Review Article
Colchicine for prevention of postoperative
atrial fibrillation following cardiac surgery:
a meta-analysis of randomized controlled trials
Zhi Li*, Hongguang Han*, Yu Liu*, Yuji Zhang, Liming Yu, Yan Zhu, Xiaodong Xue, Yuanchen He, Huishan
Wang
Department of Cardiovascular Surgery, General Hospital of Shenyang Military Command, Shenyang, China. *Equal
contributors.
Received November 16, 2017; Accepted May 3, 2018; Epub February 15, 2019; Published February 28, 2019
Abstract: The most common complication following cardiac surgery, postoperative atrial fibrillation (POAF) increases hospital costs and morbidity. Inflammation may be an important pathogenic factor for POAF. Colchicine, an ancient and natural anti-inflammatory drug, has potential antiarrhythmic properties which have aroused interest regarding its use for POAF prevention. To evaluate effects of colchicine on POAF prevention following cardiac surgery, published randomized controlled trials (RCTs), reporting the effects of colchicine on POAF prevention, were searched up to
the end of January 2017 in Cochrane library, PubMed, and EMBASE databases. Four RCTs, involving 1272 patients (636 in colchicine group, 636 in controls group), were included in the meta-analysis. Data are expressed as odds
ratio (OR) or mean difference (MD) with 95% confidence interval (CI). Colchicine significantly reduced incidence of POAF following cardiac surgery (OR = 0.6, 95% CI = 0.45 - 0.79; P<0.001), especially Coronary Artery Bypass Grafting (CABG) (OR = 0.5, 95% CI = 0.31 - 0.80; P<0.01), and shortened length of hospitalization (MD = -1.35, 95% CI = -1.76 - -0.94; P<0.001), compared with controls. Although colchicine therapy has been associated with increased incidence of gastrointestinal side effects (OR = 3.44, 95% CI = 2.27 - 5.22; P<0.001), it did not increase treatment discontinuation (OR = 1.45; 95% CI = 0.95 - 2.24; P = 0.09). Collectively, colchicine may prove beneficial in POAF prevention following cardiac surgery, especially CABG. However, because of the small number of studies in CABG, further research with larger RCTs is warranted to confirm the effects of colchicine for POAF prevention.
Keywords: Colchicine, atrial fibrillation, meta-analysis
Introduction
The most common complication following
car-diac surgery, postoperative atrial fibrillation (POAF) increases hospital costs and morbidity [1-3]. Therefore, POAF prevention is of great
importance for clinical management.
It has been postulated that inflammation is implicated in the development of POAF [4].
Therefore, use of an agent with anti-inflamma
-tory properties may be effective in preventing
POAF [5]. Colchicine, an ancient and natural anti-inflammatory drug, in patients with gout,
has unique antiarrhythmic properties, arousing
interest regarding its use in POAF prevention
[6].
Recently, several studies regarding effects of
colchicine in POAF prevention have been pub
-POAF prevention following cardiac surgery,
es-pecially CABG, remains unknown. This study, therefore, undertook a meta-analysis of pub-lished studies, evaluating the effects of
colchi-cine in POAF prevention following cardiac sur
-gery, to ascertain the significance of
colchicine-related adverse events. Materials and methods Search strategy
Published randomized controlled trials (RCTs),
reporting the effects of colchicine in POAF pre
ft”, “cardiac surgery”, “cardiothoracic surgery”, “heart surgery”, “cardiopulmonary bypass”,
“colchicine”, “atrial fibrillation”, “Auricular
Fib-rillation”, “Arrhythmia”, “Randomized controlled trial”, “Randomized”, and “placebo”.
Eligibility criteria and exclusion criteria
Studies were selected for meta-analysis if they
fulfilled the following entry criteria [11]: (1)
Par-ticipants: patients after heart surgery; (2) In- tervention: colchicine (no matter what regimen applied); (3) Control: control (placebo or no
col-chicine); (4) Outcome: incidence of POAF; and
(5) Study design: randomized design. No lan-guage restrictions were applied.
Data extraction and outcome measures All data were independently abstracted, in dup- licate, by two investigators (Z.L and Z.Y.J). Dis- crepancies were resolved by consensus. When necessary, original authors were contacted for supplementary information. The following data
were extracted from each study: first author’s
last name, number of patients, country, design,
colchicine dose, type of surgery, definition of AF
to meet endpoint, method of AF detection, fol-low up, cohort mean age, mean ejection frac-tion, gender, diabetes, hypertension, chronic
obstructive pulmonary disease (COPD), current
smoker, previous history of AF, prior congestive heart failure (CHF), previous cardiac surgery, CABG surgery, valvular surgery, and aortic sur-gery. Extracted data were entered into a
stan-dardized Excel file.
Risk of bias assessment
Risk of bias was evaluated on the basis of Cochrane Risk of Bias Methods [12], by two authors (Y.C.H. and L.M.Y.), and assigned a
value of ‘high’, ‘low’, or ‘unclear’ based on the
following: random sequence generation, allo- cation concealment, blinding of participants and personnel, blinding of outcome assess-ment, incomplete outcome data, and selective reporting.
Grading quality of evidence
The GRADE system was used to assess quality of evidence. Evidence from cohort studies be- gan with a grade of “Low”. The quality of
[image:2.612.93.522.69.376.2]Table 1. Characteristics of included studies
First author
(year) N Country Design Colchicine dose Type of Surgery
Definition of AF to Meet Endpoint
Method of AF
detection Follow-Up
Tabbalat, 2016 360 Jordan MC, OL 2 mg 12-24 hours before surgery, 1 mg day of surgery, and 0.5 mg bid from 1 day postoperative until discharge and 0.25 mg
bid if <70 kg or intolerant to highest dose
Cardiac surgery
AF lasting more than 5 min
ECGs monitor
2 months
Imazio, 2014 360 Italy MC, DB Colchicine: 0.5 mg bid for 1 month if ≥70 kg and 0.25 mg twice day if <70 kg or intolerant
to highest dose
Cardiac surgery AF lasting more than 30 s cECG monitor 3 months
Sarzaeem, 2014 216 Iran DB 1 mg night before and on day of surgery, 0.5 mg bid for 5 days postoperative
CABG only
AF lasting at least 10 min
ECG 6 months
Imazio, 2011 336 Italy MC, DB 1.0 mg bid on first day, then maintenance dose of 0.5 mg bid for 1 month if ≥70 kg and 0.25 mg bid if <70 kg or intolerant to highest
dose
Cardiac surgery
AF lasting more than 5 min
cECG monitor
12 months
MC = multicenter; OL = open-label; AF = atrial fibrillation; ECG = electrocardiography; bid. = Eis in die; DB = double-blind; cECG = continuous electrocardiography; CABG =
coronary artery bypass grafting.
Table 2. Baseline clinical characteristics of selected studies
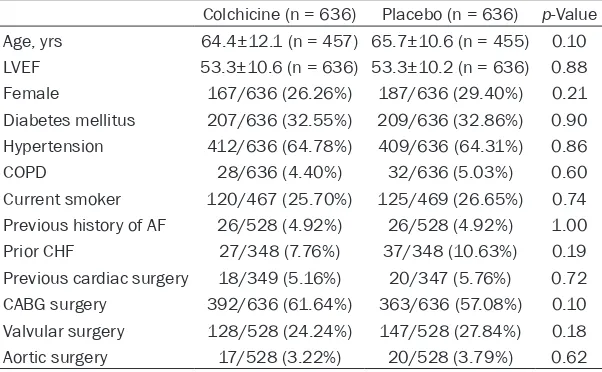
Colchicine (n = 636) Placebo (n = 636) p-Value Age, yrs 64.4±12.1 (n = 457) 65.7±10.6 (n = 455) 0.10
LVEF 53.3±10.6 (n = 636) 53.3±10.2 (n = 636) 0.88
Female 167/636 (26.26%) 187/636 (29.40%) 0.21 Diabetes mellitus 207/636 (32.55%) 209/636 (32.86%) 0.90 Hypertension 412/636 (64.78%) 409/636 (64.31%) 0.86
COPD 28/636 (4.40%) 32/636 (5.03%) 0.60
Current smoker 120/467 (25.70%) 125/469 (26.65%) 0.74 Previous history of AF 26/528 (4.92%) 26/528 (4.92%) 1.00 Prior CHF 27/348 (7.76%) 37/348 (10.63%) 0.19 Previous cardiac surgery 18/349 (5.16%) 20/347 (5.76%) 0.72 CABG surgery 392/636 (61.64%) 363/636 (57.08%) 0.10 Valvular surgery 128/528 (24.24%) 147/528 (27.84%) 0.18 Aortic surgery 17/528 (3.22%) 20/528 (3.79%) 0.62
LVEF, left ventricular ejection fraction; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; CHF, congestive heart failure; CABG, Coronary artery bypass grafting.
dence was upgraded for large magnitude of ef- fect, plausible residual confounding that would not reduce the effect size, and a dose response gradient. It was downgraded for inconsistency, indirectness, imprecision, and publication bias. Finally, quality of evidence was categorized as high, moderate, low, and very low [13].
Statistical analysis
All statistical analyses were conducted with Review Manager Version 5.3, Stata Version 12 and GRADE system. A two-tailed P-value <0.05 was considered statistically significant. Results
are expressed as odds ratios (OR) or mean dif
-ference (MD) with 95% confidence intervals (CI) (using a fixed-effect approach unless there was
ity (I2>50%) [14]. Potential publication bias was assessed using Begg’s funnel plot test [15].
Results
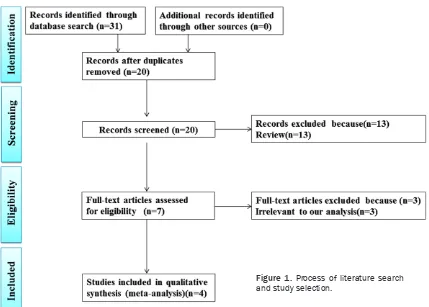
Study identification and selection
The initial search yielded 31 relevant publica-tions, of which 24 were excluded for various reasons (11 duplications, or 13 reviews) based on titles and abstracts. The remaining seven were then retrieved for full text review. Three of them were also excluded because they were irrelevant to the current analysis. Thus, four
studies were included in the final analysis [7-10]. A flow chart of studies included in the
meta-analysis is shown in Figure 1.
significant heterogeneity,
in which case a random-effects statistical model was used). Heterogeneity across studies was tested by using I2 statistic. I2 sta-tistic of 25% to 50%, 50% to 75%, and 75% to 100%
were, respectively, consid-ered to have low neity, moderate heteroge-neity, and high degree of heterogeneity. An I2 value greater than 50%
indica-tes significant heteroge
-neity. A fixed-effects
mo-del was used (I2<50%)
and random-effects mo- del was used in the case
[image:3.612.91.392.298.484.2]-Study characteristics
Characteristics of included studies are shown in Table 1 and baseline clinical characteristics of selected studies are presented in Table 2.
Of the 4 included trials, two were done in Italy
[9, 10], one in Jordan [7], and one in Iran [8]. These studies were published between 2011 and 2016. Sample sizes of these studies rang-
ed from 216 to 360 (total 1272). One study in
this meta-analysis enrolled patients under- going CABG only [8]. The remaining three stud-ies included patients undergoing CABG and/or other cardiac surgery [7, 9, 10]. Different regi-mens of colchicine were administered orally. Timing of initiation for colchicine was 1/2-3 days pre-operation or 3 day post-operation and continued for 5 days or 1 month, or until dis-charge [7-10] (Table 1). Three studies adminis-tered colchicine during the perioperative period
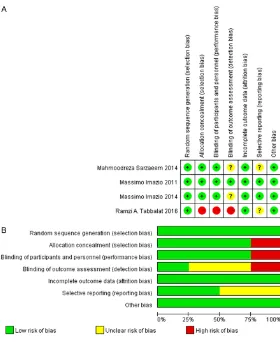
information to determine blinding of outcome assessment [9], leading to unclear risk of bias for these items. Tabbalat et al. [7] described methods of randomized but performed an open-label study, leading to high risk of bias for items such as allocation concealment, blinding of participants and personnel, and blinding of
outcome assessment. They also had no suffi
-cient information to determine selective report-ing, leading to unclear risk of bias for the two items.
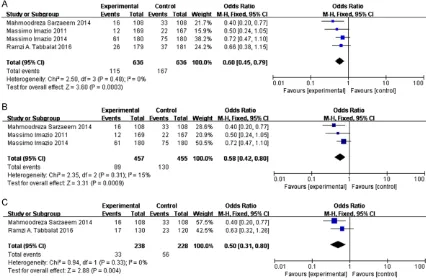
Effect of colchicine on incidence of POAF
Overall, four studies, including 1272 patients,
were included in this analysis (636 in the col-chicine group and 636 in the control group).
Meta-analysis of the two studies, using a fixed
effects model, suggested that colchicine
[image:4.612.92.372.68.410.2]sig-nificantly reduced incidence of POAF in patients undergoing cardiac surgery (OR = 0.6, 95% CI = Figure 2. Risk-of-bias analysis. A. Risk-of-bias summary: author judgments
regarding each risk-of-bias item for each included study. B. Risk-of-bias graph: author judgments regarding each risk-of-bias item presented as per-centages across all included studies.
[7-9], while one study admin-istered colchicine after sur-gery [10]. There were no
sta-tistically significant differenc -es in baseline clinical charac-teristics of selected studies (Table 2).
Methodological bias of in-cluded studies
Details concerning risks of bi- as of the included studies, ac- cording to the Cochrane as- sessment tool, are listed in Figure 2. Both of the includ- ed comparisons were RCTs, three in a double-blind design [8-10] and one in an
open-label design [7]. Overall quali -ty was good, although only one trial [10] was at low risk of bias for all quality criteria. Sarzaeem et al. described methods of randomized and double blind but had no suf-
ficient information to deter -mine blinding of outcome as- sessment and selective re- porting [8], leading to unclear risk of bias for the two items. Imazio et al. described meth-ods of randomized and
0.45 - 0.79; P<0.001; Figure 3A), especially
Coronary Artery Bypass Grafting (OR = 0.5,
95% CI = 0.31 - 0.80; P<0.01; Figure 3C),
com-pared with controls. There were no heterogene-ities among the studies (I2 = 0% and 0%, het
-erogeneity p = 0.48 and 0.33; Figure 3A and
3C). Although one of the experimental studies was an open-label study [7], when we removed the study, heterogeneity was not reduced but increased (I2 = 15%, Figure 3B). Thus, the
open-label study did not have any effect on the
accu-racy of results indicating that colchicine signifi
-cantly reduced incidence of POAF in patients
undergoing cardiac surgery.
Effect of colchicine on length of hospitalization (LOH)
Four trials [7-10] reported the effect of
colchi-cine on POAF but only 2 trials [8, 10] provided available data in LOH (expressed as mean ±
standard deviation), with a total of 288 patien-
ts. Pooled analysis, using a fixed effects mo-del, showed that colchicine significantly
reduc-ed LOH after surgery (MD = -1.35, 95% CI =
-1.76 - -0.94; P<0.001; Figure 4), with no sta-
tistical heterogeneity between the trials (I2 = 34%, heterogeneity P = 0.22).
Gastrointestinal side effects and treatment discontinuation
Pooled analysis revealed that colchicine use was associated with increased incidence of gastrointestinal side effects (anorexia, diar-rhea, nausea, vomiting, and abdominal pain),
compared to placebo (OR = 3.44, 95% CI = 2.27 - 5.22; P<0.001, I2 = 42%) (Figure 5).
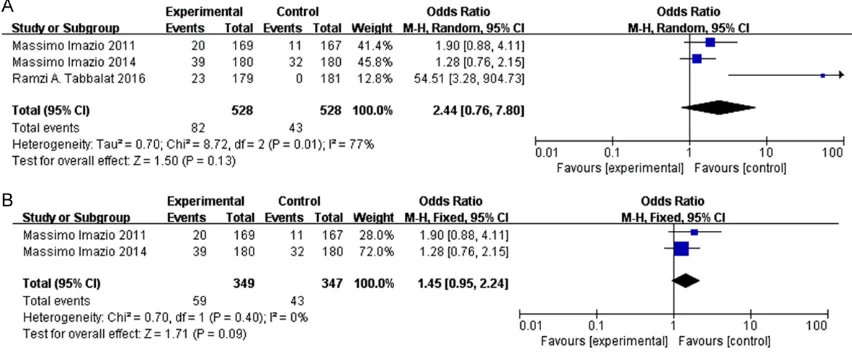
However, the rate of treatment discontinuation
was not significantly different between colchi
-cine group and placebo (OR = 2.44; 95% CI =
0.76 - 7.8; P = 0.13, I2= 77%) (Figure 6A). Due
to significant heterogeneity of the studies (I2 =
77%), sensitivity analyses were performed to
ascertain the reliability and stability of results. In sensitivity analyses, 1 study [7], whose re- search design was open-label, was removed
[image:5.612.92.518.74.354.2]and results were dramatically changed (OR = Figure 3. Forest plots from meta-analyses for the effects of colchicine on incidence of POAF undergoing cardiac surgery (A), the effects of colchicine on incidence of POAF undergoing cardiac surgery after removing 1 study in
open-label study (B) and CABG (C). The effect size of each study is proportional to the statistical weight. A diamond
1.45; 95% CI = 0.95 - 2.24; P = 0.09, I2 = 0%)
(Figure 6B).
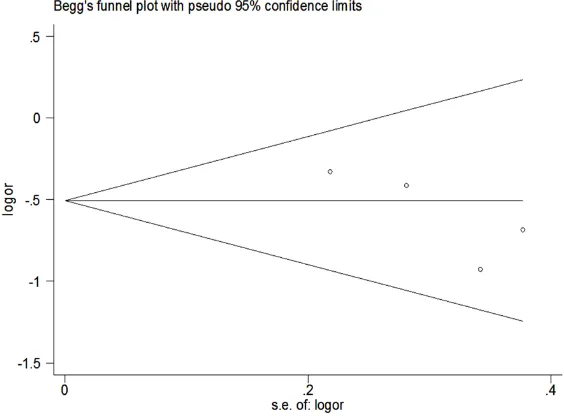
Publication bias
Begg’s funnel plot of the included studies did not suggest any significant publication bias
re-garding the effects of ranolazine on incidence
of AF (Begg’s test p = 0.145, Figure 7).
Quality of evidence
Following the GRADE system, evidence quality was assessed by reviewing whether the studies
had limitations or flaws. The results of the four
studies were not completely consistent, down-grading the quality of evidence for this out-come. Two studies reported that colchicine had
[image:6.612.91.522.72.140.2]no significant protective effects on POAF [7, 9] Figure 4. Forest plots from meta-analyses for the effects of colchicine on length of hospitalization. The effect size of each study is proportional to the statistical weight. A diamond indicates the overall summary estimate for the
[image:6.612.89.524.203.294.2]analysis; the width of the diamond represents the 95% CI. CI, confidence interval.
Figure 5. Forest plots from meta-analyses for the effects of colchicine on gastrointestinal side effects. The effect size of each study is proportional to the statistical weight. A diamond indicates the overall summary estimate for the
analysis; the width of the diamond represents the 95% CI. CI, confidence interval.
Figure 6. Forest plots from meta-analyses for the effects of colchicine on treatment discontinuation (A) and the effects of colchicine on treatment discontinuation after removing 1 study in open-label study (B). The effect size of each study is proportional to the statistical weight. A diamond indicates the overall summary estimate for the
[image:6.612.92.518.359.535.2]but the other two reported that colchicine did
have a preventive effect on POAF [8, 10]. It was
a moderate type of evidence that colchicine
had a preventive effect on POAF (Table 3).
Discussion
This study, by pooling results of available ran-domized controlled trials, found that colchicine
could significantly reduce incidence of POAF
following cardiac surgery, especially CABG, and
shorten LOH, compared with controls. Although
colchicine therapy has been associated with increased incidence of gastrointestinal side ef- fects, it was not increased to the point of tre- atment discontinuation. Therefore, colchicine
may prove beneficial for POAF prevention fol -lowing cardiac surgery.
It has been postulated that inflammatory pro
-cesses triggered by cardiac surgery have be-
en implicated in the development of POAF [4].
Therefore, use of an agent with anti-inflamma
-tory properties may be effective in preventing
POAF [5]. Colchicine is an ancient and natural anti-inflammatory drug [6]. Recently, several studies, regarding effects of colchicine in POAF
prevention, have been published [7-10]. Some studies have shown that colchicine can prevent
POAF. In 2011, Imazio et al. found that a signifi
-cant reduction in incidence of POAF was noted
ed the effects of colchicine therapy for POAF prevention in 360 patients. POAF occurred in
14.5% of patients on colchicine (p = 0.14), com
-pared with 20.5% of patients with no colchici-ne [7]. Colchicicolchici-ne failed to significantly reduce incidence of POAF. Therefore, the role of col-chicine in POAF prevention, following cardiac
surgery, especially CABG, remains unknown. This study, therefore, performed meta-analysis
of published studies to assess the efficacy of colchicine in POAF prevention for patients after
heart surgery. The results of this meta-analy- sis suggest that colchicine administration is
effective and safe for POAF prevention, follow
-ing cardiac surgery, especially CABG. Interest-
ingly, Wang’s [16] findings were not consistent with our findings, as they found that colchicine therapy was not associated with a significant protective effects on POAF. It is believed that
the reasons for this are multifaceted and are mainly attributable to the selection of studies: First, the studies they included were missing
Sarzaeem’s [8] findings in 2014. Second, the
two included articles [17, 18] were not direct studies about the effects of colchicine on pre-vention of AF, thus, there was no inaccurate record of occurrence of AF. Third, the heteroge-neity of their research was high (I2 = 47.3%),
whereas this present study does not show het-erogeneity (I2 = 0%). It is worth mentioning that Figure 7. Funnel plots (with pseudo 95% CIs) of all the individual studies
in the meta-analyses for the effects of colchicine on POAF incidence. POAF, postoperative atrial fibrillation; OR, odds ratio, SE, standard error; CI, confi -dence interval.
in patients treated with 1 mg
colchicine, twice daily (12.0%
vs. 22.0%, p<0.05), in 336
patients [10]. In 2014, Sarza-
eem et al. reported their find
-ings regarding 216 patients treated with colchicine vs.
pla-cebo; POAF was significantly
reduced in the colchicine gro-
up (14.8% vs. 30.6%, p<0.01) [8]. One other study has
sh-own that colchicine cannot
effectively prevent POAF. In
2014, Imazio et al. evaluated
the efficacy of colchicine in
preventing AF, after cardiac surgery, in 360 patients [9]. Their results, however, indi-cated that colchicine has no
significant protective effect on POAF, inconsistent with
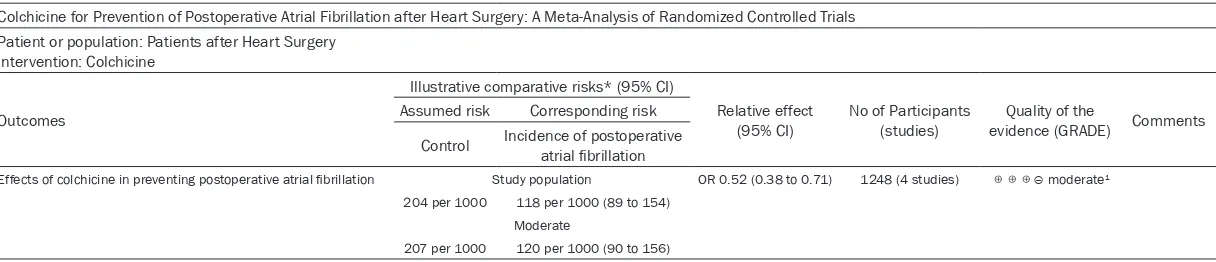
[image:7.612.92.374.75.283.2]Table 3. Summary of findings table using GRADE methodology for colchicine on prevention of POAF
Colchicine for Prevention of Postoperative Atrial Fibrillation after Heart Surgery: A Meta-Analysis of Randomized Controlled Trials Patient or population: Patients after Heart Surgery
Intervention: Colchicine
Outcomes
Illustrative comparative risks* (95% CI)
Relative effect
(95% CI) No of Participants (studies) evidence (GRADE)Quality of the Comments Assumed risk Corresponding risk
Control Incidence of postoperative atrial fibrillation
Effects of colchicine in preventing postoperative atrial fibrillation Study population OR 0.52 (0.38 to 0.71) 1248 (4 studies) ⊕⊕⊕⊝ moderate1
204 per 1000 118 per 1000 (89 to 154) Moderate
207 per 1000 120 per 1000 (90 to 156)
*The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of
the findings of this current study were also con
-firmed by Lee [5], Kreidieh [19], and Trivedi
[20]. However, this present research is slightly different from the three studies mentioned
above. Not only does this study find colchicine
playing a preventive role in POAF, following car
-diac surgery, but also in POAF following CABG.
Similarly, due to an increase in the number of selected studies, none of the above studies
showed heterogeneity. Although Trivedi’s find
-ings also showed no statistically significant het
-erogeneity [20], only one of the included three studies regarded cardiac surgery [10] and the remaining two concerned radiofrequency abla-tion [21, 22]. This was not enough to show that
colchicine has a preventive effect on POAF fol
-lowing cardiac surgery.
Although POAF may be transient, it can
increa-se hospital stays, thus, increasing health-care
costs [23]. This study’s results found that
pa-tients receiving colchicine had shorter overall hospital stays. Therefore, colchicine can not
only prevent POAF but also reduce patient
health-care costs.
A statistically higher incidence of gastrointesti-nal side effects (anorexia, diarrhea, nausea, vomiting, and abdominal pain) in the colchicine group has attracted a high degree of attention. Fortunately, rates of treatment discontinuation
have shown no significant statistical differenc
-es in the colchicine group. Th-ese findings are
similar to another meta-analysis performed on
colchicine for POAF [5]. Colchicine is currently
in Class IIb recommendations for reduction of
POAF [24, 25]. One strategy to reduce in gastro
-intestinal side effects may be to optimize dos-age and timing of colchicine therapy, avoiding pre-operative administration. Another strategy is to choose the right patients, especially those
at higher risk of POAF, such as those with heart failure, advanced age, and COPD [26] instead
of routine administration to all patients under-going cardiac surgery. Further studies may be required in determining the optimal treatment protocol to reduce incidence of gastrointestinal
intolerance, so that patients may benefit from
adjunctive prophylaxis with colchicine.
Several potential limitations of this meta-analy-sis merit consideration. First, overestimation of the treatment effect is more likely in smaller studies compared with larger samples. Seco- nd, these studies lacked homogeneity
regard-ing the dose of colchicine. This may lead to potential underestimation and/or
overestima-tion of the true incidence of POAF. Third, it was
possible that missing and unpublished data may have led to bias in effect size.
In conclusion, despite various limitations, this present study is clinically valuable because it reveals that colchicine leads to lower inciden-
ce of POAF, following cardiac surgery, especially
CABG. Additional large-scale, multicenter, ran-domized controlled parallel trials are necessary
to confirm these positive results.
Acknowledgements
This work was supported in part by a Grant- in-Aid from National Natural Science Found- ation of China (Grant No. 81600311), Postdoc- toral Science Foundation of China (Grant No.
2016M593030), Doctoral Scientific Research
Foundation of Liaoning Province in China (Gr- ant No. 201601402), and Liaoning Province, Science and Technology public relations Pro- ject of China (Grant No. 201602805).
Disclosure of conflict of interest
None.
Address correspondence to: Huishan Wang, Depart- ment of Cardiovascular Surgery, General Hospital of Shenyang Military Command, 83 Wenhua Road, Shenhe District, Shenyang 110016, China. Tel: +86-24-28897382; Fax: +86-24-23912376; E-mail: hui- shanw@126.com
References
[1] Bessissow A, Agzarian J, Srinathan S, Schnei-der L, Devereaux P, Neary J, Dechert W, Gandy L, Finley C, Hanna WC, Schieman C and Shar-gall Y. The effect of colchicine administration on postoperative pleural effusion following thoracic surgery: a randomized, double blind, placebo-controlled, feasibility pilot study. Inter-act Cardiovasc Thorac Surg 2016; 23: i20. [2] Saenen JB, Vanduynhoven PH, Pintelon I,
Mil-joen HPJ, Timmermans JP, Laga S, Vergauwen W, Rodrigus I and Vrints CJ. Connexin lateral-ization during Coronary Artery Bypass Graft (CABG) operation: a target for the antiarrhyth-mic effect of colchicine in the prevention of
post-operative atrial fibrillation. Eur Heart J
2013; 34: 252-253.
Caforio AL, Finkelstein Y, Hoit B, Maisch B,
Mayosi BM, Oh JK, Ristic AD, Seferovic P, Spod -ick DH and Adler Y. Rationale and design of the colchicine for prevention of the
post-pericardi-otomy syndrome and post-operative atrial fi
-brillation (COPPS-2 trial): a randomized, place -bo-controlled, multicenter study on the use of colchicine for the primary prevention of the postpericardiotomy syndrome, postoperative
effusions, and postoperative atrial fibrillation.
Am Heart J 2013; 166: 13-19.
[4] Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP and Damiano RJ Jr. Inflam -mation of atrium after cardiac surgery is asso-ciated with inhomogeneity of atrial conduction
and atrial fibrillation. Circulation 2005; 111:
2881-2888.
[5] Lee JZ, Singh N, Howe CL, Low SW, Huang JJ,
Ortega G, Lee KS and Pandit A. Colchicine for prevention of post-operative atrial fibrillation: a
meta-analysis. JACC Clin Electrophysiol 2016; 2: 78-85.
[6] Tong DC, Wilson AM and Layland J. Colchicine in cardiovascular disease: an ancient drug with modern tricks. Heart 2016; 102: 995-1002. [7] Tabbalat RA, Hamad NM, Alhaddad IA,
Ham-moudeh A, Akasheh BF and Khader Y. Effect of colchicine on the incidence of atrial fibrillation
in open heart surgery patients: END-AF trial. Am Heart J 2016; 178: 102-107.
[8] Sarzaeem M, Shayan N, Bagheri J, Jebelli M and Mandegar M. Low dose colchicine in
pre-vention of atrial fibrillation after coronary ar -tery bypass graft: a double blind clinical trial. Tehran University Medical Journal 2014; 72: 147-154.
[9] Imazio M, Brucato A, Ferrazzi P, Pullara A, Adler Y, Barosi A, Caforio AL, Cemin R, Chirillo F,
Co-moglio C, Cugola D, Cumetti D, Dyrda O, Ferrua
S, Finkelstein Y, Flocco R, Gandino A, Hoit B,
Innocente F, Maestroni S, Musumeci F, Oh J,
Pergolini A, Polizzi V, Ristic A, Simon C, Spodick DH, Tarzia V, Trimboli S, Valenti A, Belli R and Gaita F. Colchicine for prevention of postperi-cardiotomy syndrome and postoperative atrial
fibrillation: the COPPS-2 randomized clinical
trial. JAMA 2014; 312: 1016-1023.
[10] Imazio M, Brucato A, Ferrazzi P, Rovere ME, Gandino A, Cemin R, Ferrua S, Belli R, Mae-stroni S, Simon C, Zingarelli E, Barosi A, San-sone F, Patrini D, Vitali E, Trinchero R, Spodick DH and Adler Y. Colchicine reduces
postopera-tive atrial fibrillation: results of the Colchicine
for the Prevention of the Postpericardiotomy
Syndrome (COPPS) atrial fibrillation substudy.
Circulation 2011; 124: 2290-2295.
[11] Julian PT, Higgins SG. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. 2008.
[12] Luo X, Zhang J, Castelberg R, Wu T, Yu P, He C and Wang P. The effects of traditional Chinese exercise in patients with chronic obstructive
pulmonary disease: a meta-analysis. PLoS One
2016; 11: e0161564.
[13] Viani GA, Manta GB, Stefano EJ and de Fendi LI. Brachytherapy for cervix cancer: low-dose rate or high-dose rate brachytherapy-a meta-analysis of clinical trials. J Exp Clin Cancer Res 2009; 28: 47.
[14] Sakamoto A, Hamasaki T and Kitakaze M. Peri -operative landiolol administration reduces at-
rial fibrillation after cardiac surgery: a
meta-analysis of randomized controlled trials. Adv Ther 2014; 31: 440-450.
[15] Song F and Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ 1998; 316: 471.
[16] Wang MX, Deng XL, Mu BY, Cheng YJ, Chen YJ, Wang Q, Huang J, Zhou RW and Huang CB. Ef-fect of colchicine in prevention of pericardial
effusion and atrial fibrillation: a meta-analysis.
Intern Emerg Med 2016; 11: 867-876. [17] Imazio M, Trinchero R, Brucato A, Rovere ME,
Gandino A, Cemin R, Ferrua S, Maestroni S, Zingarelli E, Barosi A, Simon C, Sansone F, Pa-trini D, Vitali E, Ferrazzi P, Spodick DH, Adler Y and Investigators C. Colchicine for the Pre-vention of the Post-pericardiotomy Syndrome
(COPPS): a multicentre, randomized,
double-blind, placebo-controlled trial. Eur Heart J 2010; 31: 2749-2754.
[18] Meurin P, Lelay-Kubas S, Pierre B, Pereira H,
Pavy B, Iliou MC, Bussiere JL, Weber H, Beugin JP, Farrokhi T, Bellemain-Appaix A, Briota L, Ta-bet JY; French Society of Cardiology. Colchicine for postoperative pericardial effusion: a multi-centre, double-blind, randomised controlled trial. Heart 2015; 101: 1711-1716.
[19] Kreidieh O, Kabach M, El Dassouki S, Martinez
J, Rosenstein R and Chait R. Colchicine versus placebo for prevention of post-procedural
atri-al fibrillation: a systematic review and
meta-analysis. Cardiology (Switzerland) 2016; 134: 227.
[20] Trivedi C and Sadadia M. Colchicine in
preven-tion of atrial fibrillapreven-tion following cardiac sur -gery: systematic review and meta-analysis. In-dian J Pharmacol 2014; 46: 590-595.
[21] Deftereos S, Giannopoulos G, Kossyvakis C, Efremidis M, Panagopoulou V, Kaoukis A, Rai
-sakis K, Bouras G, Angelidis C, Theodorakis A, Driva M, Doudoumis K, Pyrgakis V and Stefa -nadis C. Colchicine for prevention of early at-
rial fibrillation recurrence after pulmonary vein
[22] Yasuyuki Egami MN, Jun Tanouchi. Correlation between left atrial epicardial adipose tissue volume and acute effects of colchicine
treat-ment after atrial fibrillation ablation. Circula -tion 2013; 128: Issue Suppl 22.
[23] Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ Jr, Cohn
LH and Burstin HR. Predictors of atrial fibrilla -tion after coronary artery surgery. Current trends and impact on hospital resources. Cir-culation 1996; 94: 390-397.
[24] Camm AJ, Kirchhof P, Lip GY, Schotten U, Save -lieva I, Ernst S, Van Gelder IC, Al-Attar N,
Hin-dricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R,
De Sutter J, Goette A, Gorenek B, Heldal M,
Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski
P, Rutten FH; ESC Committee for Practice Guidelines. Guidelines for the management of
atrial fibrillation: the task force for the manage
-ment of atrial fibrillation of the European Soci -ety of Cardiology (ESC). Europace 2010; 12: 1360-1420.
[25] January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor
PT, Ezekowitz MD, Field ME, Murray KT, Sacco
RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for
the management of patients with atrial fibrilla -tion: a report of the American college of cardi-ology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol 2014; 64: e1-76.
[26] Fuster V, Ryden LE, Cannom DS, Crijns HJ,
Cur-tis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN,
Tamargo JL and Wann LS. 2011 ACCF/AHA/ HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the
man-agement of patients with atrial fibrillation: a