of passive film formation in Ca(OH)2
solutions, Corrosion Science 52 (2010) pp. 3875– 3882.
[3] I.Peter, M. Rosso, F.S. Gobber: Study of protective coatings for aluminum die casting molds, Applied Surface Science 358 (2015) pp. 563–571. [4] Pacyna JM.: Monitoring and assessment of metal contaminants in the air, Toxicology of Metals. Boca Raton, FL, CRC Press (1996) pp. 9–28.
[5] Gregory John Gibbson, Robert George Hansell: Journal of materials processing technology 2 0 4 (2008) pp. 184-191
[6] G. Bolelli, V. Cannillo, L. Lusvarghi, S. Ricco: Surface & Coatings Technology 200 (2006) pp.2995 – 3009
[7] Gulizia, S., et al.: Performance evaluation of PVD coatings for high pressure die casting, Surface and Coatings Technology, Vol. 140, No. 3 (2001), pp. 200–205.
[8] Dobrazanski, L.A., et al.: Structure and properties of wear resistance PVD coatings deposited onto X37CrMoV5-1 type hot work steel, Journal of Materials Processing Technology, Vol. 164 –165, (2005), pp. 843 – 849.
[9] I. Peter, et al.: Investigations on coating of dies for advanced squeeze casting process, Acta Metallurgica Slovaca, Vol. 20, 2014, No. 1, pp. 18-27. [10] H.S. Sidhu, B.S. Sidhu, S . Prakash: The role of HVOF coatings in improving hot corrosion resistance of ASTM-SA210 GrA1 steel in the presence of Na2SO4–V2O5 salt deposits, Surf. Coat. Technol. 200 (2006) pp. 5386–5394.
[11] N . Bala, H. Singh, S. Prakash: An overview of characterizations and high temperature behaviour of thermal spray NiCr coatings, Int. J. Mater. Sci. 2 (3) (2007) pp. 201-218.
[12] J.A. Picas, Y. Xiong, M. Punset , L. Ajdelsztajn, A. Forn, J.M. Schoenung: Int. Journal of Refractory Metals & Hard Materials 27 (2009) pp. 344–349
[13] S. Thiele, K. Sempf, KJ. Roessler, LM Berger, J. Spatzier: J.Thermal Spray Technol 20 (1-2) 2011 pp. 358-365.
[14] I. Lichioiu, I.Peter, B.Varga, M. Rosso, “Preparation and Structural Characterization of Rapidly Solidified Al-Cu Alloys” J. Mater. Sci. Technol., 2014, 30(4), pp. 394-400.
[15] F. Endres, et al: Electrodeposition from Ionic Liquids, WILEY-VCH Verlag GmbH & Co. KGaA, (2008), ISBN 978-3-527-31565-9.
[16] M. Rosso, F. Calosso, I. Peter: “Grain growth on galvanic deposition of aluminium”, Metalurgia International, pp. 15-18, (2011), Vol. 5.
[17] I. Peter, M. Rosso: “Simulation of electrodeposition of Al from ionic liquid”, Materials Science Forum Volume 794-796, 2014, pp 229-234. [18] R. Ghelichi, D. MacDonald, S. Bagherifard, H. Jahed, M. Guagliano and B. Jodoin: Microstructure and fatigue behaviour of cold spray coated Al5052, Acta Materialia 60 (2012) pp. 6555–6561.
corrosion properties, Nucl. Eng. Technol. 43 (2011) pp. 557–566.
[20] P. C. King, G. Bae, S. H. Zahiri, M. Jahedi and C. Lee: An experimental and finite element study of cold spray copper impact onto two aluminum substrates , J. Thermal Spray Technol. 19 (2010) pp. 620–634.
[21] R. Lupoi: Current Design and Performance of Cold spray Nozzles: Experimental and numerical observations on deposition efficiency and particle velocity, Syrface Engineering, 30 (5) (2014) pp. 316-322.
[22] Barry Aldwell, Shuo Yin, Kevin A. McDonnell, Daniel Trimble, Tanvir Hussain, Rocco Lupoi, A novel
method for metal-diamond composite coating
deposition with cold spray and formation