metal-organic papers
Acta Cryst.(2007). E63, m241–m243 doi:10.1107/S1600536806053566 Shenget al. [Mn
2(C10H12O5)2(C12H8N2)2]6H2O
m241
Acta Crystallographica Section EStructure Reports
Online
ISSN 1600-5368
Bis(
l
-cantharidinato)bis[(1,10-phenanthroline)-manganese(II)] hexahydrate
Guo-Ding Sheng, Liang Shen,* Yin-Zhi Jin and Juan Mei
Department of Chemistry, Hangzhou Teachers College, Hangzhou, People’s Republic of China
Correspondence e-mail: shenchem@hotmail.com
Key indicators
Single-crystal X-ray study T= 298 K
Mean(C–C) = 0.002 A˚ Rfactor = 0.030 wRfactor = 0.074
Data-to-parameter ratio = 16.6
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 3 December 2006 Accepted 11 December 2006
#2007 International Union of Crystallography All rights reserved
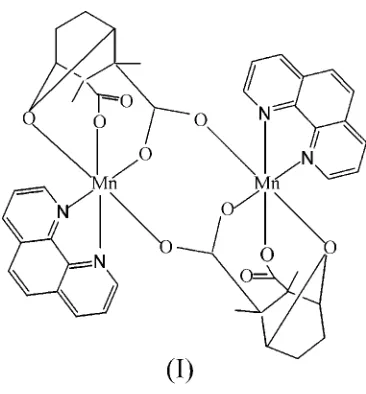
The title compound {systematic name: bis( -2,3-dimethyl-7- oxobicyclo[2.2.1]heptane-2,3-dicarboxylato)bis[(1,10-phenan-throline)manganese(II)] hexahydrate}, [Mn2(C10H12O5)2
-(C12H8N2)2]6H2O, comprises centrosymmetric dinuclear
neutral molecules in which two MnIIcenters are bridged by a single carboxylate group of each of two cantharidinate ligands, together with water molecules. In the complex, each MnII atom is in a distorted octahedral geometry, being
coordinated by two N atoms of one 1,10-phenanthroline ligand, three O atoms from one cantharidinate dianion and one carboxylate O atom from another cantharidinate dianion. Adjacent molecules link to each other through hydrogen bonds involving solvent water molecules and carboxylate groups, forming a three-dimensional network.
Comment
Cantharidine has long been used as a Chinese medicine (Li, 1957). In the past few decades, several studies showed that cantharidine and its derivatives presented potential antitumor capability against lung, colon and breast cancer (Cui et al., 1984; Liet al., 1984; Shimiet al., 1982). A few metal canthar-idinate complexes have been synthesized. Some platinum cantharidine complexes have shown effective antitumor activity. To our knowledge, one platinum and one copper complex with cantharidinate have been structurally char-acterized (Wanget al., 1997; Yinet al., 2003). We report here the preparation and structure of a manganese(II) complex with cantharidinate and 1,10-phenanthroline.
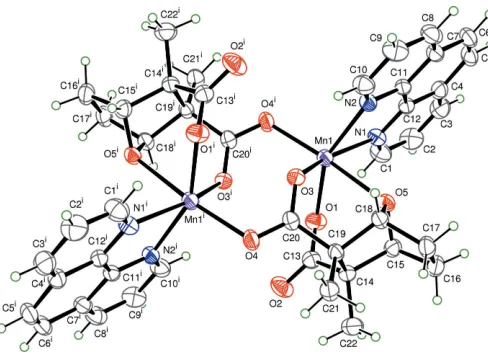
[image:1.610.240.423.516.713.2]dinuclear molecules. A cantharidinate dianion chelates an MnIIatom by two O atoms from one carboxylate group and one bridging oxo O atom. The MnIIatom is also coordinated by two N atoms of one 1,10-phenanthroline ligand and one carboxylate O atom from another cantharidinate dianion. Each Mn site exhibits a distorted octahedral coordination, withtransbond angles ranging from 153.59 (4) to 168.22 (4) and cis angles from 73.51 (4) to 105.02 (4) (Table 1). The Mn—O(carboxylate) bond distances are comparable with those in [Mn(phen)(cyclohexane-1,1-diacetato)(H2O)2]
trihy-drate (Shen et al., 2005). The Mn—O(bridging oxo) bond distance of 2.4032 (11) A˚ is much longer than those of Mn— O(carboxylate). The Mn—N bond lengths are comparable to the corresponding ones found in [Mn2(mal)(phen)3(H2O)2
Cl]-Cl (Sain et al., 2003). Cantharidinate adopts a chair confor-mation in this complex. One of the carboxylate groups behaves as a monodentate ligand, and the other carboxylate acts in a common syn–anti bridging coordination mode (Policaret al., 1999). The manganese–manganese separation is 4.441 (5) A˚ .
The hydrogen-bonding interactions (Table 2) play an important role in the solid-state structure of the title complex, (I). Adjacent molecules are linked to form a three-dimen-sional chain by hydrogen bonds between a coordinated O atom and water, and between water molecules.
Experimental
An aqueous solution (20 ml) of 2,3-dimethyl-7-oxobicyclo-[2,2,1]-heptane-2,3-dicarboxylic acid (0.214 g, 1 mmol) and Mn2(OH)2CO3 (0.102 g, 0.5 mmol) was heated to reflux temperature on a water-bath for 8 h and was then filtered. To this hot filtrate, an aqueous solution (10 ml) containing 1,10-phenanthroline (0.180 g, 1 mmol) was added. The reaction mixture was stirred at reflux temperature for 12 h. Yellow crystals were collected after cooling the reaction mixture. Yellow single crystals of (I) were obtained by recrystallizing from water.
Crystal data
[Mn2(C10H12O5)2(C12H8N2)2]6H2O
Mr= 1002.79 Monoclinic,C2=c a= 17.806 (5) A˚
b= 20.600 (5) A˚
c= 11.982 (3) A˚
= 90.812 (11)
V= 4395 (2) A˚3
Z= 4
Dx= 1.515 Mg m 3
MoKradiation
= 0.65 mm1
T= 298 (1) K Needle, yellow 0.300.150.10 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
!scans
Absorption correction: multi-scan (ABSCOR; Higashi, 1995)
Tmin= 0.830,Tmax= 0.937
21189 measured reflections 5033 independent reflections 3904 reflections withF2> 2(F2)
Rint= 0.029 max= 27.5
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.030
wR(F2) = 0.074
S= 1.00 5033 reflections 303 parameters
H-atom parameters constrained
w= 1/[0.0003Fo 2
+(Fo 2
)]/(4Fo 2
) (/)max< 0.001
max= 0.35 e A˚ 3
min=0.34 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
Mn1—O1 2.1206 (11) Mn1—O3 2.1276 (10) Mn1—O4i 2.1222 (11) Mn1—O5 2.4032 (11) Mn1—N1 2.3042 (12)
Mn1—N2 2.2353 (11) O1—C13 1.2824 (18) O2—C13 1.2344 (18) O3—C20 1.2533 (16) O4—C20 1.2597 (16)
O1—Mn1—O3 95.40 (4) O1—Mn1—O4i
104.27 (4) O1—Mn1—N1 92.69 (4) O1—Mn1—N2 153.59 (4) O3—Mn1—O4i
91.41 (4) O3—Mn1—N1 159.30 (4) O3—Mn1—N2 91.27 (4) O4i
—Mn1—N1 105.02 (4) O4i
—Mn1—N2 101.08 (4) O4i
—Mn1—O5 168.22 (4) O5—Mn1—O1 78.08 (4) O5—Mn1—O3 76.85 (4) O5—Mn1—N1 86.27 (4) O5—Mn1—N2 78.63 (4) N1—Mn1—N2 73.51 (4)
Symmetry code: (i)xþ1 2;yþ
[image:2.610.48.292.69.249.2]1 2;zþ1.
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O6—H601 O9i
0.91 2.04 2.949 (2) 174 O7—H701 O9i
0.90 1.91 2.809 (2) 176 O8—H801 O2 0.91 1.84 2.7466 (18) 176 O8—H802 O7ii
0.92 1.95 2.836 (2) 161 O9—H901 O1 0.91 1.87 2.7710 (17) 178 O9—H902 O8 0.92 1.95 2.772 (2) 147
Symmetry codes: (i)xþ1 2;yþ
1
2;zþ1; (ii)xþ 1 2;y
1 2;z.
All the water H atoms were located in a difference Fourier map and the remaining H atoms were placed in calculated positions, with C—H = 0.93–0.98 A˚ and O—H = 0.90–0.92 A˚. All H atoms were included in the final cycle of refinement in riding mode, withUiso(H) = 1.2Ueq(C,O).
Data collection: PROCESS-AUTO (Rigaku, 1998); cell refine-ment:PROCESS-AUTO; data reduction:CrystalStructure(Rigaku/ Figure 1
[image:2.610.314.562.565.639.2]al., 1999); program(s) used to refine structure:CRYSTALS (Better-idge et al., 2003); molecular graphics: ORTEP-3 for Windows
(Farrugia, 1997); software used to prepare material for publication:
CrystalStructure(Rigaku/MSC, 2004).
We express our gratitude to the Zhejiang Provincial Natural Science Foundation of China for financial Support through Project No. M203077. We gratefully acknowledge Professor Jian-Ming Gu for the structure analysis and advice.
References
Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999).J. Appl. Cryst.32, 115–119.
Betteridge, P. W., Carruthers, J. R., Cooper, R. L., Prout, K. & Watkin, D. J. (2003).J. Appl. Cryst.36, 1487.
Cui, Z. Y., Xu, S. Y., Wang, S. Q., Huang, D. T. & Wang, G. S. (1984).Chin. Pharm. Bull.19, 567–570.
Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Higashi, T. (1995).ABSCOR. Rigaku Corporation, Tokyo, Japan.
Li, D. H., Li, H. K., Chang, S. K., Hao, X. G., Ma, K. S. & Wang, Z. L. (1984).
Nat. Med. J. China,18, 785–788.
Li, S. Z. (1957).Encyclopedia of Chinese Medical Herbs,40, 1528–1529. Policar, C., Lambert, F., Cesario, M. & Morgenstern-Badarau, I. (1999).Eur. J.
Inorg. Chem.pp. 2201–2207.
Rigaku (1998).PROCESS-AUTO. Version 1.06. Rigaku Corporation, Tokyo, Japan.
Rigaku/MSC (2004). CrystalStructure. Version 3.6.0. Rigaku/MSC, The Woodlands, Texas, USA.
Sain, S., Maji, T. K., Mostafa, G., Lu, T. H. & Chaudhuri, N. R. (2003).Inorg. Chim. Acta,351, 12–20.
Shen, L., Yan, L.-C., Jin, Z.-M. & Zhang, Y.-J. (2005).Acta Cryst.E61, m1419– m1421.
Shimi, I. R., Zaki, Z., Shoukry, S. & Medhat, A. M. (1982).Eur. J. Cancer Clin. Oncol.18, 785–789.
Wang, Y. H., Huang, Z. X. & Wu, G. (1997).Polyhedron,1, 57–59. Yin, F. L., Shen, J. & Li, R. C. (2003).Acta Chem. Sinica,4, 556–561.
metal-organic papers
Acta Cryst.(2007). E63, m241–m243 Shenget al. [Mn
supporting information
Acta Cryst. (2007). E63, m241–m243 [https://doi.org/10.1107/S1600536806053566]
Bis(
µ
-cantharidinato)bis[(1,10-phenanthroline)manganese(II)] hexahydrate
Guo-Ding Sheng, Liang Shen, Yin-Zhi Jin and Juan Mei
bis(µ-2,3-dimethyl-7-oxobicyclo[2.2.1]heptane-2,3- dicarboxylato)bis[(1,10-phenanthroline)manganese(II)]
hexahydrate
Crystal data
[Mn2(C10H12O5)2(C12H8N2)2]·6H2O
Mr = 1002.79
Monoclinic, C2/c
Hall symbol: -C 2yc
a = 17.806 (5) Å
b = 20.600 (5) Å
c = 11.982 (3) Å
β = 90.812 (11)°
V = 4395 (2) Å3
Z = 4
F(000) = 2088.00
Dx = 1.515 Mg m−3
Mo Kα radiation, λ = 0.71075 Å Cell parameters from 17413 reflections
θ = 3.0–27.5°
µ = 0.65 mm−1
T = 298 K Needle, yellow 0.30 × 0.15 × 0.10 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Detector resolution: 10.00 pixels mm-1
ω scans
Absorption correction: multi-scan (ABSCOR; Higashi, 1995)
Tmin = 0.830, Tmax = 0.937
21189 measured reflections
5033 independent reflections 3904 reflections with F2 > 2σ(F2)
Rint = 0.029
θmax = 27.5°
h = −23→23
k = −26→26
l = −15→14
Refinement
Refinement on F2
R[F2 > 2σ(F2)] = 0.030
wR(F2) = 0.074
S = 1.00 5033 reflections 303 parameters
H-atom parameters constrained
w = 1/[0.0003Fo2 + σ(Fo2)]/(4Fo2)
(Δ/σ)max < 0.001
Δρmax = 0.35 e Å−3
Δρmin = −0.34 e Å−3
Special details
Refinement. Refinement using all reflections. The weighted R-factor (wR) and goodness of fit (S) are based on F2. R
-factor (gt) are based on F. The threshold expression of F2 > 2.0 σ(F2) is used only for calculating R-factor (gt).
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-2
Acta Cryst. (2007). E63, m241–m243
O2 0.28038 (6) 0.09050 (6) 0.38385 (9) 0.0455 (3) O3 0.17367 (6) 0.24311 (5) 0.53552 (8) 0.0299 (2) O4 0.17555 (6) 0.22806 (6) 0.35272 (8) 0.0338 (2) O5 0.13254 (6) 0.12974 (5) 0.65226 (6) 0.0281 (2) O6 0.000000 (10) 0.29861 (12) 0.250000 (10) 0.0930 (9) O7 0.000000 (10) 0.47324 (10) 0.250000 (10) 0.0642 (6) O8 0.43323 (8) 0.07894 (6) 0.36334 (12) 0.0589 (4) O9 0.43373 (8) 0.10863 (9) 0.58903 (12) 0.0714 (5) N1 0.27147 (6) 0.14483 (6) 0.82519 (10) 0.0309 (3) N2 0.16934 (6) 0.24152 (6) 0.79571 (9) 0.0282 (3) C1 0.32145 (10) 0.09838 (9) 0.84055 (13) 0.0414 (4) C2 0.33484 (11) 0.06794 (9) 0.94276 (14) 0.0473 (5) C3 0.29435 (11) 0.08682 (8) 1.03268 (13) 0.0426 (4) C4 0.24000 (9) 0.13520 (8) 1.02118 (12) 0.0329 (4) C5 0.19501 (10) 0.15837 (9) 1.11146 (12) 0.0378 (4) C6 0.14336 (10) 0.20472 (9) 1.09595 (12) 0.0375 (4) C7 0.13074 (9) 0.23361 (8) 0.98846 (12) 0.0309 (3) C8 0.07725 (10) 0.28202 (9) 0.96785 (12) 0.0389 (4) C9 0.07090 (10) 0.30905 (9) 0.86346 (13) 0.0422 (4) C10 0.11869 (10) 0.28751 (8) 0.77998 (12) 0.0359 (4) C11 0.17556 (8) 0.21388 (6) 0.89842 (11) 0.0263 (3) C12 0.23003 (8) 0.16342 (6) 0.91417 (11) 0.0272 (3) C13 0.24742 (9) 0.10079 (6) 0.47205 (12) 0.0289 (3) C14 0.16239 (9) 0.08531 (6) 0.47822 (11) 0.0267 (3) C15 0.14330 (9) 0.06695 (6) 0.60028 (11) 0.0296 (3) C16 0.06492 (10) 0.03735 (8) 0.61249 (12) 0.0372 (4) C17 0.01408 (9) 0.09706 (8) 0.59338 (12) 0.0350 (4) C18 0.07212 (8) 0.15092 (8) 0.57799 (11) 0.0269 (3) C19 0.11037 (8) 0.14838 (6) 0.46227 (10) 0.0247 (3) C20 0.15695 (8) 0.21127 (6) 0.44957 (11) 0.0251 (3) C21 0.05506 (9) 0.14505 (8) 0.36363 (12) 0.0328 (4) C22 0.14496 (10) 0.03072 (8) 0.39478 (12) 0.0387 (4)
H1 0.3494 0.0852 0.7796 0.050*
H2 0.3708 0.0353 0.9494 0.057*
H3 0.3029 0.0674 1.1018 0.051*
H5 0.2020 0.1407 1.1823 0.045*
H6 0.1150 0.2185 1.1561 0.045*
H8 0.0461 0.2959 1.0247 0.047*
H9 0.0354 0.3411 0.8487 0.051*
H10 0.1147 0.3065 0.7097 0.043*
H15 0.1827 0.0411 0.6370 0.036*
H18 0.0529 0.1941 0.5965 0.032*
H161 0.0555 0.0041 0.5567 0.045*
H162 0.0582 0.0191 0.6863 0.045*
H171 −0.0174 0.0919 0.5273 0.042*
H172 −0.0172 0.1053 0.6574 0.042*
H211 0.0189 0.1114 0.3766 0.039*
H213 0.0297 0.1860 0.3557 0.039*
H221 0.1743 −0.0069 0.4138 0.046*
H222 0.1572 0.0448 0.3209 0.046*
H223 0.0925 0.0201 0.3975 0.046*
H601 0.0230 0.3250 0.3010 0.112*
H701 0.0200 0.4481 0.3040 0.078*
H801 0.3826 0.0823 0.3665 0.071*
H802 0.4438 0.0423 0.3225 0.071*
H901 0.3835 0.1141 0.5812 0.086*
H902 0.4526 0.0941 0.5222 0.085*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-4
Acta Cryst. (2007). E63, m241–m243
Geometric parameters (Å, º)
Mn1—O1 2.1206 (11) C16—C17 1.542 (2)
Mn1—O3 2.1276 (10) C17—C18 1.529 (2)
Mn1—O4i 2.1222 (11) C18—C19 1.5543 (18)
Mn1—O5 2.4032 (11) C19—C20 1.547 (2)
Mn1—N1 2.3042 (12) C19—C21 1.5292 (19)
Mn1—N2 2.2353 (11) O6—H601 0.911
O1—C13 1.2824 (18) O6—H601ii 0.911
O2—C13 1.2344 (18) O7—H701 0.899
O3—C20 1.2533 (16) O7—H701ii 0.899
O4—C20 1.2597 (16) O8—H801 0.906
O5—C15 1.4495 (17) O8—H802 0.920
O5—C18 1.4535 (17) O9—H901 0.905
N1—C1 1.318 (2) O9—H902 0.923
N1—C12 1.3602 (18) C1—H1 0.930
N2—C10 1.320 (2) C2—H2 0.930
N2—C11 1.3591 (17) C3—H3 0.930
C1—C2 1.393 (2) C5—H5 0.930
C2—C3 1.362 (2) C6—H6 0.930
C3—C4 1.395 (2) C8—H8 0.930
C4—C5 1.437 (2) C9—H9 0.930
C4—C12 1.4167 (19) C10—H10 0.930
C5—C6 1.337 (2) C15—H15 0.980
C6—C7 1.434 (2) C16—H161 0.970
C7—C8 1.399 (2) C16—H162 0.970
C7—C11 1.411 (2) C17—H171 0.970
C8—C9 1.372 (2) C17—H172 0.970
C9—C10 1.395 (2) C18—H18 0.980
C11—C12 1.432 (2) C21—H211 0.960
C13—C14 1.550 (2) C21—H212 0.960
C14—C15 1.5530 (19) C21—H213 0.960
C14—C19 1.605 (2) C22—H221 0.960
C14—C22 1.534 (2) C22—H222 0.960
C15—C16 1.532 (2) C22—H223 0.960
O1···O9 2.7710 (17) C10···H9xiv 3.317
O2···O8 2.7466 (18) C10···H801i 3.206
O2···C2iii 3.475 (2) C12···H221xi 3.374
O4···C5iv 3.2506 (18) C13···H2iii 3.575
O4···C6iv 3.1584 (17) C13···H801 2.761
O6···O7 3.597 (3) C13···H901 2.751
O6···O9i 2.949 (2) C15···H222xi 3.511
O6···O9v 2.949 (2) C16···H161xviii 3.051
O6···C21 3.575 (2) C16···H162xiv 3.303
O6···C21ii 3.575 (2) C16···H171xviii 3.251
O7···O6 3.597 (3) C16···H172xiv 3.217
O7···O8vii 2.836 (2) C16···H222xi 3.418
O7···O9i 2.809 (2) C16···H223xviii 3.044
O7···O9v 2.809 (2) C17···H161xviii 3.008
O8···O2 2.7466 (18) C17···H162xiv 3.361
O8···O7viii 2.836 (2) C17···H172xiv 2.990
O8···O9 2.772 (2) C17···H223xviii 3.073
O8···C9i 3.565 (2) C18···H172xiv 3.461
O8···C9ix 3.367 (2) C20···H6iv 3.587
O8···C10i 3.366 (2) C21···H5iv 3.425
O9···O1 2.7710 (17) C21···H6iv 3.113
O9···O6i 2.949 (2) C21···H6xiv 3.391
O9···O7i 2.809 (2) C21···H211ii 3.224
O9···O8 2.772 (2) C21···H212ii 3.088
O9···C13 3.587 (2) C21···H213ii 3.128
N1···C6x 3.572 (2) C22···H5iv 3.567
N2···C5x 3.354 (2) C22···H15iii 3.499
C2···O2xi 3.475 (2) C22···H162iii 3.093
C3···C8x 3.540 (2) C22···H171xviii 3.531
C3···C9x 3.438 (2) H1···O7i 2.965
C4···C7x 3.553 (2) H1···O9 2.793
C4···C11x 3.580 (2) H221···N1iii 3.499
C5···O4xii 3.2506 (18) H221···C1iii 3.355
C5···N2x 3.354 (2) H221···C2iii 3.138
C5···C11x 3.501 (2) H221···C3iii 3.037
C6···O4xii 3.1584 (17) H221···C4iii 3.157
C6···N1x 3.572 (2) H221···C12iii 3.374
C6···C12x 3.533 (2) H221···H2iii 3.567
C7···C4x 3.553 (2) H221···H3iii 3.426
C7···C12x 3.453 (2) H221···H15iii 3.396
C8···C3x 3.540 (2) H221···H162iii 3.406
C9···O8i 3.565 (2) H221···H171xviii 3.380
C9···O8xiii 3.367 (2) H221···H172xviii 3.548
C9···C3x 3.438 (2) H222···C5iv 3.503
C10···O8i 3.366 (2) H222···C15iii 3.511
C11···C4x 3.580 (2) H222···C16iii 3.418
C11···C5x 3.501 (2) H222···H5iv 2.708
C12···C6x 3.533 (2) H222···H15iii 2.868
C12···C7x 3.453 (2) H222···H162iii 2.712
C13···O9 3.587 (2) H223···C16xviii 3.044
C21···O6 3.575 (2) H223···C17xviii 3.073
C21···C21ii 3.333 (2) H223···H161xviii 2.745
Mn1···H901 3.244 H223···H162xviii 2.965
O1···H801 3.095 H223···H162iii 2.717
O1···H901 1.867 H223···H171xviii 2.820
O1···H902 3.173 H223···H172xviii 2.980
O2···H2iii 3.145 H601···O4 3.421
O2···H3iv 3.442 H601···O7 3.139
supporting information
sup-6
Acta Cryst. (2007). E63, m241–m243
O2···H801 1.842 H601···O9v 3.276
O2···H802 3.170 H601···C8xiv 3.433
O2···H901 3.013 H601···H1i 3.095
O2···H902 3.466 H601···H6iv 3.255
O3···H901i 3.407 H601···H6xiv 3.340
O4···H5iv 2.767 H601···H8iv 3.395
O4···H6iv 2.584 H601···H8xiv 2.511
O4···H601 3.421 H601···H213 2.941
O4···H901i 3.512 H601···H213ii 3.545
O5···H172xiv 3.131 H601···H701 2.535
O6···H6iv 2.872 H601···H701ii 2.926
O6···H6xiv 2.872 H601···H901i 2.504
O6···H8iv 2.833 H601···H902i 2.725
O6···H8xiv 2.833 H701···O6 3.165
O6···H213 2.693 H701···O8vi 3.193
O6···H213ii 2.693 H701···O8vii 3.469
O6···H701 3.165 H701···O9i 1.912
O6···H701ii 3.165 H701···O9v 3.201
O6···H901i 3.393 H701···C1i 3.468
O6···H901v 3.393 H701···H1i 2.636
O7···H1i 2.965 H701···H1v 3.123
O7···H1v 2.965 H701···H2v 3.217
O7···H2i 3.346 H701···H601 2.535
O7···H2v 3.346 H701···H601ii 2.926
O7···H601 3.139 H701···H802vi 2.381
O7···H601ii 3.139 H701···H802vii 2.552
O7···H801vi 3.384 H701···H901i 2.533
O7···H801vii 3.384 H701···H902i 2.303
O7···H802vi 1.951 H801···O1 3.095
O7···H802vii 1.951 H801···O2 1.842
O7···H901i 3.392 H801···O7viii 3.384
O7···H901v 3.392 H801···O9 2.857
O7···H902i 3.166 H801···C2iii 3.340
O7···H902v 3.166 H801···C10i 3.206
O8···H2iii 2.807 H801···C13 2.761
O8···H9i 3.085 H801···H2iii 2.628
O8···H9ix 2.462 H801···H3iv 3.470
O8···H10i 2.654 H801···H9i 3.373
O8···H701viii 3.193 H801···H9ix 3.155
O8···H701xv 3.469 H801···H10i 2.467
O8···H802xvi 3.235 H801···H901 2.654
O8···H901 2.861 H801···H902 2.242
O8···H902 1.954 H802···O2 3.170
O9···H1 2.793 H802···O7viii 1.951
O9···H2iii 3.577 H802···O8xvi 3.235
O9···H8ix 2.917 H802···O9 3.480
O9···H9ix 3.577 H802···C2iii 3.328
O9···H601xvii 3.276 H802···H2iii 2.572
O9···H701i 1.912 H802···H9i 3.183
O9···H701xvii 3.201 H802···H9ix 2.917
O9···H801 2.857 H802···H10i 3.305
O9···H802 3.480 H802···H701viii 2.381
N1···H6x 3.471 H802···H701xv 2.552
N1···H221xi 3.499 H802···H802xvi 2.670
N2···H5x 3.345 H802···H902 2.622
C1···H8x 3.579 H901···Mn1 3.244
C1···H221xi 3.355 H901···O1 1.867
C1···H701i 3.468 H901···O2 3.013
C1···H901 3.328 H901···O3i 3.407
C2···H8x 3.535 H901···O4i 3.512
C2···H221xi 3.138 H901···O6i 3.393
C2···H801xi 3.340 H901···O7i 3.392
C2···H802xi 3.328 H901···O8 2.861
C3···H15xi 3.540 H901···C1 3.328
C3···H221xi 3.037 H901···C13 2.751
C4···H221xi 3.157 H901···H1 2.533
C5···H212xii 3.054 H901···H2iii 3.465
C5···H222xii 3.503 H901···H8ix 3.512
C6···H171xiv 3.538 H901···H601i 2.504
C6···H211xiv 3.489 H901···H701i 2.533
C6···H212xii 3.013 H901···H801 2.654
C6···H213xiv 3.168 H902···O1 3.173
C7···H18xiv 3.506 H902···O2 3.466
C7···H171xiv 3.552 H902···O7i 3.166
C7···H213xiv 3.573 H902···O8 1.954
C8···H18xiv 3.032 H902···C8ix 3.449
C8···H213xiv 3.482 H902···C9ix 3.485
C8···H601xiv 3.433 H902···H2xix 3.383
C8···H902xiii 3.449 H902···H2iii 3.156
C9···H3x 3.416 H902···H8ix 2.812
C9···H9xiv 3.216 H902···H9ix 2.891
C9···H10xiv 3.407 H902···H601i 2.725
C9···H18xiv 3.275 H902···H701i 2.303
C9···H902xiii 3.485 H902···H801 2.242
C10···H3x 3.581 H902···H802 2.622
C10···H5x 3.542
O1—Mn1—O3 95.40 (4) O5—C18—C19 101.90 (10)
O1—Mn1—O4i 104.27 (4) C17—C18—C19 112.87 (12)
O1—Mn1—N1 92.69 (4) C14—C19—C18 100.39 (10) O1—Mn1—N2 153.59 (4) C14—C19—C20 112.35 (11) O3—Mn1—O4i 91.41 (4) C14—C19—C21 114.83 (11)
supporting information
sup-8
Acta Cryst. (2007). E63, m241–m243
O4i—Mn1—N2 101.08 (4) O3—C20—O4 123.42 (13)
O4i—Mn1—O5 168.22 (4) O3—C20—C19 118.61 (11)
O5—Mn1—O1 78.08 (4) O4—C20—C19 117.96 (11)
O5—Mn1—O3 76.85 (4) H601—O6—H601ii 106.6
O5—Mn1—N1 86.27 (4) H701—O7—H701ii 109.5
O5—Mn1—N2 78.63 (4) H801—O8—H802 107.2
N1—Mn1—N2 73.51 (4) H901—O9—H902 108.8
Mn1—O1—C13 125.87 (9) N1—C1—H1 118.1
Mn1—O3—C20 119.31 (9) C2—C1—H1 118.1
Mn1i—O4—C20 117.86 (9) C1—C2—H2 120.6
C15—O5—C18 96.08 (10) C3—C2—H2 120.6
Mn1—N1—C1 128.35 (10) C2—C3—H3 120.0
Mn1—N1—C12 113.74 (9) C4—C3—H3 120.0
C1—N1—C12 117.89 (12) C4—C5—H5 119.3
Mn1—N2—C10 125.09 (9) C6—C5—H5 119.3
Mn1—N2—C11 116.25 (9) C5—C6—H6 119.3
C10—N2—C11 118.48 (12) C7—C6—H6 119.3
N1—C1—C2 123.80 (15) C7—C8—H8 120.1
C1—C2—C3 118.75 (17) C9—C8—H8 120.1
C2—C3—C4 120.03 (15) C8—C9—H9 120.6
C3—C4—C5 123.82 (14) C10—C9—H9 120.6
C3—C4—C12 117.42 (13) N2—C10—H10 118.4
C5—C4—C12 118.75 (14) C9—C10—H10 118.4
C4—C5—C6 121.50 (14) O5—C15—H15 112.9
C5—C6—C7 121.34 (14) C14—C15—H15 112.9
C6—C7—C8 123.53 (14) C16—C15—H15 112.9
C6—C7—C11 118.96 (14) C15—C16—H161 111.4
C8—C7—C11 117.49 (13) C15—C16—H162 111.4
C7—C8—C9 119.83 (15) C17—C16—H161 111.4
C8—C9—C10 118.77 (16) C17—C16—H162 111.4
N2—C10—C9 123.27 (14) H161—C16—H162 109.5
N2—C11—C7 122.14 (13) C16—C17—H171 111.4
N2—C11—C12 118.01 (12) C16—C17—H172 111.4 C7—C11—C12 119.84 (12) C18—C17—H171 111.4
N1—C12—C4 122.10 (13) C18—C17—H172 111.4
N1—C12—C11 118.35 (12) H171—C17—H172 109.5
C4—C12—C11 119.54 (12) O5—C18—H18 113.1
O1—C13—O2 123.92 (14) C17—C18—H18 113.1
O1—C13—C14 117.23 (12) C19—C18—H18 113.1
C14—C15—C16 113.50 (12) H221—C22—H222 109.5 C15—C16—C17 101.65 (12) H221—C22—H223 109.5 C16—C17—C18 101.55 (12) H222—C22—H223 109.5 O5—C18—C17 101.77 (11)
O1—Mn1—O3—C20 16.28 (10) C5—C4—C12—N1 178.81 (14) O3—Mn1—O1—C13 −22.09 (12) C5—C4—C12—C11 −0.2 (2) O1—Mn1—O4i—C20i 51.85 (11) C12—C4—C5—C6 1.3 (2)
O4i—Mn1—O1—C13 −114.93 (12) C4—C5—C6—C7 −0.3 (2)
O1—Mn1—N1—C1 23.69 (14) C5—C6—C7—C8 179.74 (17) O1—Mn1—N1—C12 −154.53 (9) C5—C6—C7—C11 −2.0 (2) N1—Mn1—O1—C13 138.83 (12) C6—C7—C8—C9 177.27 (16) O1—Mn1—N2—C10 −117.50 (13) C6—C7—C11—N2 −176.56 (14) O1—Mn1—N2—C11 57.55 (15) C6—C7—C11—C12 3.1 (2) N2—Mn1—O1—C13 81.81 (15) C8—C7—C11—N2 1.8 (2) O3—Mn1—O4i—C20i −44.09 (10) C8—C7—C11—C12 −178.55 (14)
O4i—Mn1—O3—C20 120.76 (10) C11—C7—C8—C9 −1.0 (2)
O3—Mn1—N1—C1 136.71 (14) C7—C8—C9—C10 −0.3 (2) O3—Mn1—N1—C12 −41.52 (17) C8—C9—C10—N2 1.0 (2) N1—Mn1—O3—C20 −96.27 (15) N2—C11—C12—N1 −1.4 (2) O3—Mn1—N2—C10 −12.65 (12) N2—C11—C12—C4 177.65 (13) O3—Mn1—N2—C11 162.40 (10) C7—C11—C12—N1 178.95 (13) N2—Mn1—O3—C20 −138.13 (10) C7—C11—C12—C4 −2.0 (2) O4i—Mn1—N1—C1 −81.85 (14) O1—C13—C14—C15 −27.46 (17)
O4i—Mn1—N1—C12 99.92 (10) O1—C13—C14—C19 82.75 (15)
N1—Mn1—O4i—C20i 148.65 (10) O1—C13—C14—C22 −150.41 (13)
O4i—Mn1—N2—C10 79.03 (13) O2—C13—C14—C15 150.55 (13)
O4i—Mn1—N2—C11 −105.92 (10) O2—C13—C14—C19 −99.24 (15)
N2—Mn1—O4i—C20i −135.65 (10) O2—C13—C14—C22 27.60 (18)
N1—Mn1—N2—C10 −178.41 (13) C13—C14—C15—O5 83.87 (13) N1—Mn1—N2—C11 −3.36 (9) C13—C14—C15—C16 −168.19 (12) N2—Mn1—N1—C1 −179.21 (14) C13—C14—C19—C18 −116.63 (12) N2—Mn1—N1—C12 2.57 (9) C13—C14—C19—C20 −2.59 (14) Mn1—O1—C13—O2 132.53 (13) C13—C14—C19—C21 120.76 (13) Mn1—O1—C13—C14 −49.57 (17) C15—C14—C19—C18 −1.11 (13) Mn1—O3—C20—O4 −119.08 (13) C15—C14—C19—C20 112.92 (11) Mn1—O3—C20—C19 61.55 (15) C15—C14—C19—C21 −123.72 (12) Mn1i—O4—C20—O3 14.25 (19) C19—C14—C15—O5 −34.76 (13)
Mn1i—O4—C20—C19 −166.39 (9) C19—C14—C15—C16 73.17 (14)
supporting information
sup-10
Acta Cryst. (2007). E63, m241–m243
C12—N1—C1—C2 −0.9 (2) C16—C17—C18—C19 74.85 (14) Mn1—N2—C10—C9 174.67 (12) O5—C18—C19—C14 36.49 (13) Mn1—N2—C11—C7 −176.57 (11) O5—C18—C19—C20 −81.13 (13) Mn1—N2—C11—C12 3.80 (16) O5—C18—C19—C21 159.74 (11) C10—N2—C11—C7 −1.2 (2) C17—C18—C19—C14 −71.88 (13) C10—N2—C11—C12 179.19 (13) C17—C18—C19—C20 170.50 (11) C11—N2—C10—C9 −0.3 (2) C17—C18—C19—C21 51.37 (17) N1—C1—C2—C3 −0.0 (2) C14—C19—C20—O3 −90.97 (14) C1—C2—C3—C4 0.8 (2) C14—C19—C20—O4 89.64 (14) C2—C3—C4—C5 −179.65 (17) C18—C19—C20—O3 18.59 (17) C2—C3—C4—C12 −0.7 (2) C18—C19—C20—O4 −160.80 (12) C3—C4—C5—C6 −179.71 (17) C21—C19—C20—O3 141.71 (13) C3—C4—C12—N1 −0.2 (2) C21—C19—C20—O4 −37.69 (17) C3—C4—C12—C11 −179.22 (14)
Symmetry codes: (i) −x+1/2, −y+1/2, −z+1; (ii) −x, y, −z+1/2; (iii) x, −y, z−1/2; (iv) x, y, z−1; (v) x−1/2, −y+1/2, z−1/2; (vi) x−1/2, y+1/2, z; (vii) −x+1/2,
y+1/2, −z+1/2; (viii) x+1/2, y−1/2, z; (ix) x+1/2, −y+1/2, z−1/2; (x) −x+1/2, −y+1/2, −z+2; (xi) x, −y, z+1/2; (xii) x, y, z+1; (xiii) x−1/2, −y+1/2, z+1/2; (xiv) −x, y, −z+3/2; (xv) −x+1/2, y−1/2, −z+1/2; (xvi) −x+1, y, −z+1/2; (xvii) x+1/2, −y+1/2, z+1/2; (xviii) −x, −y, −z+1; (xix) −x+1, y, −z+3/2.
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O(6)—H(601)···O(9)i 0.91 2.04 2.949 (2) 174
O(7)—H(701)···O(9)i 0.90 1.91 2.809 (2) 176
O(8)—H(801)···O(2) 0.91 1.84 2.7466 (18) 176 O(8)—H(802)···O(7)viii 0.92 1.95 2.836 (2) 161
O(9)—H(901)···O(1) 0.91 1.87 2.7710 (17) 178 O(9)—H(902)···O(8) 0.92 1.95 2.772 (2) 147