EXPERIENCE & REASON
Short communications of factual material are published here. Comments and criticisms appear as Commentaries or Letters to the Editor.
“In Medicine one must pay attention not to plausible theorizing but to experience and reason together.. . .
I agree that theorizing is to be approved, provided that it is based on facts, and systematically makes its deductions from what is observed.. . .But conclusions drawn from unaided reason can hardly be serviceable; only those drawn from observed fact.”
—Hippocrates,Precepts
Hypocalcemic Seizures and Secondary Bilateral
Femoral Fractures in an Adolescent With Primary
Vitamin D Deficiency
David Schnadower, MD, MPHa, Chhavi Agarwal, MDb, Sharon E. Oberfield, MDb, Ilene Fennoy, MD, MPHb, Martin Pusic, MD, MAa
Divisions ofaPediatric Emergency Medicine andbPediatric Endocrinology, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical
Center, New York, New York
The authors have indicated they have no financial relationships relevant to this article to disclose.
ABSTRACT
Nutritional rickets and osteomalacia are reemerging in Western societies, particularly in young children and in adolescents of African or Asian descent. Hypocalcemic seizures resulting from vitamin D deficiency are rare in adolescents, whereas fractures caused by seizures without evidence of direct trauma have not yet been reported in this population. We present an unusual case of secondary bilateral femoral fractures caused by hypocalcemic seizures in a 17-year-old boy with primary vitamin D deficiency. We examine the epidemiology and the clinical presentation of rickets and osteomalacia in the adolescent population, the risk of secondary injuries in patients with seizures, and the evaluation and management of hypocalcemic seizures and primary vitamin D deficiency.
A
LTHOUGH THERE AREno national data on the inci-dence of rickets or osteomalacia in the United States, several reports have suggested that primary vita-min D deficiency is reemerging in Western societies, particularly in young children and possibly inadoles-cents of African or Asian origin.1–3 Most adolescents
with vitamin D deficiency are asymptomatic. Here we present an unusual case of secondary bilateral femoral fractures caused by hypocalcemic seizures in a 17-year-old black boy with primary vitamin D deficiency.
CASE REPORT
A 17-year-old black boy was brought to the pediatric emergency department during the wintertime after hav-ing a first seizure while sitthav-ing on a couch at home. It was witnessed by the patient’s mother and described as a 5-minute generalized self-resolving seizure with rhyth-mic shaking movements of all extremities and backward
rolling of the eyes. It occurred at 5 AM after a night
without sleep. He had been watching television. The
patient did not fall from the couch or experience any trauma during the seizure. Paramedics found the patient unresponsive and administered 50 mL of 50% dextrose and 100 mg of thiamine intravenously. On arrival to the emergency department, the patient was somnolent but able to answer questions appropriately. He complained of severe bilateral thigh pain. He reported having felt somewhat weak for the previous few days, although he had been able to play basketball, as he did most days of the week, on the day before admission. He denied any trauma. He had no upper respiratory symptoms, fever, nausea, or vomiting, reported no recent changes in
vi-Key Words:adolescents, rickets, hypocalcemia, seizures, fractures Abbreviation:PTH, parathyroid hormone
www.pediatrics.org/cgi/doi/10.1542/peds.2006-1170
doi:10.1542/peds.2006-1170
Accepted for publication Jun 12, 2006
Address correspondence to David Schnadower, MD, MPH, Division of Pediatric Emergency Medicine, 622 West 168th St, PH-137, New York, NY 10032. E-mail: ds2194@columbia.edu
sion or gait, and denied ingestions, medication use, or illicit drug use. His diet consisted of mostly “junk food” and canned soft drinks, with very few fresh fruits, veg-etables, or cereals, and less than 1 serving of dairy prod-ucts per day. He had not traveled recently. He had no significant past medical history, including no history of seizures, and there were no individuals with seizures in his immediate family.
In the emergency department, his vital signs were: temperature, 97.5°F; heart rate, 107 beats per minute; respiratory rate, 20 breaths per minute; blood pressure, 139/63 mm Hg; oxygen saturation, 99% on room air; and pain score, 7/10. He was a slender black adolescent boy with prominent musculature. His physical examina-tion was normal except for the musculoskeletal and neurologic components. His extremities revealed con-tracted quadriceps bilaterally and exquisite pain on pal-pation of both thighs. The pain prevented him from sitting or walking. On neurologic examination, he was somnolent but arousable and oriented to person, time, and place. He had a generalized increase in muscle tone. Deep-tendon reflexes were brisk all over. Chvostek and Trousseau signs were negative.
One hour after his arrival, the patient had a second generalized tonic-clonic seizure that lasted⬃4 minutes and stopped after intravenous administration of 2 mg of lorazepam. Chemistries were significant for a calcium level of 4.5 mg/dL (reference range: 8.7–10 mg/dL) and ionized calcium level of 0.6 mM/L (reference range: 1.12–1.32 mM/L). His sodium level was 140 mM/L (reference range: 136 –146 mM/L), potassium level was 4.0 mM/L (reference range: 3.6 –5 mM/L), phosphorus level was 5.0 mg/dL (reference range: 2.5– 4.3 mg/dL), and magnesium level was 1.5 mg/dL (ref-erence range: 1.5–2.3 mg/dL). An electrocardiogram showed a normal sinus rhythm with a QTc of 413 milliseconds. He received intravenous calcium gluconate and was transferred to the PICU. Laboratory evalua-tion revealed normal renal funcevalua-tion with a creatinine level of 1.0 mg/dL (reference range: 0.6 –1.2 mg/dL) and normal hepatic function (aspartate aminotransfer-ase level: 36 U/L [reference range: 12–38 U/L]; alanine aminotransferase level, 16 U/L [reference range: 7– 41 U/L]). His alkaline phosphatase level was 338 U/L (ref-erence range: 33–96 U/L), parathyroid hormone (PTH) level was 515 pg/mL (reference range: 8 –51 pg/mL),
vitamin D 25-hydroxy level was ⬍5 ng/mL (reference
range: 20 –57 ng/mL), and vitamin D 1,25-dihydroxy
level was ⬍4 pg/mL (reference range: 15–75 pg/mL).
Additional workup for malabsorption was negative (negative serology markers for celiac disease and stool negative for fat). The patient was diagnosed with pri-mary vitamin D deficiency.
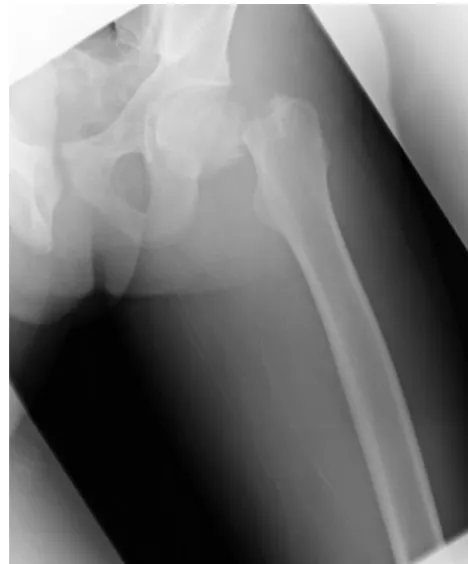
Radiographs of his legs revealed bilateral femoral-neck fractures (see Figs 1 and 2). A long-bone survey showed normal bone mineralization. Dual radiograph
absorptiometry was not performed because of the ur-gency of surgical management as recommended by the orthopedic surgeons. He underwent open reduction and internal fixation of both femoral heads (see Fig 3). He was discharged on hospital day 12 to a physical rehabilitation facility on calcitriol, calcium carbonate, multivitamins, and ferrous sulfate. Subsequently, he was transitioned to ergocalciferol. A diet with increased calcium intake of at least 2 to 4 servings of dairy per day and daily vitamin D (400 IU) supplementation was recommended and, to the best of our knowledge, has been followed. Seven months after admission the patient had normal PTH and vitamin D levels, and all his medications were discontinued except a standard multivitamin preparation.
DISCUSSION
Vitamin D deficiency seems to be an unrecognized and prevalent problem in adolescents. School-based studies in the United Kingdom and Finland suggest that a sig-nificant proportion of adolescent girls may have subclin-ical vitamin D deficiency.4,5 A recent cross-sectional
study of 307 healthy teenagers attending an annual physical examination in an adolescent clinic in the northeastern United States showed that vitamin D defi-ciency was present in 24% of the subjects and that the prevalence was highest in black adolescents.3The
patho-physiology of rickets and osteomalacia in this population
FIGURE 1
may stem from a combination of increased metabolic demands as a result of rapid growth and puberty, poor vitamin D or calcium intake, high soft drink consump-tion with increased phosphorous content, decreased
physical activity, and diminished exposure to sunlight, particularly in individuals with darker skin pigmenta-tion.6
The clinical presentation of rickets and osteomalacia in the adolescent population differs from the presenta-tion in younger patients. Whereas toddlers generally exhibit bony deformities soon after weight-bearing age, most adolescents are asymptomatic. When sympto-matic, they tend to present with signs of hypocalcemia such as neuromuscular irritability and, rarely, seizures. Radiographs of the long bones in these patients may not necessarily show radiologic changes of rickets or osteomalacia. It is thought that hypocalcemic symptoms secondary to vitamin D deficiency occur largely in pa-tients with rapid growth rates such as children younger than 1 year and adolescents. In a retrospective review of 65 hospitalized children with vitamin D deficiency in
the United Kingdom, Ladhani et al7reported that
hy-pocalcemic symptoms occurred exclusively in children younger than 3 years or older than 10 years. Narchi et al8
reported 21 cases of symptomatic rickets in adolescents from Saudi Arabia. Most of their patients presented with carpopedal spasm, limb pain, or weakness. The incidence of seizures in adolescents with vitamin D deficiency is unknown. In the Ladhani et al series, 16 patients pre-sented with seizures, but it is unclear how many were in the older age group, whereas none of the adolescent patients in the Narchi et al group had seizures.
Patients with a history of epilepsy seem to be at a higher risk for injuries, including head and dental trauma, lacerations, burns, sprains, and fractures. Sur-veys and population studies indicate that close to 20% of patients who experience a seizure sustain some kind of injury, and overall, 30% to 35% of patients with sei-zures have experienced secondary injury as a result of a seizure during their lifetime.9–11The data in children are
limited. A small case-control study in Canada showed that most cognitively intact children with epilepsy have a similar risk of serious injuries compared with their
peers without epilepsy.12 A prospective study of 198
consecutive children aged 1 to 16 years with newly diagnosed and untreated seizures noted that serious in-juries were uncommon; 12% of their patients with sei-zures sustained injuries, but only 2% required medical attention.13
However, a recent meta-analysis reported that pa-tients with epilepsy are twice as likely to sustain a
frac-ture as patients without epilepsy,14 which may be a
result of (1) increased risk of trauma, (2) decreased bone density caused by the use of antiepileptic drugs, and/or (3) comorbidities. Most of the fractures sustained during seizures are caused by direct trauma and typically in-volve the skull, nasal bones, and clavicles, but in rare instances, fractures can be caused by the muscular ten-sion of the seizure itself. In these cases, the proximal humerus and the shoulder are more commonly
affect-FIGURE 2
Right femoral-neck fracture.
FIGURE 3
ed.15Although bilateral femoral fractures caused by
elec-troconvulsive therapy were occasionally encountered in the 1950s and 1960s,16,17femoral fractures caused by the
muscular tension of a seizure itself seem to be unusual and are described in only a handful of case reports in older adults, patients with renal failure, and skeletally
immature patients.18–22 To the best of our knowledge,
this is the first reported case of nontraumatic bilateral femoral fractures in an adolescent resulting from hy-pocalcemic seizures caused by primary vitamin D defi-ciency.
Laboratory testing after a first unexplained nonfe-brile seizure should be considered, particularly in pa-tients with suggestive clinical findings such as vomiting, diarrhea, or dehydration, failure to return to baseline alertness, or increased muscle tone or fractures such as in our patient. The workup should include obtain-ing electrolyte levels, includobtain-ing calcium, magnesium, and phosphorous. Toxicology screening should be con-sidered if there is a question of drug exposure or sub-stance abuse.23 The differential diagnosis of
hypocal-cemia in adolescence includes vitamin D deficiency, hypoparathyroidism, hypomagnesemia, malabsorption,
and renal and hepatic failure, among others.24 Once
hypocalcemia is found, additional laboratory investiga-tions such as obtaining a basic metabolic panel, liver-function tests, and PTH and vitamin D 25-hydroxy and 1,25-dihydroxy levels should be performed. A workup for malabsorption should be undertaken if it is suggested by history or initial laboratory results. The diagnosis of primary vitamin D deficiency is made when low vitamin D levels along with a compatible history are accompa-nied by high levels of PTH, in the absence of other metabolic or gastrointestinal abnormalities. Dual-beam radiograph– based photon absorptiometry is the most sensitive routine method of detecting and quantifying bone loss and may be considered for patients with vita-min D deficiency.25
Hypocalcemic seizures should be treated with intra-venous calcium. In general, calcium gluconate is pre-ferred to calcium chloride because it is less irritating and is less likely to cause tissue necrosis if extravasation occurs. Intravenous therapy with calcium should be continued as long as the patient is symptomatic. Mag-nesium should be replaced if low levels are identified,
and vitamin D replacement in the form of vitamin D2
(ergocalciferol) may be initiated intramuscularly initially and continued orally as long as the patient does not have malabsorption. Phosphate replacement is usually not necessary for vitamin D deficiency, because low levels are a result of the elevated PTH level, which resolves once adequate calcium and vitamin D are supplied. It is important to monitor serum calcium, phosphate, alka-line phosphatase, PTH, and vitamin D levels and the urinary calcium/creatinine ratio during treatment to monitor response and avoid complications of
hypocal-cemia or hypercalhypocal-cemia. Every effort should be made to prevent this disease by encouraging adequate diet, sun exposure, and vitamin D supplementation for patients at risk.26
CONCLUSIONS
This case illustrates that emergency medicine physicians should carefully evaluate patients with seizures for sec-ondary injuries, both at presentation and after the pa-tient recovers from the postictal stage. Although symp-tomatic rickets and osteomalacia are rare in the adolescent population, pediatricians, general practitio-ners, and policy-makers should be aware that subclinical vitamin D deficiency is on the rise in Western urban societies, particularly in individuals of African or Asian origin. Additional research into primary prevention of primary vitamin D deficiency in this population is war-ranted.
ACKNOWLEDGMENT
We thank Dr Peter Dayan for careful review of the manuscript.
REFERENCES
1. Welch TR, Bergstrom WH, Tsang RC. Vitamin D-deficient rickets: the reemergence of a once-conquered disease.J Pediatr. 2000;137:143–145
2. Scanlon K. CDC vitamin D expert panel meeting: October 11– 12, 2001; Atlanta, Georgia. Available at: www.cdc.gov/nccdphp/ dnpa/nutrition/pdf/Vitamin㛭D㛭Expert㛭Panel㛭Meeting.pdf. Ac-cessed June 7, 2006
3. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med.2004;158:531–537
4. Lehtonen-Veromaa M, Mottonen T, Irjala K, et al. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur J Clin Nutr. 1999;53: 746 –751
5. Das G, Crocombe S, McGrath M, Berry J, Mughal Z. Hypo-vitaminosis D among healthy adolescent girls attending an inner city school.Arch Dis Child.2006;91:569 –572
6. Moncrieff MW, Lunt HR, Arthur LJ. Nutritional rickets at puberty.Arch Dis Child.1973;48:221–224
7. Ladhani S, Srinivasan L, Buchanan C, Allgrove J. Presentation of vitamin D deficiency.Arch Dis Child.2004;89:781–784 8. Narchi H, El Jamil M, Kulaylat N. Symptomatic rickets in
adolescence.Arch Dis Child.2001;84:501–503
9. van den Broek M, Beghi E. Accidents in patients with epilepsy: types, circumstances, and complications—a European cohort study.Epilepsia.2004;45:667– 672
10. Buck D, Baker GA, Jacoby A, Smith DF, Chadwick DW. Pa-tients’ experiences of injury as a result of epilepsy.Epilepsia. 1997;38:439 – 444
11. Neufeld MY, Vishne T, Chistik V, Korczyn AD. Life-long history of injuries related to seizures.Epilepsy Res.1999;34: 123–127
12. Kirsch R, Wirrell E. Do cognitively normal children with epi-lepsy have a higher rate of injury than their nonepileptic peers?J Child Neurol.2001;16:100 –104
newly diagnosed and untreated epilepsy.Epilepsia.2002;43: 764 –767
14. Vestergaard P. Epilepsy, osteoporosis and fracture risk: a meta-analysis.Acta Neurol Scand.2005;112:277–286
15. Finelli PF, Cardi JK. Seizure as a cause of fracture.Neurology. 1989;39:858 – 860
16. Kelly JP. Fractures complicating electro-convulsive ther-apy and chronic epilepsy.J Bone Joint Surg Br.1954;36-B: 70 –79
17. Powell HD. Stimultaneous bilateral fractures of the neck of the femur.J Bone Joint Surg Br.1960;42-B:236 –252
18. Kause J, Parr MJ. Bilateral subcapital neck of femur fractures after eclamptic seizures.Br J Anaesth.2004;92:590 –592 19. Ribacoba-Montero R, Salas-Puig J. Simultaneous bilateral
frac-tures of the hip following a grand mal seizure: an unusual complication.Seizure.1997;6:403– 404
20. Undar L, Topcu S, Percin S. Simultaneous bilateral fractures of the femoral neck and superior pubis ramus following renal failure-induced hypocalcaemic convulsions. Br J Clin Pract. 1990;44:774 –776
21. Vanderhooft E, Swiontkowski M. Bilateral femoral neck frac-tures following a grand mal seizure.Ann Emerg Med.1994;24: 1188 –1191
22. Blanco JS, Dahir G, McCrystal K. Bilateral femoral neck frac-tures secondary to hypocalcemic seizures in a skeletally imma-ture patient.Am J Orthop.1999;28:187–188
23. Hirtz D, Ashwal S, Berg A, et al. Practice parameter: evaluating a first nonfebrile seizure in children—report of the quality standards subcommittee of the American Academy of Neurol-ogy, The Child Neurology Society, and The American Epilepsy Society.Neurology.2000;55:616 – 623
24. Guise TA, Mundy GR. Clinical review 69: evaluation of hy-pocalcemia in children and adults. J Clin Endocrinol Metab. 1995;80:1473–1478
25. Singh J, Moghal N, Pearce SH, Cheetham T. The investigation of hypocalcaemia and rickets.Arch Dis Child.2003;88:403– 407 26. Lehtonen-Veromaa MK, Mottonen TT, Nuotio IO, Irjala KM, Leino AE, Viikari JS. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr.2002;76:1446 –1453
THE IG NOBEL PRIZE
“What does it take to win an Ig Nobel Prize? There is but a single criterion: Do something that first makes people LAUGH, and then makes them THINK. (WHAT people think is entirely up to them.) Until recently we used more elaborate language to describe this. I am mildly embarrassed that it took us about twelve years to boil it down to a simple phrase that anyone can understand. An Ig Nobel-winning achievement can be good or bad, important or trivial, valuable or worthless. Or all of those. It just has to make people laugh, and then make them think.“
DOI: 10.1542/peds.2006-1170
2006;118;2226
Pediatrics
Pusic
David Schnadower, Chhavi Agarwal, Sharon E. Oberfield, Ilene Fennoy and Martin
Adolescent With Primary Vitamin D Deficiency
Hypocalcemic Seizures and Secondary Bilateral Femoral Fractures in an
Services
Updated Information &
http://pediatrics.aappublications.org/content/118/5/2226 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/118/5/2226#BIBL This article cites 25 articles, 10 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/nutrition_sub
Nutrition
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2006-1170
2006;118;2226
Pediatrics
Pusic
David Schnadower, Chhavi Agarwal, Sharon E. Oberfield, Ilene Fennoy and Martin
Adolescent With Primary Vitamin D Deficiency
Hypocalcemic Seizures and Secondary Bilateral Femoral Fractures in an
http://pediatrics.aappublications.org/content/118/5/2226
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.