Hydroxyl Radical Generation in Oxygen-treated Infants
Gert Lubec, MD*; John A. Widness, MD‡; Michael Hayde, MD*; Daniel Menzel, MD*; and Arnold Pollak, MD*
ABSTRACT. Objective. Because the hydroxyl radical is capable of oxidizing phenylalanine to O-tyrosine, we sought to determine whether increased levels of O -ty-rosine are found in urine of infants treated with supple-mental oxygen.
Methods. A total of 39 consecutively admitted neo-nates to an intensive care unit were included. Twenty-seven received supplemental oxygen therapy for respira-tory disease, and 12 did not. Urinary O-tyrosine levels were determined on two or more occasions using high-performance liquid chromatography with results ex-pressed as a percentage of the urinary phenylalanine concentration. Using simple and stepwise multiple linear regression analyses, urinaryO-tyrosine was examined for associations with relevant clinical conditions and labora-tory measurements.
Results. Infants supplemented with oxygen showed significantly higher mean6SEM urinaryO-tyrosine lev-els (0.40% 6 0.028) compared with those remaining in room air (0.18%60.012). Mean daily FIO2was the clinical and laboratory variable most highly correlated with uri-nary O-tyrosine (r 5 0.66). In the stepwise regression, significant associations were also found for renal frac-tional sodium excretion and Apgar score at 5 minutes.
Conclusions. Hydroxylation at the O position of phenylalanine, a specific direct marker for the hy-droxyl radical attack, was strongly associated with ox-ygen treatment in neonates. This finding increases our understanding of the pathogenesis of oxygen injury and suggests a basis for developing therapeutic ap-proaches. Pediatrics 1997;100:700 –704; hydroxyl radi-cal, oxygen,O-tyrosine, infants.
T
he role of oxygen in the development of neo-natal lung injury and disease is unequivocal. The principal hypothesis put forth to explain this process is the oxygen free radical theory.1 In 1981, Freeman and Crapo2 showed that hyperoxia caused increased oxygen radical production in rat lungs. Two years later, they demonstrated that su-peroxide dismutase supplementation of media in which endothelial cells were grown prevented oxy-gen injury.3Based on these encouraging results, su-peroxide dismutase treatment was tested in newborn infants with lung disease, and some benefit was ob-served.4,5These and subsequent studies strengthened the free radical hypothesis, but without providingdirect evidence regarding the specific type of oxygen species or free radical(s) responsible.6 – 8
The first direct evidence that superoxide and hy-droxyl radicals (zOH) are involved in hyperoxia came from studies of Zweier et al.9These authors demon-strated that exposure to high oxygen concentrations specifically leads to zOH production in pulmonary endothelial cells grown in tissue culture. Their pio-neering in vitro work encouraged us to investigate the involvement of zOH in a premature neonatal animal model of lung disease. Based on the principle that zOH attack of phenylalanine results in o -hy-droxylation with the generation of o-tyrosine, we demonstrated the presence of this reactive species in lung tissue of preterm guinea pigs maintained for up to 4 weeks in a high-oxygen environment.10 In the present study, we extend these observations to hu-man infants treated with supplemental oxygen for clinical indications by demonstrating oxygen dose-dependent elevations of urinaryo-tyrosine.
METHODS
The study was approved by the hospital’s ethics committee, and parental consent was obtained. A total of 39 infants, including 27 receiving supplemental oxygen for treatment of various clinical conditions, were the subjects of this study. Consecutive admission to the neonatal intensive care unit was the only criterion for enrollment. All study subjects had two or more 24-hour urine collections foro-tyrosine measurement before death or discharge. The mean6SEM number of days during which urineo-tyrosine measurements were made was significantly greater in the group receiving oxygen supplementation compared with the room air group (3.460.28 vs 2.160.08, respectively [P,.01]).
Twenty-four hour urine collections were performed using ad-hesive urinary collection bags, starting as early as 12 hours of age. Only complete collections were included. No preservatives were added. Samples were kept at 4°C until completion of collection and then frozen at240°C until analyzed. The protocol required two to four complete 24-hour urinary collections. All infants re-ceived orally 5 mg vitamin E per kilogram daily in two doses (Ephynal, Hoffmann-La Roche, Basel, Switzerland) while receiv-ing supplemental oxygen. Vitamins A and C were not supple-mented in their intravenous alimentation. Enterally fed infants receiving formula obtained 105 mg of vitamin A and 16mg of vitamin C per 100 mL.
Hospital charts were reviewed for determination of birth weight, gestational age at birth, 5-minute Apgar score, and clinical diagnoses. Specific clinical criteria were applied in defining clini-cal diagnoses encountered among infants studied. Respiratory distress syndrome was diagnosed using standard clinical and radiologic criteria,11patent ductus arteriosus by echocardiogram,
and sepsis by a positive blood or spinal fluid culture and a clinical course consistent with sepsis. Pneumonia was defined by clinical criteria, a supportive chest radiograph, and laboratory findings. Intraventricular hemorrhage was diagnosed by cranial ultrasound or findings at autopsy. Criteria applied for the diagnosis of bron-chopulmonary dysplasia were continuous oxygen treatment be-fore death or at 28 days of life and a chest radiograph consistent with chronic lung disease.
From the *Department of Pediatrics, Division of Neonatology, University of Vienna, Vienna, Austria; and the ‡Department of Pediatrics, University of Iowa, Iowa City, Iowa.
Received for publication Sep 3, 1996; accepted Mar 26, 1997.
Data Management and Statistical Analyses
In addition to clinical information, hospital charts were re-viewed for Fio
2and laboratory information. Fio2data from birth
to the last day that urinaryo-tyrosine data were available were recorded to calculate mean daily Fio
2. With the exception of peak
plasma bilirubin, all laboratory data used in the statistical corre-lation analyses were the average of all values from all days that 24-hour urinary o-tyrosine collections were performed. pH and Pco
2determinations included both capillary and arterial samples,
whereas Pao2values were only from arterial samples. From these, the single lowest pH and Pao
2values recorded and the single
highest Pco2value on a given study day were averaged. Other laboratory determinations included urinary creatinine and plasma and urinary sodium levels. With the exception of that for o-tyrosine and phenylalanine, all analyses were performed in the hospital‘s clinical laboratory.
Statistical analyses were performed using StatView 4.5 (Abacus Concepts, Inc, Berkeley, CA). Between-group comparisons of con-tinuous variables were performed using Student’s unpaired t test, whereas discontinuous variables were compared using Fisher‘s exact test. Simple and stepwise multiple linear regressions were applied in testing for associations between study variables. Unless otherwise stated, results are presented as the mean6SEM. All testing was performed using two-tailed significance testing, with analevel of P,.05 considered as significant.
Determination of UrinaryO-Tyrosine and Phenylalanine
Aliquots of frozen 24-hour urine collections were thawed on ice, centrifuged, charcoal-treated,12 and subjected to anion
ex-change.13Urinaryo-tyrosine and phenylalanine were determined by reversed-phase high-performance liquid chromatography sep-aration on a Supersphere 100 (Analytichem International, Harbor City, CA), 4mm, 10034.6 mm column using fluorescence detec-tion as reported by Ishimitsu et al.14The pump used was a
Shi-madzu LC6A (ShiShi-madzu Corporation, Kyoto, Japan), the sampler was a SIL and SCL-6B (Shimadzu), and the integrator was a CR4AX. A Jasco fluorometer 820 FP detector was used to measure emission absorbance at 305 nm after excitation at 275 nm. The intraassay variation coefficient was 1.7%, the interassay 2.4%. o-tyrosine was identified using commercially available standards (T3504, Sigma, St Louis, MO) and identity verified by electrospray mass-spectrometry.
RESULTS
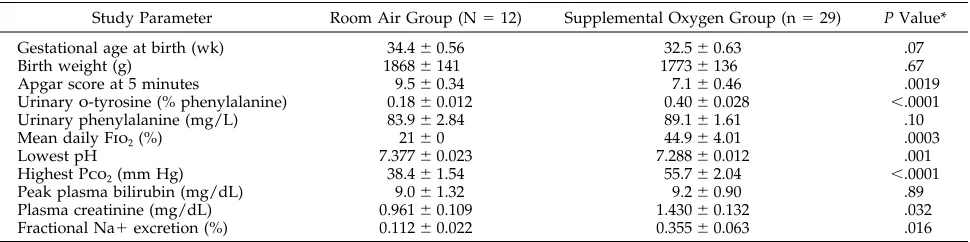
Compared with infants in the the room air group, the mean percentage of urinaryo-tyrosine was sig-nificantly greater in infants receiving supplemental oxygen therapy (P, .0001) (Table 1). There was no difference between the two groups in the 24-hour urinary phenylalanine excretion. Although infants on supplemental oxygen demonstrated a tendency for being born at an earlier gestational age, there was no association of gestational age and mean urinary o-tyrosine for either this group or the room air group (Fig 1). The supplemental oxygen group had
signif-icantly lower 5-minute Apgar scores, lower daily pH values, higher daily Pco2levels, higher plasma cre-atinine concentrations, and higher renal fractional sodium excretion. There was no difference for the mean daily protein intake between the room air group and the supplemental oxygen group (2.28 6 0.46 vs 2.05 6 0.21 g per kilogram of body weight, respectively).
As a group, the oxygen-supplemented group dem-onstrated a more complicated hospital course (Table 2). Infants in this group were more likely to have developed bronchopulmonary dysplasia, to have manifest respiratory distress syndrome, to have de-veloped a clinically significant patent ductus arteri-osus, to have received aminoglycoside treatment, and to have died.
Correlation analysis of all 39 study subjects in both groups demonstrated that the mean urinary o -ty-rosine was significantly associated with several of the laboratory parameters. The most highly corre-lated of these was mean Fio
2(r50.66, P,.001) (Fig 2). When examined separately for only the 27 infants in the supplemental oxygen group, a significant as-sociation was also observed (r50.49, P,.01) (data not shown). Urinaryo-tyrosine was also significantly associated with Apgar score at 5 minutes (r5 0.53, P,.001), the highest Pco
2(r50.37, P,.05), plasma creatinine concentration (r50.39, P,.05), and renal fractional sodium excretion (r 5 0.55, P , .001) (Table 3).
Fig 1. Relationship between mean 24-hour urinaryo-tyrosine (ex-pressed as percentage of phenylalanine) and gestational age at birth in weeks.
TABLE 1. Mean6SEM Clinical and Laboratory Data by Study Group
Study Parameter Room Air Group (N512) Supplemental Oxygen Group (n529) P Value*
Gestational age at birth (wk) 34.460.56 32.560.63 .07
Birth weight (g) 18686141 17736136 .67
Apgar score at 5 minutes 9.560.34 7.160.46 .0019
Urinaryo-tyrosine (% phenylalanine) 0.1860.012 0.4060.028 ,.0001
Urinary phenylalanine (mg/L) 83.962.84 89.161.61 .10
Mean daily Fio
2(%) 2160 44.964.01 .0003
Lowest pH 7.37760.023 7.28860.012 .001
Highest Pco
2(mm Hg) 38.461.54 55.762.04 ,.0001
Peak plasma bilirubin (mg/dL) 9.061.32 9.260.90 .89
Plasma creatinine (mg/dL) 0.96160.109 1.43060.132 .032
Fractional Na1excretion (%) 0.11260.022 0.35560.063 .016
Using stepwise multiple regression with urinary o-tyrosine entered as the dependent variable, mean daily Fio2 was the most highly correlated variable. Fio
2 was followed in order by renal fractional so-dium excretion and 5-minute Apgar score. The re-maining variables shown in Table 1 did not contrib-ute additional significance.
Urinary o-tyrosine levels among the oxygen-treated infants were examined with respect to the kinetics of urinaryo-tyrosine. The period from birth to the first urinary o-tyrosine measurement taken was examined. Twenty-three of the 27 oxygen-sup-plemented infants had urinary o-tyrosine measure-ments performed within 2 days of birth. The mean urinaryo-tyrosine for this group (0.34%60.025) was significantly greater than that for the 11 room air-treated infants sampled within the same interval (0.18 6 0.019%) (P5 .0003). Although most infants experienced a very gradual decline in urinary o-tyrosine after being removed from supplemental oxygen (ie, over several weeks), interpretation was complicated by marked variability in the concentra-tion of oxygen received by individual infants for variable periods of time. Thus, rigorous analysis of the decline in urinaryo-tyrosine after curtailment of oxygen supplementation was not possible.
DISCUSSION
Hydroxyl radical attack may well be the primary mechanism of injury responsible for the acute and chronic complications in human infants receiving
long-term oxygen therapy. Our findings of increased urinary o-tyrosine levels provide direct evidence that infants receiving oxygen therapy experience hy-droxyl radical attack. This is in agreement with re-sults of our previous animal studies, in which pre-mature guinea pigs were exposed to hyperoxia for 4 weeks.10Aromatico-hydroxylation was confirmed in these animals by demonstrating specific zOH attack on free and/or bound (protein-derived) phenylala-nine, leading to the formation of o-tyrosine, a reli-able, dose-dependent marker specific for hydroxyl radical attack.15–17 The biochemical consequences of zOH attack are protein oxidation with subsequent impairment of protein function, lipid peroxidation with modification and destruction of membrane lip-ids, and DNA oxidation and breakage. The patho-physiologic manifestation of these events is an alter-ation of important biomolecules resulting in cell injury, dysfunction, benign and malignant prolifera-tion, and death.
Both the dose-dependent increase in urinary o-tyrosine concentration correlating with Fio
2 and the stepwise regression indicating that Fio2was the variable most closely associated with urinary o -ty-rosine suggest that oxygen exposure is a primary mechanism forzOH generation. Our findings are in agreement with those of Schlenzig and coworkers,8 who observed increased urinary malondialdehyde, a marker of lipid peroxidation in low birth weight infants receiving oxygen treatment. The hydroxyl radical attack of polyunsaturated fatty acids inevita-bly leads to the oxidative break of these fatty acids, resulting in the production of malondialdehyde and other alkenals. A greater zOH attack would have been expected in immature infants, because lower antioxidant enzyme activities (eg, glutathione perox-idase, superoxide dismutase, or catalase) have been shown in immature individuals.18,19 Because there was no correlation with either gestational age or birth weight with o-tyrosine (Figure 1), immaturity per se was not found to be a major contributor to our o-tyrosine findings.
Fractional sodium excretion, the second most highly correlating variable with urinary o-tyrosine, is probably only indirectly associated. More likely, along with the elevated plasma creatinine levels, it reflects the greater severity of illness within the group of infants on supplemental oxygen. If an im-mature glomerular–tubular reabsorption system is to
TABLE 2. Clinical Diagnoses and Conditions by Study Group
Diagnosis Room Air Group
(N512)
Supplemental Oxygen Group
(N529)
P Value*
Condition Present Condition Present
N % N %
Bronchopulmonary dysplasia 0 0.0 8 29.6 .042
Respiratory distress syndrome 0 0.0 21 77.8 ,.0001
Symptomatic patent ductus arteriosis 0 0.0 14 51.9 .003
Sepsis, meningitis, or pneumonia 2 16.7 8 29.6 .46
Intraventricular hemorrhage 0 0.0 5 18.5 .30
Aminoglycoside treatment 3 25.0 19 70.4 .035
Death 0 0.0 8 29.6 .042
* Room air group compared with supplemental oxygen group.
be incriminated as an explanation for increased uri-nary o-tyrosine, one would have anticipated in-creased urinary phenylalanine losses. This was not found, thus making preferential urinary o-tyrosine losses within the group of infants receiving supple-mental oxygen unlikely. Similarly, the frequent oc-curence of patent ductus arteriosus within the group of infants on supplemental oxygen is probably not the direct result of pathophysiologic processes asso-ciated with increased oxygen free radicals. In con-trast, hydroxyl radical attack may well be the pri-mary mechanism of injury responsible for chronic complications such as bronchopulmonary dysplasia experienced by infants receiving long-term oxygen treatment.
The significant association of the Apgar score at 5 minutes of life and urinary o-tyrosine could be of importance because of the known association of hy-poxic ischemia reperfusion with free radical genera-tion in neonates.20 However, because many of the oxygen-treated infants did not experience birth as-phyxia, yet had elevated urinaryo-tyrosine levels in the first days of life, it does not appear that free radical generation during hypoxia-reperfusion injury was a primary generator of free radicals in these infants. This does not exclude a role of free radicals in the higher incidence of intraventricular hemor-rhage observed among infants in the present study. Similarly, although gentamicin is known to generate hydroxyl radicals21 and was also associated with higher o-tyrosine levels, it could also be a mere re-flection of the severity of illness in the oxygen-treated group. Finally, infection, which has also been associated with hydroxyl radical generation as a re-sult of the inflammatory process,22 did not occur more commonly among infants on supplemental oxygen.
Based on limited kinetic data, we observed that within 2 days of birth, urinaryo-tyrosine levels pre-sumably increase after exposure to oxygen treat-ment. Because urinaryo-tyrosine was not measured in these infants before oxygen treatment, our sugges-tion that o-tyrosine levels rise rapidly with oxygen treatment must remain tentative. What appeared to have been a very slow return to baseline urinary o-tyrosine levels after the curtailment of oxygen ther-apy may have been the result of increased metabo-lism of oxygen-damaged proteins containing o -ty-rosine.
Oxidative stress among infants receiving oxygen therapy has been reported previously without docu-mentation of the oxygen free radical or oxygen spe-cies involved. Identification of the oxygen spespe-cies involved is crucial, because it provides a valuable clue to developing therapeutic strategies. Our find-ing of o-hydroxylation of phenylalanine in associa-tion with oxygen treatment of infants provides evi-dence for direct involvement of hydroxyl radicals in this process. Although other variables likely to be associated with hydroxyl radical formation were not examined, we hypothesize that a rational therapeutic approach using hydroxyl radical scavengers may offer protective benefit when coadministered with oxygen. TABLE 3. Correlation Matrix for All Study Subjects (N 5 39)
Urinary o-tyrosine Mean F io 2 Gestational Age Birth Weight Apgar at 5 Min Lowest pH [38]
Lowest Pao
2
[23]
Highest Pco
ACKNOWLEDGMENTS
This study was supported by the Red Bull Company, Salzburg, Austria, Verein zur Durchfu¨hrung der wissenschaftlichen Forschung auf dem Gebiet der Neonatologie und pa¨diatrischen Intensivmedizin, and the March of Dimes Birth Defects Founda-tion (grant FY 95– 0220).
We thank Mrs Maria Babich for excellent technical assistance.
REFERENCES
1. Frank L. Effects of oxygen on the newborn. Fed Proc. 1985;44:2328 –2834 2. Freeman BA, Crapo DJ. Hyperoxia causes increased oxygen radical production in rat lungs and rat mitochondria. J Biol Chem. 1981;256: 10986 –10991
3. Freeman BA, Young SL, Crapo JD. Liposome mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol
Chem. 1983;258:12534 –12540
4. Evans HE, Rosenfeld W, Jhaveri R, Concepcion L, Zabaleta I. Oxidant mediated lung disease in newborn infants. Free Rad Biol Med. 1986;2: 369 –372
5. Gerdes JS. Superoxide dismutase in prevention of bronchopulmonary dysplasia. J Pediatr. 1985;106:1057
6. Walther FJ, Kuipers IM, Pavlova Z, Willebrand D, Abuchowski A, Viau AT. Mitigation of pulmonary oxygen toxicity in premature lambs with intravenous antioxidants. Exp Lung Res. 1990;16:177–189
7. Pitkaenen OM, Hallmann M, Andersson SM. Correlation of free oxygen radical induced lipid peroxidation with outcome in very low birth weight infants. J Pediatr. 1990;116:760 –764
8. Schlenzig JS, Bervoets K, Loewenich Vv, Boehles H. Urinary malondi-aldehyde concentration in preterm neonates: is there a relationship to disease entities of neonatal intensive care? Acta Paediatr. 1993;82:202–205 9. Zweier JL, Duke SS, Kuppusamy P, Sylvester JT, Gabrielson EW. Elec-tron paramagnetic resonance evidence that cellular oxygen toxicity is caused by the generation of superoxide and hydroxyl free radicals.
FEBS Lett. 1989;252:12–16
10. Kelly F, Lubec G. Hyperoxic injury of immature guinea pig lung is mediated via hydroxyl radicals. Pediatr Res. 1995;38:286 –291 11. Rudolph AJ, Smith CA. Idiopathic respiratory distress syndrome of the
newborn. J Pediatr. 1960;57:905–921
12. Bartosch B, Vycudilik W, Popow C, Lubec G. Urinary 3-hydroxyproline excretion in Alport syndrome: a noninvasive screening test? Arch Dis
Child. 1991;66:259 –262
13. Lubec B, Arbeiter K. The determination of the urinary D/L trans 3-hy-droxyproline ratio: a noninvasive screening test for Alport syndrome.
J Pediatr. 1993;123:748 –751
14. Ishimitsu S, Fujimoto S, Ohara A. High performance liquid chromato-graphic determination ofm-tyrosine ando-tyrosine in rat urine. J
Chro-matogr. 1989;489:377–383
15. Karam LM, Bergtold DS, Simic MG. Biomarkers of OH radical damage in vivo. Free Radic Res Commun. 1991;12:11–16
16. Ibe FI, Grinter R, Massey R, Homer R. Detection of o-tyrosine in
irradiated chicken by reverse-phase HPLC and fluorescence detection.
Food Addit Contam. 1991;8:787–792
17. Lubec G. The hydroxl radical: from chemistry to disease. J Invest Med. 1996;44:324 –346
18. Langley SC, Kelly FJ. Different response of the glutathione system to fasting in neonatal and adult guinea pigs. Biochem Pharmacol. 1992;44: 1489 –1494
19. Sosenko LRS, Frank L. Guinea pig lung development antioxidant en-zymes and premature survival in high O2. Am J Physiol. 1987;252:
693– 698
20. Saugstad OD. Oxygen toxicity in the neonatal period. Acta Paediatr
Scand. 1990;79:881– 892
21. Miller RA, Britigan BE. The formation and biological significance of phagocyte-derived oxidants. J Invest Med. 1995;43:39 – 49
22. Du XH, Yang CL. Mechanism of gentamicine nephrotoxicity in rats and the protective effect of zinc-induced metallothionine synthesis. Nephrol
DOI: 10.1542/peds.100.4.700
1997;100;700
Pediatrics
Gert Lubec, John A. Widness, Michael Hayde, Daniel Menzel and Arnold Pollak
Hydroxyl Radical Generation in Oxygen-treated Infants
Services
Updated Information &
http://pediatrics.aappublications.org/content/100/4/700
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/100/4/700#BIBL
This article cites 22 articles, 3 of which you can access for free at:
Subspecialty Collections
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_
Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.100.4.700
1997;100;700
Pediatrics
Gert Lubec, John A. Widness, Michael Hayde, Daniel Menzel and Arnold Pollak
Hydroxyl Radical Generation in Oxygen-treated Infants
http://pediatrics.aappublications.org/content/100/4/700
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.