NIDCAP: A Systematic Review and Meta-analyses of
Randomized Controlled Trials
abstract
BACKGROUND AND OBJECTIVE:The“synactive”theory of neurobehavioral development forms the basis of the Newborn Individualized Develop-mental Care and Assessment Program (NIDCAP). Our objective was to assess the effectiveness of NIDCAP in improving outcomes in preterm infants.
METHODS:Medline, CINAHL, Embase, PsychInfo, The Cochrane Library, Pediatric Academic Societies’ Abstracts and Web of Science were searched in July 2010 and February 2012. The studies selected were randomized controlled trials testing the effectiveness of NIDCAP on medical and neurodevelopmental outcomes. The authors abstracted baseline characteristics of infants and outcomes. The risk of bias was assessed by using Cochrane criteria. RevMan 5.1 was used to synthe-size data by the use of relative risk and risk difference for dichoto-mous outcomes and mean or standardized mean difference for continuous outcomes.
RESULTS:Eleven primary and 7 secondary studies enrolling 627 neo-nates were included, with 2 of high quality. The composite primary out-comes of death or major sensorineural disability at 18 months corrected age or later in childhood (3 trials, 302 children; relative risk 0.89 [95% confidence interval 0.61 to 1.29]) and survival free of disabil-ity at 18 months corrected age or later in childhood (2 trials, 192 infants; relative risk 0.97 [95% confidence interval 0.69 to 1.35]), were not significantly different between the NIDCAP and control groups. With the sensitivity analysis that excluded the 2 statistically heterogeneous outlying studies, there were no significant differences between groups for short-term medical outcomes.
CONCLUSIONS:This systematic review including 627 preterm infants did not find any evidence that NIDCAP improves long-term neurodevelopmental or short-term medical outcomes. Pediatrics 2013;131:e881–e893
AUTHORS:Arne Ohlsson, MD, MSc, FRCPCa,band Susan E.
Jacobs, MBBS, MD, FRACPc,d,e
aProfessor Emeritus Departments of Paediatrics, Obstetrics and Gynaecology, Health Policy, Management and Evaluation, University of Toronto, Ontario Canada;bHonorary Consultant Department of Paediatrics, Mount Sinai Hospital, Toronto, Ontario, Canada;cNeonatal Services, Royal Women’s Hospital, Melbourne, Victoria, Australia;dCritical Care and Neurosciences, Murdoch Childrens Research Institute, Melbourne, Victoria, Australia; andeDepartment of Obstetrics and Gynaecology, University of Melbourne, Melbourne, Victoria, Australia
KEY WORD
Newborn Individualized Developmental Care and Assessment Program (NIDCAP)
ABBREVIATIONS
APIB—Assessment of Premature Infant Behavior BSID—Bayley Scales of Infant Development BWH—Brigham Women’s Hospital CA—corrected age
CHB—Children’s Hospital Boston CHO—Children’s Hospital Oakland CI—confidence interval CLD—chronic lung disease MD—mean difference MDI—Mental Development Index
NIDCAP—Newborn Individualized Developmental Care and As-sessment Program
PDI—Psychomotor Development Index PMA—postmenstrual age
PRISMA—preferred reporting items for systematic reviews and meta-analyses
RCT—randomized controlled trial RR—relative risk
WISC-R—Wechsler Intelligence Scale for Children–Revised WPPSI-R—Wechsler Preschool and Primary Scale For Children– Revised
Drs Ohlsson and Jacobs made equal contributions to the conception, the design, the acquisition of data, the analysis and the interpretation of the data. Both authors participated in the drafting of the article and revised it critically for important intellectual content; and made thefinal approval of the version to be published. Both authors take public responsibility for the content of the article.
www.pediatrics.org/cgi/doi/10.1542/peds.2012-2121 doi:10.1542/peds.2012-2121
Accepted for publication Oct 25, 2012
Address correspondence to Arne Ohlsson, MD, MSc, FRCPC, 105 Pinnacle St North, Brighton, Ontario, K0K 1H0, Canada. E-mail: aohlsson@mtsinai.on.ca
The “synactive” theory of neuro-behavioral development, introduced by Als in the late 1970s, forms the basis of the Newborn Individualized Devel-opmental Care and Assessment Pro-gram (NIDCAP).1,2 It requires trained
and certified caregivers to use the As-sessment of Premature Infant Behavior (APIB) tool to observe 91 neonatal behaviors every 2 minutes for 1 hour before, during, and after a caregiving intervention. After the assessment, recommendations for caregiving are provided to the bedside nurses and the infant’s family. Although individualized, the caregiving principles to reduce stress and promote physiologic stabil-ity are often generalized to include alteration of the environment (lower ambient light and sound), aids to promote flexion and self-regulation, clustering of care, and parental in-volvement in the care of their infant.
The first published NIDCAP study hy-pothesized that the respiratory and functional states of the very low birth weight infants at risk for broncho-pulmonary dysplasia would be im-proved by preventing inappropriate sensory input.3 This phase-lag study
lacked power, enrolling only 16 infants over 2 years. Baseline characteristics favored the NIDCAP group, suggesting very selective enrollment. Some out-comes reported had occurred in the NIDCAP group before initiation of the study intervention.4
Since then, several small unmasked randomized controlled trials (RCTs) assessing the effectiveness of NIDCAP in improving either short-term medical and/or neurodevelopmental outcomes have been published. Since 1993, sys-tematic reviews of NIDCAP have been conducted.4–11 Reviewers agree that,
based on small sample sizes and poor study quality, there was insufficient high-quality evidence regarding the NIDCAP on which to base clinical prac-tice and that further well-conducted
RCTs were needed. Several larger tri-als were published from 2009 to 2012 justifying this update of our previous reviews.4,5,8
OBJECTIVES
To assess the effectiveness of NIDCAP in improving short-term medical and long-term neurodevelopmental out-comes in preterm infants based on published RCTs.
QUESTIONS
1. Does NIDCAP compared with stan-dard care improve neurodevelop-mental and medical outcomes in preterm infants?
2. Should NIDCAP become the stan-dard of care in the NICU? What is the evidence?
Primary Long-Term Outcome
a. The composite of death or major sensorineural disability at 18 months corrected age (CA) or later in childhood. Major sensorineural disability is defined as neuromotor impairment (cerebral palsy or Gross Motor Function Classification Score 3 to 512or standardized
mo-tor assessment.2 SDs below the mean on Bayley Scales of Infant Development [BSID] II13
Psychomo-tor Development Index [PDI] or other standardized motor assess-ment), and/or cognitive delay
de-fined as a Mental Developmental Index (MDI) score on the BSID II, or Wechsler Intelligence Scale for Children-Revised (WISC-R)14 or
Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R)15 .1 SD below the
mean, and/or aided sensorineural hearing loss, and/ or legal blind-ness. Numerator equals death and major disability in survivors
assessed, and denominator equals all randomly selected infants with known outcome.
OR
a. Survival free of any disability at 18 months CA or later in childhood defined as no neuromotor impair-ment (no cerebral palsy or Gross Motor Function Classification Score 0 to 1, standardized motor assess-ment,1SD below mean on BSID II PDI or other standardized motor assessment), AND no cognitive de-lay (MDI score on the BSID II, or WISC-R or WPPSI-R ,1 SD below mean), AND no aided hearing loss AND no legal blindness. Numerator equals healthy children among survivors assessed; denominator equals all randomly selected in-fants with known outcome.
Secondary Long-Term Outcomes
Neurodevelopmental assessment scores by a validated instrument and growth at or beyond 9 months CA. The incidence each of neuromotor impairment, ce-rebral palsy, hearing impairment with amplification, and visual impairment at or beyond 9 months CA. Health-related quality of life at or beyond 12 months CA.
Secondary Short-Term Outcomes
In hospital deaths, chronic lung disease (CLD) (supplemental oxygen at 36 weeks postmenstrual age [PMA]), necrotizing enterocolitis, intraven-tricular hemorrhage, retinopathy of prematurity, nosocomial sepsis, length of hospitalization, PMA at discharge, days on assisted ventilation via an en-dotracheal tube, daily weight gain and growth at 2 weeks CA, APIB16 and
Prechtl general movements assess-ments17 performed at 2 weeks CA,
providers were not included. We did not include results of neurophysiolog-ical or neuroimaging studies because we consider them to be surrogate outcomes. If NIDCAP was shown to de-crease adverse outcomes, we planned to include reported cost-effectiveness analyses.
METHODS
We followed the “Preferred reporting items for systematic reviews and meta-analyses” (PRISMA) statement for reporting of this systematic review18
and the format of our previous sys-tematic reviews.4,5,8 To be included in
the review, the intervention had to be NIDCAP as described by Als1–3and
ap-plied to low-birth-weight or preterm infants while in the hospital. The in-tervention had to be tested in a RCT design and compared with standard care. At least 1 of the short- or long-term outcomes described above had to be reported. No language restrictions were applied.
Search Strategy
Manual searches were conducted of personalfiles and reference lists from our previously published reviews on NIDCAP.4,5,8 Electronic literature
searches were performed on July 30, 2010 of Medline, CINAHL, Embase, PsychInfo, The Cochrane Library, and the Pediatric Academic Societies’ Ab-stract Archives from 2000 to 2010. The search in Medline included the search terms: NIDCAP[All Fields] OR (Neonatal [All Fields] AND Individualized[All Fields] AND Developmental[All Fields] AND Care[All Fields] AND (“program evaluation”[MeSH Terms] OR (“ pro-gram”[All Fields] AND “evaluation”[All Fields]) OR “program evaluation”[All Fields] OR (“assessment”[All Fields] AND “program”[All Fields]) OR “ as-sessment program”[All Fields]). On the same day, the Web of Science was searched for articles that quoted the
first published RCT of NIDCAP.19 One
author (A.O.) identified from titles and abstracts potentially relevant studies from the printouts of the searches and the second author (S.J.) verified the inclusions. Discrepancies (in total, 4) were resolved through consensus and did not require involving an arbitrator. The searches were repeated on Feb-ruary 5, 2012, and 1 additional study was identified for inclusion.
One author (A.O.) abstracted data from the included studies to a predesigned abstraction form and entered the data into RevMan 5.1.20The second author
(S.J.) checked for accuracy, and dis-crepancies were resolved, if needed, through discussion between the authors.
Risk of bias in the included RCTs was assessed by both authors by using the Cochrane Collaboration criteria21: (a)
randomization sequence generation; (b) allocation concealment; (c) blind-ing of participants and personnel, and blinding of outcome assessments; (d) incomplete outcome data addressed; (e) free of selective outcome reporting; and (f) free of other sources of bias. Possible responses were: high risk, low risk, and unclear risk for bias. In ad-dition, we included information about trial registration and when the trial was registered in relation to re-cruitment.
If at least 2 studies reported the same outcome, meta-analyses by the use of RevMan 5.1 were performed.20 The
fixed-effects model was used. For di-chotomous outcomes, the relative risk (RR) is reported. If the RR was statis-tically significant, the risk difference was to be reported. If the risk differ-ence was statistically significant, we planned to report the number needed to treat to benefit or to harm. For continuous outcomes, the mean dif-ference (MD) is reported. The stan-dardized mean difference was used when in different studies the same
construct was measured by using dif-ferent scales of measurement. For all estimates, the 95% confidence inter-vals (CI) are reported. Heterogeneity was measured by using the I2test and I2values of,25%, 25% to 49%, 50% to 74%, and $75% were assigned not important, low, moderate, and high heterogeneity, respectively.22
Sensitiv-ity analyses were conducted in an at-tempt to explain heterogeneity. When means and SDs were not reported in the primary reports, we contacted the authors to provide the information, or means and SDs were estimated by Dr Shafagh Fallah, Statistician, from reported medians, ranges and sample sizes.
RESULTS
The search strategy identified 11 tri-als19,23–32and 7 secondary (follow-up)
studies.33–39Theflow of the searches
and the number of included19,23–39and
excluded40–45studies are described in
Fig 1. As per Cochrane convention, trials are identified in the text by the name of thefirst author and year of publication as well as the reference number from the list of references. The McAnulty 2009 trial28 included
data from 3 trials conducted at the Brigham Women’s Hospital (BWH); Als 1994 study,19which was conducted at
the BWH; the trial at the BWH site in Als 2003 3-site study26; and 1 trial at the
BWH not previously published. As did the authors, we report separately on the 2 additional sites in the Als 2003 study26 conducted at Children’s
Hos-pital Boston (CHB) (Als CHB 2003) and at Children’s Hospital Oakland (CHO) (Als CHO 2003). Five trials including 6 reports were excluded because 1 trial did not report results per groups randomized,40one trial (2 reports)41,42
tested only part of the NIDCAP intervention (covers and nesting), 1 trial43 tested a developmental
and 2 trials tested the NIDCAP in-tervention during stressful/painful interventions.44,45
We obtained unpublished data or clar-ification of aspects of the trials from several authors/coauthors for this or previous reviews (Als, Buehler, Fleisher, Maguire, Tyebkhan, and Westrup).
Characteristics of the included studies are presented in Supplemental Ap-pendix 1, and the “Risk of Bias” is reported in Supplemental Appendix 2. The 11 identified trials included 627 neonates for which baseline criteria were reported. There were no statisti-cally significant differences between the NIDCAP and the control group for 2 important baseline criteria, birth weight and PMA at birth (birth weight MD 9 g [95% CI223 to 42] and PMA at birth MD 0.18 weeks [95% CI20.06 to 0.43]).
The quality of the included trials varied (Supplemental Appendix 2). Adequate randomization sequence generation was reported in 2 studies.29,30 There
was low risk of bias for allocation concealment because all but 2 stud-ies26,28 reported the use of sealed
envelopes. There was high risk of bias in all studies for blinding, because the NIDCAP intervention cannot be blinded to personnel, parents, and assessors of most outcomes. There was a low risk for bias regarding addressing incomplete outcome data in 4 studies,23,28–30with
unclear risk in 3 studies19,24,31and high
risk in 4 studies.25–27,32The risk of
se-lective reporting was unclear for all studies, because no study was entered into a trials registry until after the last infant had been recruited. The risk of other sources of bias was unclear in 6 studies19,26–28,31,32 and low in 5
studies.23–25,29,30In many of the studies
by Als’group there was a long delay (up to 20 years, or more) between infant recruitment and study publication. The 2 studies by Peters et al30and Maguire
et al29 published in 2009 were of the
highest quality and had the largest sample sizes.
Primary and Secondary Long-Term Outcomes
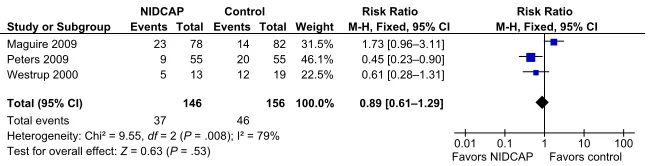
Of the 2 primary outcomes,“The com-posite of death or major sensorineural disability at 18 months CA or later in childhood” did not differ significantly between the groups (3 trials 302 chil-dren; RR 0.89 [95% CI 0.61 to 1.29]). There was high heterogeneity for this outcome (I2 = 79%). Our second pri-mary outcome, “Survival free of dis-ability at 18 months CA or later in childhood,” was not significantly dif-ferent between the 2 groups (2 trials, 192 infants RR 0.97 [95% CI 0.69 to 1.35]). There was no heterogeneity for this outcome (I2= 0%) (Table 1; Fig 2).
Secondary long-term outcomes at or beyond 18 months CA did not dif-fer significantly between the groups for visual impairment, sensorineural hearing loss, or cerebral palsy. One study found no significant difference in health-related quality of life at 12 months of age.39
Neurodevelopmental Outcomes From 4 Months CA to 8 Years of Age
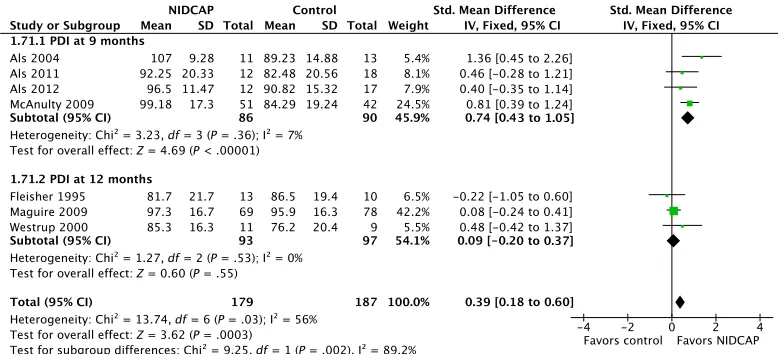
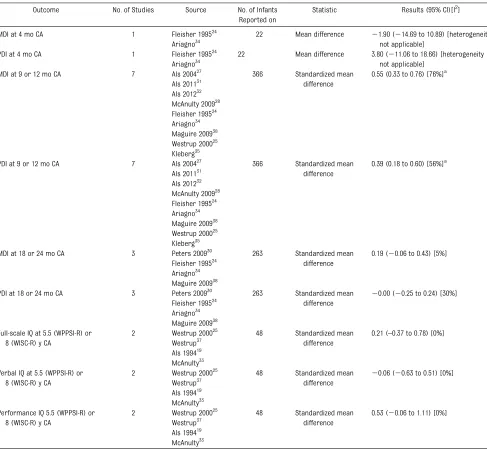
There were no significant differences in BSID-MDI and BSID-PDI scores at 4 months CA. At age 9 or 12 months CA, the BSID-MDI and BSID-PDI scores were significantly higher in the NIDCAP group. As seen in Figs 3 and 4 and Table 2, the statistically significant differences were seen at 9 months CA, but not at 4, 12, 18, or 24 months CA. There was high and moderate heterogeneity for these outcomes (I2 of 76% and 56%, re-spectively). Full-scale, verbal, or per-formance IQ at 5.5 or 8 years of age did not differ significantly between the NIDCAP and control groups.
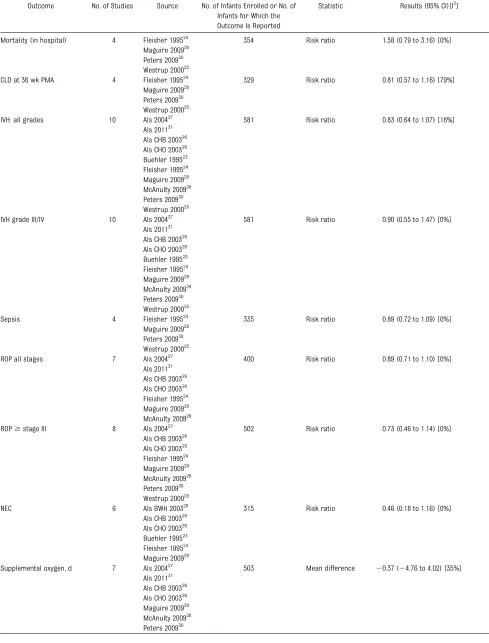
Short-term Medical Outcomes
No significant differences were found in the rates of in hospital deaths, CLD at 36 weeks PMA, intraventricular hemor-rhage (all grades and grades III/IV), sepsis, retinopathy of prematurity (all FIGURE 1
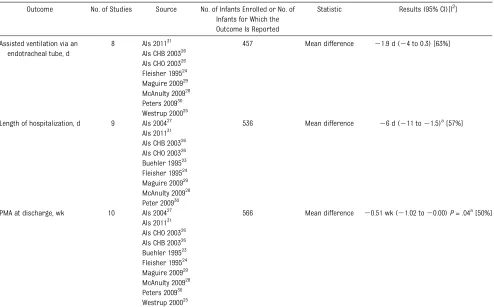
stages and stages$3) and necrotizing enterocolitis, nor in the duration of supplemental oxygen and days of assisted ventilation via an endotra-cheal tube (Table 3). There was a sta-tistically significant decrease in length of hospitalization (MD26 days [95% CI
211 to 21.5 days]) with moderate heterogeneity for this outcome (I2 = 57%). A sensitivity analysis excluding the 2 clear outliers, Als CHO 2003 (MD
243 days) and McAnulty 2009 (MD244 days) (Fig 5), resulted in a non-significant MD of24.1 days (95% CI2 8.8 to 0.58) with low heterogeneity (I2= 35%). PMA at discharge was signifi -cantly reduced (MD 20.51 week [2 1.02 to 20.00]), with moderate het-erogeneity for this outcome (I2= 50%). A sensitivity analysis excluding the 2 clear outliers, Als CHO 2003 (MD 26 weeks) and McAnulty 2009 (MD 24.9 weeks) (Fig 6), resulted in a non-significant MD of 0.36 weeks (20.88 to 0.15) with no important heterogeneity (I2 = 9%). Thus, the between-study heterogeneity could be explained by the 2 outliers.
Growth
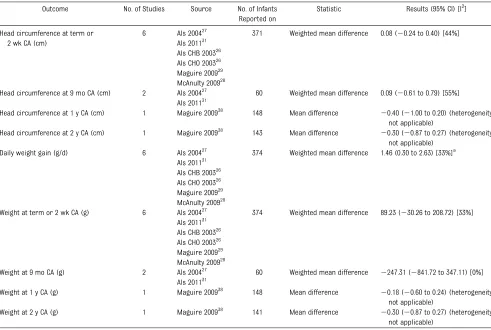
There were no significant differences in head circumference at term or 2 weeks CA, nor at 9 months, 1 year and 2 years CA. There was a significant difference in daily weight gain in hospital of 1.5 g (95% CI 0.3 to 2.6), but no difference in weight at term, 2 weeks, 9 months, 1 year, and 2 years CA (Table 4).
Prechtl and APIB Scores
In the studies by Als group, the results of Prechtl and APIB scores performed at 2 weeks CA favored the NIDCAP group (Supplemental Appendix 3). In the latest study by Als et al,32different categories
for the APIB assessment compared with previous studies were used, such that
we were not able to include this study (Supplemental Appendix 1). The results favored the NIDCAP group. In the study by Maguire 2009 a definitely abnormal Prechtl score was more common in the NIDCAP group.38
Sleep Outcomes
Percentage of time spent in quite sleep at 32 and 36 weeks PMA, percentage during day and night time at 36 weeks PMA, and at 3 months of age did not differ significantly between groups (Supplemental Appendix 4).
DISCUSSION
We set out to answer 2 questions: (1) Does NIDCAP improve long-term
FIGURE 2
Death or disability at 18 months CA or later in childhood. M-H, Mantel-Haenszel. TABLE 1 Primary and Secondary Long-term Neurosensory Outcomes at 18 Months CA or Later
Outcome No. of Studies Source No. of Infants Reported on Statistic Results (95% CI)[I2] Primary outcomes
Death or major sensorineural disability
3 Maguire 200938
302 Relative risk 0.89 (0.61 to 1.29) [79%] Peters 200930
Westrup 200025 Westrup 200437
Survival free of any disability 2 Maguire 200938
192 Relative risk 0.97 (0.69 to 1.35) [0%] Westrup 200025
Westrup 200437 Secondary outcomes
Visual impairment 2 Peters 200930 127 Relative risk 4.0 (0.18 to 89.95) (heterogeneity not applicable, because there were no cases in either group in the study by Peters 2009)
Westrup 200025 Westrup 200437
Sensorineural hearing loss 3 Als 199419 149 Relative risk 0.61 (0.14 to 2.65) [0%] McAnulty 201033
Peters 200930 Westrup 200025 Westrup 200437 Cerebral palsy 3 Als 199419
149 Relative risk 0.22 (0.04 to 1.21 [0%] McAnulty 201033
neurodevelopmental and short-term medical outcomes in preterm infants? and (2) Should NIDCAP be the standard of care for all preterm infants? What is the evidence? This systematic review and meta-analyses demonstrate that the answer to both questions is no.
This is the largest systematic review of the effectiveness of NIDCAP to improve short-term medical and long-term neurodevelopmental outcomes. There were no statistically significant differ-ences in the 2 primary outcomes of “Death or disability at 18 months CA or later in life,” nor in “Survival free of disability at 18 months CA or later in life.” We found small increases in the
BSID-MDI and the BSID-PDI scores at 9 months CA, which were not seen at 4, 12, 18, or 24 months CA. These small transient differences are not clinically important, and there was significant statistical heterogeneity for these out-comes (I2 of 76% [high] and 56% [moderate] respectively) reducing the robustness of the point estimates. Likewise, a daily weight gain of 1.5 g (95% CI 0.3 to 2.6 g) during hospitali-zation is not clinically important, es-pecially because there were no differences in weight at term, 2 weeks, 9 months, 1 year, and at 2 years CA (Table 4). The only other significant
findings were a 6-day reduction in
hospitalization (95% CI 211 to 21.5 days) with a related 0.5-week reduction in PMA at discharge (95% CI21.02 to
20.00). There was moderate hetero-geneity for both of these outcomes, with the same 2 clear outliers
identi-fied for both outcomes (Als CHO 2003 and McAnulty 2009) (Figs 5 and 6). In sensitivity analyses excluding these 2 studies, statistical significance dis-appeared for both outcomes. At study entry, the PMA favored the NIDCAP group by 0.18 weeks (95% CI20.06 to 0.43), offsetting thefindings in PMA at discharge. There were no statistically significant differences in any other medical and neurodevelopmental FIGURE 3
Bayley scales of infant development: mental development index at 9 or 12 months corrected age.
FIGURE 4
outcomes between the NIDCAP and control groups. Given the large number of outcomes, one would expect a few to reach statistical significance by chance. Because NIDCAP was not ef-fective in reducing adverse outcomes, performing cost-effectiveness analyses became redundant.
The quality of the included trials varied, with the 2 largest trials having the lowest risk of bias.29,30These 2 studies
reported conflicting results.46,47 The
intervention did not appear to differ in the Canadian study, which showed shorter length of hospitalization,30
compared with the Dutch study.29
The NIDCAP intervention cannot be blinded. There were remarkably long time periods between the conduct and publication of many trials.19,26,28,31None
of the RCTs were registered before the recruitment of the last infant, making it impossible to ascertain whether the selection of primary outcomes was
made at the design stage of the trial or after data had been collected.
Other published systematic reviews, comprising fewer studies and infants, have not identified a clear benefit of NIDCAP.4–11 In our first review
pub-lished in 1993, only 1 phase-lag NIDCAP study (not an RCT) of 16 infants was identified.4In our second review
pub-lished in 2002, including 5 RCTs enrolling 136 infants, we found a sig-nificant benefit of NIDCAP on number of TABLE 2 Neurodevelopmental Outcomes From 4 Months to 8 Years CA
Outcome No. of Studies Source No. of Infants Reported on
Statistic Results (95% CI)[I2]
MDI at 4 mo CA 1 Fleisher 199524
22 Mean difference 21.90 (214.69 to 10.89) [heterogeneity not applicable]
Ariagno34 PDI at 4 mo CA 1 Fleisher 199524
22 Mean difference 3.80 (211.06 to 18.66) [heterogeneity not applicable]
Ariagno34 MDI at 9 or 12 mo CA 7 Als 200427
366 Standardized mean difference
0.55 (0.33 to 0.76) [76%]a Als 201131
Als 201232 McAnulty 200928 Fleisher 199524 Ariagno34 Maguire 200938 Westrup 200025 Kleberg35
PDI at 9 or 12 mo CA 7 Als 200427 366 Standardized mean difference
0.39 (0.18 to 0.60) [56%]a Als 201131
Als 201232 McAnulty 200928 Fleisher 199524 Ariagno34 Maguire 200938 Westrup 200025 Kleberg35 MDI at 18 or 24 mo CA 3 Peters 200930
263 Standardized mean difference
0.19 (20.06 to 0.43) [5%] Fleisher 199524
Ariagno34 Maguire 200938
PDI at 18 or 24 mo CA 3 Peters 200930 263 Standardized mean difference
20.00 (20.25 to 0.24) [30%] Fleisher 199524
Ariagno34 Maguire 200938 Full-scale IQ at 5.5 (WPPSI-R) or
8 (WISC-R) y CA
2 Westrup 200025 48 Standardized mean difference
0.21 (–0.37 to 0.78) [0%] Westrup37
Als 199419 McAnulty33 Verbal IQ at 5.5 (WPPSI-R) or
8 (WISC-R) y CA
2 Westrup 200025 48 Standardized mean difference
20.06 (20.63 to 0.51) [0%] Westrup37
Als 199419 McAnulty33 Performance IQ 5.5 (WPPSI-R) or
8 (WISC-R) y CA
2 Westrup 200025
48 Standardized mean difference
0.53 (20.06 to 1.11) [0%] Westrup37
Als 199419 McAnulty33
TABLE 3 Short-term Medical Outcomes
Outcome No. of Studies Source No. of Infants Enrolled or No. of Infants for Which the Outcome Is Reported
Statistic Results (95% CI)[I2]
Mortality (in hospital) 4 Fleisher 199524
354 Risk ratio 1.58 (0.79 to 3.16) [0%] Maguire 200929
Peters 200930 Westrup 200025 CLD at 36 wk PMA 4 Fleisher 199524
329 Risk ratio 0.81 (0.57 to 1.16) [79%] Maguire 200929
Peters 200930 Westrup 200025
IVH: all grades 10 Als 200427 581 Risk ratio 0.83 (0.64 to 1.07) [16%] Als 201131
Als CHB 200326 Als CHO 200326 Buehler 199523 Fleisher 199524 Maguire 200929 McAnulty 200928 Peters 200930 Westrup 200025 IVH grade III/IV 10 Als 200427
581 Risk ratio 0.90 (0.55 to 1.47) [0%] Als 201131
Als CHB 200326 Als CHO 200326 Buehler 199523 Fleisher 199524 Maguire 200929 McAnulty 200928 Peters 200930 Westrup 200025
Sepsis 4 Fleisher 199524 335 Risk ratio 0.89 (0.72 to 1.09) [0%] Maguire 200929
Peters 200930 Westrup 200025
ROP all stages 7 Als 200427 400 Risk ratio 0.89 (0.71 to 1.10) [0%] Als 201131
Als CHB 200326 Als CHO 200326 Fleisher 199524 Maguire 200929 McAnulty 200928 ROP$stage III 8 Als 200427
502 Risk ratio 0.73 (0.46 to 1.14) [0%] Als CHB 200326
Als CHO 200326 Fleisher 199524 Maguire 200929 McAnulty 200928 Peters 200930 Westrup 200025
NEC 6 Als BWH 200326 315 Risk ratio 0.46 (0.18 to 1.16) [0%] Als CHB 200326
Als CHO 200326 Buehler 199523 Fleisher 199524 Maguire 200929 Supplemental oxygen, d 7 Als 200427
503 Mean difference 20.37 (24.76 to 4.02) [35%] Als 201131
TABLE 3 Continued
Outcome No. of Studies Source No. of Infants Enrolled or No. of Infants for Which the Outcome Is Reported
Statistic Results (95% CI)[I2]
Assisted ventilation via an endotracheal tube, d
8 Als 201131 457 Mean difference 21.9 d (24 to 0.3) [63%] Als CHB 200326
Als CHO 200326 Fleisher 199524 Maguire 200929 McAnulty 200928 Peters 200930 Westrup 200025
Length of hospitalization, d 9 Als 200427 536 Mean difference 26 d (211 to21.5)a[57%] Als 201131
Als CHB 200326 Als CHO 200326 Buehler 199523 Fleisher 199524 Maguire 200929 McAnulty 200928 Peter 200930 PMA at discharge, wk 10 Als 200427
566 Mean difference 20.51 wk (21.02 to20.00)P= .04a [50%] Als 201131
Als CHO 200326 Als CHB 200326 Buehler 199523 Fleisher 199524 Maguire 200929 McAnulty 200928 Peters 200930 Westrup 200025
IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity; NEC, necrotizing enterocolitis. aIndicates statistically significantfinding.
FIGURE 5
Length of hospitalization (days).
FIGURE 6
days of supplemental oxygen, days on assisted ventilation, and improved neurodevelopmental outcome at 9 or 12 months but not at 2 years CA.5The
2006 update of Symington and Pinelli’s Cochrane review included 5 trials,6one
of which was the 1986 phase-lag study by Als et al.3Outcomes were reported
for a maximum of 4 trials with a total of 105 infants, with a reduction in days on assisted ventilation.6Our 2007 update,
published in Swedish, included a total of 6 studies with a maximum of 330 infants reported in any 1 outcome.8We
noted significant reductions in CLD at 36 weeks PMA, days on assisted venti-lation, days in hospital, PMA at dis-charge, and daily weight gain. Wallin and Eriksson conducted a systematic review, but not meta-analysis, and in-cluded only studies that showed a sta-tistically significant difference in an
outcome.9 They concluded: “….the
scientific evidence on the effects of NIDCAP is limited. Shortcomings in de-sign and methods in the reviewed studies hamper far-reaching claims on the effectiveness of the method.” Vanderveen et al10included 5 NIDCAP
trials with a maximum of 43 infants for any reported outcome. They found a significant increase in BSID-MDI at 12 months CA in the NIDCAP group, but not at 24 months CA or in WPPSI-R at 5 years of age. BSID-PDI was not signifi -cantly better at any age. In our current review with the largest number of in-cluded infants and maximum number of 566 infants reported in any 1 out-come, the number of statistically sig-nificant outcomes are fewer and cannot be considered to be clinically important.
Thefindings and conclusions of these systematic reviews are in sharp contrast to the statements by propo-nents of NIDCAP.48 The NIDCAP
Fed-eration International claims that “Research has documented the ben-eficial effect of NIDCAP in terms of shorter intensive care and overall hospital stay, better weight gain and improved behavioral outcomes that endure beyond infancy. Studies have also documented that the NIDCAP approach enhances brain structure and function when measured by so-phisticated medical techniques such as EEG and MRI.”48We did not include
neurophysiological or neuroimaging outcomes as they are surrogate biomarkers for long-term neuro-development, and were only reported in a limited number of trials with possible selection bias (all randomly TABLE 4 Growth
Outcome No. of Studies Source No. of Infants Reported on
Statistic Results (95% CI) [I2]
Head circumference at term or 2 wk CA (cm)
6 Als 200427
371 Weighted mean difference 0.08 (20.24 to 0.40) [44%] Als 201131
Als CHB 200326 Als CHO 200326 Maguire 200929 McAnulty 200928 Head circumference at 9 mo CA (cm) 2 Als 200427
60 Weighted mean difference 0.09 (20.61 to 0.79) [55%] Als 201131
Head circumference at 1 y CA (cm) 1 Maguire 200938 148 Mean difference 20.40 (21.00 to 0.20) (heterogeneity not applicable)
Head circumference at 2 y CA (cm) 1 Maguire 200938 143 Mean difference 20.30 (20.87 to 0.27) (heterogeneity not applicable)
Daily weight gain (g/d) 6 Als 200427 374 Weighted mean difference 1.46 (0.30 to 2.63) [33%]a Als 201131
Als CHB 200326 Als CHO 200326 Maguire 200929 McAnulty 200928 Weight at term or 2 wk CA (g) 6 Als 200427
374 Weighted mean difference 89.23 (230.26 to 208.72) [33%] Als 201131
Als CHB 200326 Als CHO 200326 Maguire 200929 McAnulty 200928 Weight at 9 mo CA (g) 2 Als 200427
60 Weighted mean difference 2247.31 (2841.72 to 347.11) [0%] Als 201131
Weight at 1 y CA (g) 1 Maguire 200938 148 Mean difference 20.18 (20.60 to 0.24) (heterogeneity not applicable)
Weight at 2 y CA (g) 1 Maguire 200938 141 Mean difference 20.30 (20.87 to 0.27) (heterogeneity not applicable)
assigned infants did not undergo all the tests).
We stated in our 2002 systematic review that “modification of the extrauterine NICU environment and caregiving according to each infant’s current physiologic and neurobehavioral func-tioning is a rational and intuitive ap-proach to caring for preterm infants and their families and to supporting infant development.”5 The NIDCAP is 1
clinical framework and training pro-gram to provide individualized de-velopmental care to vulnerable preterm infants. NIDCAP is resource-consuming, labor-intensive, and expensive both to implement and maintain, because it requires developmental specialists, regular APIB assessments, and training of nursing staff.49 Gibbins et al50
re-cently proposed a new conceptual de-velopmental care model “of a shared
surface, manifested most obviously by the skin that forms the critical link be-tween the body/organism and the envi-ronment and becomes the focal point of human interactions.”To our knowledge, this model has not been rigorously in-vestigated. Innovative interventions to promote development in preterm infants should be tested in large well-designed RCTs and their results pub-lished in a timely fashion. Before any further research of developmental care is undertaken, consideration should be given to reports that many NIDCAP behaviors are rarely or never seen among preterm infants,51 that only
a few are associated with stressful/ painful interventions,52 and that
clus-tering of care can result in important behavioral and autonomic reactions.53
Two commonly recommended in-terventions as part of NIDCAP are
incubator covers and nesting, which have not been shown to be effective in improving developmental outcomes.42
Cycled lighting, as opposed to dim lighting or near darkness, may have beneficial effects on infants’fussing and crying behavior and growth in thefirst weeks of life.54,55
Because we were not able to identify any clear benefits of NIDCAP for long-term neurodevelopmental outcomes, nor for any short-term medical outcomes, we cannot recommend the implementation of NIDCAP in its present form as standard care in preterm infants.
ACKNOWLEDGMENT
We are thankful to Dr Shafagh Fallah, Statistician, who estimated means and SDs from medians, ranges, and sample sizes when reported.
REFERENCES
1. Als H. Toward a synactive theory of
de-velopment: promise for the assessment of infant individuality. Infant Ment Health J. 1982;3(4):229–243
2. Als H. A synactive model of neonatal
be-havioral organization: framework for the assessment of neurobehavioral devel-opment in the premature infant and for
support of infants and parents in the neonatal intensive care environment.Phys Occup Ther Pediatr. 1986;6:3–55
3. Als H, Lawhon G, Brown E, et al. In-dividualized behavioral and environmental
care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: neonatal intensive care unit and
developmental outcome. Pediatrics. 1986; 78(6):1123–1132
4. Lacy JB, Ohlsson A. Behavioral outcomes of environmental or care-giving hospital-based interventions for preterm infants:
a critical overview.Acta Paediatr. 1993;82 (4):408–415
5. Jacobs SE, Sokol J, Ohlsson A. The Newborn Individualized Developmental Care and
As-sessment Program is not supported by meta-analyses of the data [published cor-rection appears inJ Pediatr. 2002;141:451–
2].J Pediatr. 2002;140(6):699–706
6. Symington A, Pinelli JM. Distilling the
evi-dence on developmental care: a systematic review. Adv Neonatal Care. 2002;2(4):198– 221
7. Symington AJ, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2006;(2):CD001814
8. Ohlsson A, Jacobs SE. Meta-regression can indicate if further NIDCAP studies are
jus-tified [in Swedish]. Lakartidningen. 2007; 104(3):134–137
9. Wallin L, Eriksson M. Newborn individual development care and assessment pro-gram (NIDCAP): A systematic review of the literature. Worldviews Evid Based Nurs. 2009;6(2):54–69
10. Vanderveen JA, Bassler D, Robertson CM, Kirpalani H. Early interventions involving
parents to improve neurodevelopmental outcomes of premature infants: a meta-analysis.J Perinatol. 2009;29(5):343–351
11. Legendre V, Burtner PA, Martinez KL, Crowe TK. The evolving practice of developmental care in the neonatal unit: a systematic re-view.Phys Occup Ther Pediatr. 2011;31(3): 315–338
12. Palisano R, Rosenbaum P, Walter S, Russell
D, Wood E, Galuppi B. Development and
reliability of a system to classify gross
motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4): 214–223
13. Bayley N. Bayley Scales of Infant De-velopment II. San Antonio, TX: Psychological Corporation; 1993
14. Wechsler D.Wechsler Intelligence Scale for Children-Revised, WISC-R. New York, NY: Psychological Corporation; 1974
15. Wechsler D. Wechsler Preschool and
Pri-mary Scale of Intelligence-Revised. Swedish version. Stockholm: Psykologförlaget AB; 1999
16. Als H, Lester BM, Tronick EZ, Brazelton TB. Manual for the assessment of preterm infants’behaviour (APIB). In: Fitzgerald HE, Lester BM, Yogman MW, eds. Theory and Research in Behavioral Pediatrics. New
York, NY: Plenum Press; 1982:65–132
17. Prechtl HFR. The Neurological Examination
of the Full-Term Infant: a Manual for Clinical Use. 2nd ed. Clinics in Developmental Medicine. No. 63. Philadelphia, PA: Lippin-cott; 1977
18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:
19. Als H, Lawhon G, Duffy FH, McAnulty GB, Gibes-Grossman R, Blickman JG. Indivi-dualized developmental care for the very low-birth-weight preterm infant. Medical and neurofunctional effects. JAMA. 1994; 272(11):853–858
20. RevMan Manager Version 5.1 [Computer program]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011
21. Higgins JPT, Green S, eds.Cochrane Hand-book for Systematic Reviews of Inter-ventions Version 5.1.0. The Cochrane Collaboration; 2011 [updatedMarch 2011]. Available at: www.cochrane-handbook.org. Accessed January 3, 2013
22. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560
23. Buehler DM, Als H, Duffy FH, McAnulty GB, Liederman J. Effectiveness of individualized developmental care for low-risk preterm infants: behavioral and electrophysiologic evidence. Pediatrics. 1995;96(5 pt 1):923– 932
24. Fleisher BE, VandenBerg K, Constantinou J, et al. Individualized developmental care for very-low-birth-weight premature infants. Clin Pediatr (Phila). 1995;34(10):523– 529
25. Westrup B, Kleberg A, von Eichwald K, Stjernqvist K, Lagercrantz H. A randomized, controlled trial to evaluate the effects of the newborn individualized devel-opmental care and assessment program in a Swedish setting.Pediatrics. 2000;105(1 pt 1):66–72
26. Als H, Gilkerson L, Duffy FH, et al. A three-center, randomized, controlled trial of in-dividualized developmental care for very low birth weight preterm infants: medical, neurodevelopmental, parenting, and care-giving effects [published correction appears in J Dev Behav Pediatr. 2004;25: 224–225].J Dev Behav Pediatr. 2003;24(6): 399–408
27. Als H, Duffy FH, McAnulty GB, et al. Early experience alters brain function and structure.Pediatrics. 2004;113(4):846–857
28. McAnulty G, Duffy FH, Butler S, et al. In-dividualized developmental care for a large sample of very preterm infants: health, neurobehaviour and neurophysiology.Acta Paediatr. 2009;98(12):1920–1926
29. Maguire CM, Walther FJ, Sprij AJ, Le Cessie S, Wit JM, Veen S; Leiden Developmental Care Project. Effects of individualized de-velopmental care in a randomized trial of preterm infants ,32 weeks. Pediatrics. 2009;124(4):1021–1030
30. Peters KL, Rosychuk RJ, Hendson L, Coté JJ, McPherson C, Tyebkhan JM. Improvement
of short- and long-term outcomes for very low birth weight infants: Edmonton NIDCAP trial.Pediatrics. 2009;124(4):1009–1020
31. Als H, Duffy FH, McAnulty GB, et al. Is the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) effective for preterm infants with in-trauterine growth restriction?J Perinatol. 2011;31(2):130–136
32. Als H, Duffy FH, McAnulty G, et al. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction.J Perinatol. 2012;32(10): 797–803
33. McAnulty GB, Duffy FH, Butler SC, Bernstein JH, Zurakowski D, Als H. Effects of the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) at age 8 years: preliminary data.Clin Pediatr (Phila). 2010;49(3):258–270
34. Ariagno RL, Thoman EB, Boeddiker MA, et al. Developmental care does not alter sleep and development of premature infants. Pediatrics. 1997;100(6). Available at: www.pediatrics.org/cgi/content/full/100/ 6/e9
35. Kleberg A, Westrup B, Stjernqvist K, Lagercrantz H. Indications of improved cognitive development at one year of age among infants born very prematurely who received care based on the Newborn In-dividualized Developmental Care and As-sessment Program (NIDCAP). Early Hum Dev. 2002;68(2):83–91
36. Westrup B, Hellström-Westas L, Stjernqvist K, Lagercrantz H. No indications of in-creased quiet sleep in infants receiving care based on the newborn individualized developmental care and assessment pro-gram (NIDCAP). Acta Paediatr. 2002;91(3): 318–322, discussion 262–263
37. Westrup B, Böhm B, Lagercrantz H, Stjernqvist K. Preschool outcome in chil-dren born very prematurely and cared for according to the Newborn Individualized Developmental Care and Assessment Pro-gram (NIDCAP). Acta Paediatr. 2004;93(4): 498–507
38. Maguire CM, Walther FJ, van Zwieten PH, Le Cessie S, Wit JM, Veen S. Follow-up out-comes at 1 and 2 years of infants born less than 32 weeks after Newborn Individualized Developmental Care and Assessment Pro-gram.Pediatrics. 2009;123(4):1081–1087
39. van der Pal SM, Maguire CM, Bruil J, et al. Health-related quality of life of very pre-term infants at 1 year of age after two developmental care-based interventions. Child Care Health Dev. 2008;34(5):619–625
40. Philbin MK, Ballweg DD, Tsakiri S, et al. Hospital cost savings and physiologic benefit
of developmentally supportive care for very low birth weight newborns 222 [abstract]. Pediatr Res. 1998;43:40A
41. Maguire CM, Veen S, Sprij AJ, Le Cessie S, Wit JM, Walther FJ; Leiden Developmental Care Project. Effects of basic devel-opmental care on neonatal morbidity, neuromotor development, and growth at term age of infants who were born at,32 weeks.Pediatrics. 2008;121(2). Available at: www.pediatrics.org/cgi/content/full/121/2/ e239
42. Maguire CM, Walther FJ, van Zwieten PH, Le Cessie S, Wit JM, Veen S; Leiden De-velopmental Care Project. No change in developmental outcome with incubator covers and nesting for very preterm infants in a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2009;94 (2):F92–F97
43. Parker SJ, Zahr LK, Cole JG, Brecht ML. Outcome after developmental intervention in the neonatal intensive care unit for mothers of preterm infants with low so-cioeconomic status.J Pediatr. 1992;120(5): 780–785
44. Catelin C, Tordjman S, Morin V, Oger E, Sizun J. Clinical, physiologic, and biologic impact of environmental and behavioral interventions in neonates during a routine nursing procedure.J Pain. 2005;6(12):791– 797
45. Kleberg A, Warren I, Norman E, et al. Lower stress responses after Newborn Indivi-dualized Developmental Care and Assess-ment Program care during eye screening examinations for retinopathy of pre-maturity: a randomized study. Pediatrics. 2008;121(5). Available at: www.pediatrics. org/cgi/content/full/121/5/e1267
46. Ohlsson A. NIDCAP: new controversial evi-dence for its effectiveness.Pediatrics. 2009; 124(4):1213–1215
47. Als H. NIDCAP: testing the effectiveness of a relationship-based comprehensive in-tervention.Pediatrics. 2009;124(4):1208–1210
48. NIDCAP Federation International. Available at: www.nidcap.org/about.aspx Accessed February 9, 2012
49. Als H. Developmental care in the newborn intensive care unit.Curr Opin Pediatr. 1998; 10(2):138–142
50. Gibbins S, Hoath SB, Coughlin M, Gibbins A, Franck L. The universe of developmental care: a new conceptual model for applica-tion in the neonatal intensive care unit.Adv Neonatal Care. 2008;8(3):141–147
52. Holsti L, Grunau RE, Oberlander TF, Whitfield MF. Specific Newborn Individualized De-velopmental Care and Assessment Pro-gram movements are associated with acute pain in preterm infants in the neo-natal intensive care unit.Pediatrics. 2004; 114(1):65–72
53. Holsti L, Grunau RE, Oberlander TF, Whitfield MF. Prior pain induces heightened motor responses during clustered care in pre-term infants in the NICU. Early Hum Dev. 2005;81(3):293–302
54. Guyer C, Huber R, Fontijn J, et al. Cycled light exposure reduces fussing and crying
in very preterm infants. Pediatrics. 2012; 130(1). Available at: www.pediatrics.org/ cgi/content/full/130/1/e145
55. Morag I, Ohlsson A. Cycled light in the in-tensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev. 2011;(1):CD006982
(Continued fromfirst page)
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275). Copyright © 2013 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have nofinancial relationships relevant to this article to disclose.
DOI: 10.1542/peds.2012-2121 originally published online February 18, 2013;
2013;131;e881
Pediatrics
Arne Ohlsson and Susan E. Jacobs
Trials
NIDCAP: A Systematic Review and Meta-analyses of Randomized Controlled
Services
Updated Information &
http://pediatrics.aappublications.org/content/131/3/e881
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/131/3/e881#BIBL
This article cites 46 articles, 18 of which you can access for free at:
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2012-2121 originally published online February 18, 2013;
2013;131;e881
Pediatrics
Arne Ohlsson and Susan E. Jacobs
http://pediatrics.aappublications.org/content/131/3/e881
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
http://pediatrics.aappublications.org/content/suppl/2013/02/13/peds.2012-2121.DCSupplemental
Data Supplement at:
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.