79 Blackwell Science, LtdOxford, UKPPEPaediatric and Perinatal Epidemiology0269-5022Blackwell Publishing Ltd, 20052017986Original ArticleReproducibility of hormone assays in umbilical cord bloodI. Baik
et al.
Correspondence:
Dr Inkyung Baik, Division of Biostatistics and
Epidemiology, Department of Cancer Biology, University of Massachusetts Medical School, 364 Plantation Street, LRB #427, Worcester, MA 01605, USA.
E-mail:
inkyung.baik@umassmed.edu
Reproducibility of assays for steroid hormones, prolactin and
insulin-like growth factor-1 in umbilical cord blood
Inkyung Baika,c, Qin Liua, Susan Sturgeonc, Edward J. Stanek IIIc, William Okuliczb and Chung-Cheng Hsieha,c
aCancer Research Center and Department of Cancer Biology, bDepartment of Physiology and ILAT Steroid RIA Laboratory, University of
Massachusetts Medical School, Worcester, and cBiostatistics and Epidemiology Program, Department of Public Health, University of Massachusetts,
Amherst, MA, USA
Summary
Baik I, Liu Q, Sturgeon S, Stanek EJ III, Okulicz W and Hsieh C-C. Reproducibility of assays for steroid hormones, prolactin and insulin-like growth factor-1 in umbilical cord blood. Paediatric and Perinatal Epidemiology 2006; 20: 79–86.
We assessed the reproducibility of measurements of plasma hormone and binding protein levels in umbilical cord blood collected from 30 male and female babies. They were delivered as singleton births from full-term pregnancies (gestational age ≥ 37 weeks) in a cord blood donation programme. We assayed three plasma rep-licates from each cord blood sample at two points in time. Plasma oestradiol, uncon-jugated oestriol, testosterone, progesterone, prolactin, sex-hormone binding globulin (SHBG), insulin-like growth factor-1 (IGF-1), and IGF binding protein-3 (IGFBP-3) levels were measured in duplicates in the same batch (batch 1). In addition, another set of assays was conducted for each cord blood 1 year apart in a different batch (batch 2). Means and standard deviations for each hormone and binding protein were similar in replicates assayed in batch 1 and 2. Pearson’s correlation coefficients were 0.9 or higher in duplicates assayed in batch 1. The correlation coefficients were between 0.77 and 0.96 for between-batch assays. Intra-class correlation coefficients (ICC) were higher than 0.9 for assay of SHBG [95% CI 0.92, 1.0] and progesterone [95% CI 0.87, 0.97] and between 0.8 and 0.9 for assay of oestradiol, unconjugated oestriol, prolactin, IGF-1, and IGFBP-3. The lowest ICC value was found for testosterone (ICC = 0.74; [95% CI 0.56, 1.0]). These data indicate a high reproducibility of cord blood hormone mea-surements; minimal differences were observed between the calibrated and the original regression coefficients for the association of hormones/binding proteins with percent of CD34+ cells in mononuclear cells.
Keywords: reproducibility, steroid hormones, prolactin, IGF-1, umbilical cord blood.
Introduction
Several studies have reported reproducibility of hor-mone assays in blood samples of non-pregnant adult women.1–11 In these studies, it was pointed out that
variations within individuals over time1–3,6,7,9,11 and
between laboratories4,5,8,10 in hormone assays could be
a source of measurement error and attenuate a true association between hormone levels and outcome (e.g. cancer risk). In particular, poor reproducibility of mea-surement of steroid hormones and binding proteins,
such as oestrogen,1,5,7,8,11 progesterone,6,8 and
sex-hor-mone-binding globulin (SHBG),5 has been observed.
There is increasing interest in, and accumulating evi-dence for, a possible role of intrauterine environment in cancer risk.12–20 Specifically, it has been speculated
that high exposure to oestrogens and other growth-stimulating hormones in utero may affect the susce-ptibility to carcinogenesis in the target cells of the offspring and increase cancer risk later in life.21,22 Based
80 I. Baik et al.
measure umbilical cord blood hormone levels to indi-cate in utero hormone exposures and to link them with perinatal factors of cancer risk.23–25 Two studies have
evaluated reproducibility of hormone assay in cord blood.26,27 These studies observed high reproducibility
in measurement of oestrogens,26,27 progesterone,26 and
SHBG27 in cord blood, in contrast to reproducibility
studies in adults.1,5–8,11 High intra-class correlations
could be due to the relatively large differences in oestrogen levels among cord blood samples as com-pared with that in adult samples. Another factor that may contribute to the relatively high intra-class corre-lations is the short freezer storage time for between-batch assays, where hormones were measured in specimens stored 4 months apart.27
We are currently investigating the relationship between perinatal factors for cancer risk, hormone levels in cord blood, and stem cell potential to probe possible biological mechanisms that may underlie an intrauterine component in cancer risk. To evaluate the accuracy of hormone assays in cord blood, we have conducted a reproducibility study over a 1-year period and measured hormones and binding proteins that have not been evaluated in cord blood before. We report here the reproducibility results of plasma levels of oestradiol, oestriol, testosterone, SHBG, progester-one, prolactin, insulin-like growth factor-1 (IGF-1), and IGF-binding protein-3 (IGFBP-3), all measured in a sin-gle laboratory. In addition, we illustrate the application of the reproducibility results to correct regression coef-ficients of the association between levels of cord blood hormone/binding protein and a measure of stem cell potential observed in our preliminary data.
Methods
Study population
The study samples were from participants in a cord blood donation programme that harvests and stores haematopoietic stem cells from umbilical cord blood for transplantation purposes. Eligible participants were pregnant women (age ≥ 18 years) who delivered a singleton birth (gestational age ≥ 37 weeks) at UMASS Memorial Medical Center and St. Vincent’s Hospital (Worcester, MA). Those who reported drug use, blood diseases such as haemophilia or other clot-ting factor deficiencies, cancer, AIDS, sexually trans-mitted diseases, hepatitis, and parasitic blood diseases were excluded. Each participant signed a Human
Subjects Committee-approved informed consent form prior to collection of cord blood. Among cord blood donors, our study participants were identified from those whose cord blood samples were not accepted for storage in the cord blood bank mainly because of insufficient volume (<85 mL) or incomplete informa-tion on the label of the collecinforma-tion bag (e.g. missing exact time of delivery). These biospecimens, though not suitable for clinical purpose, are appropriate for the purpose of this study. The study protocol was approved by the Institutional Review Board of the American Red Cross, UMass Medical School, UMass Memorial Medical Center, and St. Vincent’s Hospital.
Specimen collection
Umbilical cord blood was collected from infants deliv-ered according to standard obstetric practices, while the placenta was still in utero. For infant births requir-ing caesarean section, cord blood was collected after the delivery of the placenta. Cord blood was drained from the umbilical vein using a 16-gauge needle and was collected in a plastic bag containing 35 mL of cit-rate-phosphate-dextrose anticoagulant (Baxter Health Care, Deerfield, IL). After collection, cord blood speci-mens were processed within 24 h from the time of blood collection. They were centrifuged at 20°C for 30 min at 1400 r.p.m. and plasma from each specimen was separated. At least 20 mL of plasma was collected and aliquots containing 2 mL plasma in each cryovial were prepared and stored at −70°C until the time of analysis.
Assay schedule and laboratory methods
Three plasma replicates from each of the 30 partici-pants were included in the reproducibility study and were assayed at two points in time. Two of the three replicates were prepared after thawing 12 mL plasma (six cryovial aliquots) and placed randomly to conceal which sample corresponded to which duplicate sam-ple. They were sent to the Institutional Laboratory Assay Trust Steroid RIA Laboratory of the University of Massachusetts Medical School in March 2003 and assayed in the same batch (Batch 1). These samples were stored for at most 5 months prior to assay. After 1 year, the third replicate of each cord blood sample was prepared from 6 mL plasma (three cryovial ali-quots) and sent to the same laboratory (Batch 2). For each assay run, duplicates from each of the three
con-Reproducibility of hormone assays in umbilical cord blood 81 trol samples (each control sample was formed by
pooled plasma from two subjects’ samples) were assayed. If intra-assay coefficient variations (CV) for hormones and binding proteins measured in controls were more than 15%, the entire run was repeated.
Oestradiol was measured by radioimmunoassay using kits from the Diagnostic Systems Laboratories, Inc. (DSL, Webster, TX). Testosterone was measured by solid-phase 125I radioimmunoassay using kits from the
Diagnostic Products Corporation (DPC, Los Angeles, CA). Unconjugated oestriol, progesterone, prolactin, and SHBG were measured using chemiluminescent immunoassay methodologies from the DPC. IGF-1 and IGFBP-3 were measured by immunoradiometric assay using kits obtained from the DSL. The laboratory reported intra- and inter-assay CVs from routine assays; intra- and inter-assay CVs are 3.4% and 6.8% for oestradiol, 6.6% and 9.2% for unconjugated oestriol, 5.1% and 10.1% for testosterone, 2.0% and 4.8% for SHBG, 6.3% and 7.9% for progesterone, 5.7% and 6.4% for prolactin, 3.9% and 7.4% for IGF-1, and 1.8% and 1.9% for IGFBP-3 respectively. Measurement of CD34+ cells in cord blood followed the protocol used in a previous report.28
Statistical analysis
Descriptive statistics (mean and standard deviation) of hormone and binding protein levels are presented for Batch 1 and Batch 2. Pearson’s correlation was used to evaluate the level of concordance on measurements between replicates. Pearson’s correlation coefficients were calculated between replicates assayed in the same batch (Replicate 1 vs. Replicate 2) and between replicates assayed in different batches (Replicate 1 vs. Replicate 3 and Replicate 2 vs. Replicate 3).
Assay reproducibility was evaluated by intra- and inter-assay CVs and intra-class correlation coefficients (ICC). The CV is commonly used for determining the precision of laboratory performance.29 The intra-assay
CV is estimated by dividing the square root of the within-batch variance by the mean of a measurement and expressed in percentage. In the same way, the inter-assay CV is estimated using the square root of the between-batch variance. Thus, a CV typically depends on the sample mean in the pool, but does not take account of variability between persons. The ICC, which is equivalent to the kappa statistic for continuous variables and is also known as the reli-ability coefficient, is estimated by comparing
between-person variance with total variance in a measurement.30 The advantage of estimating the ICC
is that it provides information on the systematic dif-ferences (e.g. biological difdif-ferences) between persons30
and the potential influence of measurement error on effect estimates.4
To estimate the components of variance associated with between-person variance (σ2
α), between-batch variance (σ2
β), and within-person variance (σ2ε), a restricted maximum likelihood method was used
(PROCVARCOMP in SAS 8.0, SAS Institute, Cary, NC).31
A linear model used for this analysis is as follows:
Yijk=µ+αi+βj+εijk
αi∼N(0, σ2α), βj∼N(0, σ2β), εijk∼N(0, σ2ε)
Yijk is the measured hormone value for participant i
(i= 1–30) at replicate k (k= 1–3) in batch j (j= 1 or 2) and µ denotes the overall mean. Under this model, ICC was calculated as:
ICC =σ2
α/(σ2α+σ2β+σ2ε)
and 95% confidence interval [CI] was estimated using the ‘δ method’.32
Using ICC, we calibrated regression coefficients for the association between hormone/binding protein lev-els and a measure of stem cell potential (percent of CD34+ cells in mononuclear cells, CD34+ cell %) observed in the preliminary data that include 40 sam-ples, of which 13 samples were from the subjects who had participated in the reproducibility study. Simple linear regression was used to examine the association between hormone levels (independent variable, x) and natural log-transformed CD34+ cell % (dependent vari-able, y). To estimate calibrated regression coefficient, the observed regression coefficient was divided by ICC.33,34
Results
The reproducibility study for hormone assay in umbil-ical cord blood included triplicates from each of the 30 participants. Mean and standard deviation of plasma concentrations of hormones and binding proteins are summarised in Table 1. For each hormone, mean val-ues in Replicate 3 assayed in Batch 2 differed some-what from those of Replicates 1 and 2 assayed in Batch 1: mean plasma oestradiol, SHBG, progesterone, and IGF-1 levels of Replicate 3 were lower than those of Replicates 1 and 2, while other hormones and IGFBP-3 were higher. However, none of the differences in
82 I. Baik et al.
mean values between Replicate 3 and Replicates 1 and 2 was statistically significant.
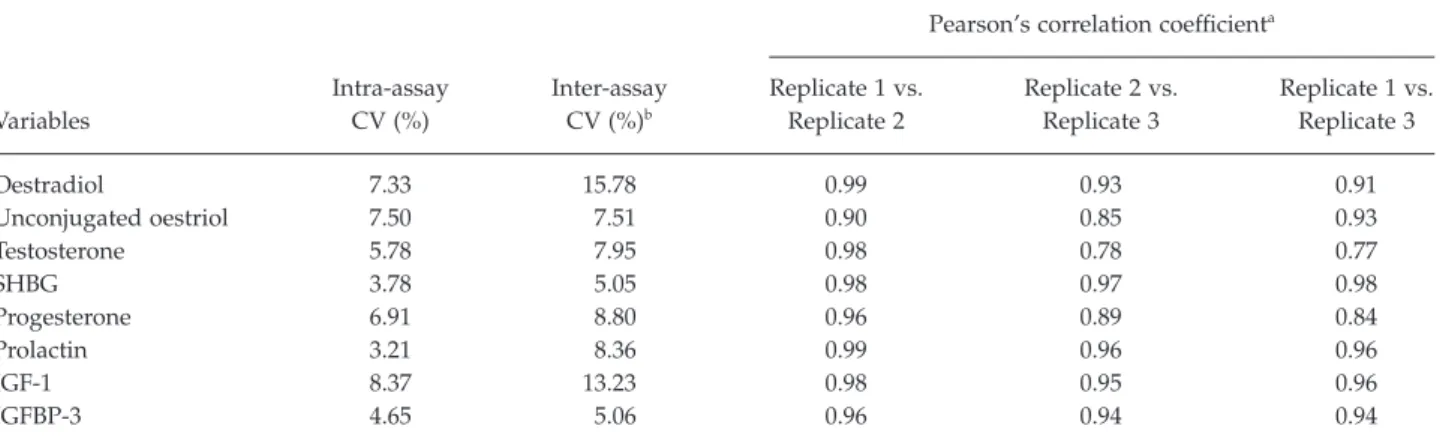
Table 2 presents intra- and inter-assay CVs and Pear-son’s correlation coefficients between replicates of each hormone and binding protein. We obtained Pearson’s correlation coefficients on the normal scale as well as on natural log-transformed scale of the hormone mea-sures and found that the correlation coefficients were the same with the two scales. Within-batch reproduc-ibility for Replicates 1 and 2 appeared acceptable, because intra-assay CVs were less than 10% for all the hormones and binding proteins measured. Within-batch assay between Replicates 1 and 2 indicated a good agreement, with Pearson’s correlation
coeffi-cients of 0.9 or higher. The inter-assay CVs were between 5.1% and 15.8% for all the hormones and binding proteins measured. The correlation coefficient between Replicate 3 and either Replicate 1 or 2 was higher than 0.9 for assay of oestradiol, unconjugated oestriol, SHBG, prolactin, IGF-1, and IGFBP-3. Lower correlation coefficients were observed in the between-batch assay for testosterone (r= 0.77 or 0.78) and progesterone (r= 0.84 or 0.89); these nonetheless indi-cate a high correlation.
Table 3 presents the estimated variance components (consisting of between-person, between-batch, and within-person variances) and the ICCs and their 95% CIs. The between-person variance component
Table 1. Comparison of mean and standard deviation (SD) of cord blood hormone and binding protein levels in triplicates from 30 participants
Variables Unit n
Mean (SD)
Batch 1 Batch 2
Replicate 1 Replicate 2 Replicate 3
Oestradiol ng/dL 30 866.7 (962.2) 853.0 (966.0) 802.8 (509.7) Unconjugated oestriol ng/mL 30 253.5 (111.8) 238.7 (117.3) 295.2 (117.7) Testosterone ng/mL 30 1.13 (0.62) 1.13 (0.59) 1.41 (0.55) SHBG nmol/L 30 24.4 (6.8) 24.3 (7.6) 23.3 (6.4) Progesterone ng/mL 29 240.5 (117.1) 241.0 (118.8) 228.1 (114.6) Prolactin ng/mL 28 207.0 (92.9) 207.5 (93.7) 262.4 (116.8) IGF-1 ng/mL 30 54.3 (37.1) 57.4 (39.0) 45.1 (35.7) IGFBP-3 ng/mL 30 1015.7 (286.1) 1010.6 (274.0) 1170.5 (329.1)
SHBG, sex hormone-binding globulin; IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3.
Table 2. Intra- and inter-assay coefficient of variation (CV) of hormones and binding proteins and Pearson’s correlation between replicates Variables Intra-assay CV (%) Inter-assay CV (%)b
Pearson’s correlation coefficienta
Replicate 1 vs. Replicate 2 Replicate 2 vs. Replicate 3 Replicate 1 vs. Replicate 3 Oestradiol 7.33 15.78 0.99 0.93 0.91 Unconjugated oestriol 7.50 7.51 0.90 0.85 0.93 Testosterone 5.78 7.95 0.98 0.78 0.77 SHBG 3.78 5.05 0.98 0.97 0.98 Progesterone 6.91 8.80 0.96 0.89 0.84 Prolactin 3.21 8.36 0.99 0.96 0.96 IGF-1 8.37 13.23 0.98 0.95 0.96 IGFBP-3 4.65 5.06 0.96 0.94 0.94
SHBG, sex hormone-binding globulin; IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3.
aP< 0.001.
Reproducibility of hormone assays in umbilical cord blood 83
accounted for a large contribution to the total variabil-ity of assay results relative to the between-batch and the person variance components. The within-person variance component, which reflects mea-surement error in laboratory assays, was a small proportion of the total variability.
The ICCs were higher than 0.9 for assay of SHBG [95% CI 0.92, 1.0] and progesterone [95% CI 0.87, 0.97] and between 0.8 and 0.9 for assay of oestradiol, uncon-jugated oestriol, prolactin, IGF-1, and IGFBP-3. The testosterone assay had the lowest ICC (0.74, [0.56, 1.0]). The results of ICCs calculated using both natural log-transformed and unlog-transformed hormone values were similar (Table 3).
The calibrated association between each of the hormones/binding proteins and percentage of CD34+ cells in mononuclear cells was calculated using the observed regression coefficient and ICC. Compared with calibrated regression coefficients, attenuation in observed coefficients was minimal; the regression co-efficients were attenuated between 4% and 24%. The regression coefficient for testosterone, which had the lowest ICC, was 0.26 [−0.04, 0.57]) and 0.35 [−0.17, 0.86] before and after the calibration.
Discussion
We evaluated the reproducibility of the assays for oestradiol, unconjugated oestriol, testosterone, SHBG, progesterone, prolactin, IGF-1, and IGFBP-3 in umbil-ical cord blood and observed a high degree of repro-ducibility in measurements from replicate samples. The variance component analysis showed that most of the variability was due to the variation between
sub-jects’ samples. Estimates of the ICC percentage, which is the percentage of variation attributable to between-person variation, were more than 90% for the SHBG and progesterone assays and between 80 and 90% for the oestradiol, unconjugated oestriol, prolactin, IGF-1, and IGFBP-3 assays. These results suggest that a single measurement of these hormones in cord plasma pro-vides a reliable measurement.
We have found two previous studies that conducted a reproducibility study for hormone assays in cord blood.26,27 Maccoby et al. evaluated reproducibility in
duplicates assayed independently for hormone mea-surements.26 In this study, duplicates appeared to be
measured in different batches, though the interval between the two assays was not described. In three cohorts including 256 male and female babies, the range of Pearson’s correlation coefficient between duplicates was 0.95–0.98 for oestrone, 0.98–0.99 for oestradiol, 0.85–0.89 for testosterone, 0.82–0.93 for androstenedione, and 0.95–0.96 for progesterone. Shi-bata et al. collected cord serum from 25 female babies and determined oestrone, total oestradiol, weakly bound oestradiol, and SHBG levels in duplicates that were measured either at the same time or at 4 months apart.27 Pearson’s correlation coefficients were 0.98 for
oestrone, 0.88 for total oestradiol, 0.90 for weakly bound oestradiol, and 0.96 for SHBG in 14 samples assayed in different batches and were higher than 0.96 in 11 samples assayed in the same batch. The estimated ICC was 0.8 for oestradiol and 0.97 for SHBG in their study and similar to our results of 0.82 for oestradiol and 0.96 for SHBG.
In our study, between-batch variations were, how-ever, examined in replicates assayed 1 year apart. In
Table 3. Variance component estimates of cord blood hormones and binding proteins
Variables Oestradiol
Unconjugated
oestriol Testosterone SHBG Progesterone Prolactin IGF-1 IGFBP-3
Variance componenta Between-person 0.75 0.55 0.35 0.07 0.23 0.22 0.62 0.07 Between-batch 0.03 0.03 0.05 0.001 0.002 0.03 0.04 0.01 Within-person 0.13 0.07 0.07 0.002 0.02 0.01 0.03 0.004 ICCa 0.82 0.83 0.74 0.96 0.92 0.86 0.89 0.83 [95% CI] of ICCa [0.72, 0.97] [0.71, 1.00] [0.56, 1.00] [0.92, 1.00] [0.87, 0.97] [0.65, 1.00] [0.76, 1.00] [0.61, 1.00] ICCb 0.86 0.82 0.76 0.96 0.89 0.83 0.92 0.82
SHBG, sex hormone-binding globulin; IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3; ICC, intra-class correlation coefficients.
aNatural log scale. bNormal scale.
84 I. Baik et al.
addition, we also evaluated additional hormones including oestriol, progesterone, prolactin, IGF-1, and IGFBP-3, which have been suggested to play a role in cancer risk related to hormone exposure in utero. We have found high reproducibility for these assays as well. ICC for testosterone was 0.74, lower than that for other hormones and binding proteins. The limited abil-ity to discriminate differences between subjects’ sam-ples with a single measurement of testosterone could be due to the lack of sensitivity at lower concentrations of testosterone in cord plasma compared with levels of oestrogens, progesterone, prolactin and IGF-1.
A number of reproducibility studies have been con-ducted for hormone assays using serum or plasma specimens from non-pregnant adult women.1–11 The
purpose of these studies is to provide evidence of reli-ability in measurements of hormone levels and even-tually to justify the association between hormone levels and risk of diseases such as cancer and osteoporosis observed in epidemiological studies. However, measurement of oestrogens,1,3–5,9–11
progest-erone,4,8 and prolactin2,7,9 in adult women has been a
concern because of the variation of assay precision in different laboratories and in multiple aliquots collected over time from the same person. In particular, the lack of reliability in hormone assays among postmeno-pausal women could be due to inter-individual vari-ability of hormone levels over time as well as due to laboratory imprecision, because multiple blood sam-ples have been drawn at different points in time1–3,7,9,11
and low blood concentrations of steroid hormones tend to reduce assay reproducibility.10 Some studies
have compared reproducibility of hormone assays between laboratories and reported considerable varia-tion and measurement error.4,5,8,10 Thus, it is important
to evaluate laboratory performance in studies assess-ing hormone levels in adult subjects.
In reproducibility studies with cord blood, a main concern is laboratory imprecision. In our study, we sent samples, stored at −70°C until analysis, to a labo-ratory dedicated to hormone assays, which uses stan-dardised assay procedures and the same brand of commercial kits for each hormone and binding pro-tein. The laboratory was blinded to the identity of replicates in our samples. In each assay run, control specimens were also assayed and if hormone levels measured in controls differed significantly the entire run would have been repeated; this did not occur dur-ing the course of this study. Another factor that might contribute to the high reproducibility is that
concentra-tions of most hormones in cord blood are much higher than levels in blood samples from non-pregnant adults. For example, concentrations in cord blood are at least two times higher for testosterone, 10 times higher for prolactin, 30 times higher for progesterone, 80 times higher for oestradiol than blood levels of these hormones in premenopausal women.6,9
Recently, hormone assessment in cord blood has been conducted by investigators who are interested in the association between intrauterine exposure to hor-mones and perinatal factors related to cancer risk.23–25
To explore potential biological mechanisms underly-ing an intrauterine component in cancer risk, we have launched a population-based study to investigate the relationship between perinatal factors of cancer risk, hormone levels in cord blood, and measures of stem cell potential. Using the preliminary data that we have to date, we calibrated regression coefficients for the association of hormone levels with percentage of CD34+ cells in mononuclear cells and found that the observed association was not attenuated when a single measurement of hormones was used. Even for test-osterone, which had the lowest value of Pearson’s correlation coefficient and ICC, there was little attenuation.
In summary, we have conducted a reproducibility study for assays of plasma oestradiol, unconjugated oestriol, testosterone, progesterone, prolactin, SHBG, IGF-1, and IGFBP-3 in cord blood and observed an overall high correlation between duplicates for within-batch assays as well as for between-within-batch assay mea-sured 1 year apart. Thus, our findings suggest that measurements of these hormones are highly reproduc-ible and a single measurement is sufficient to reliably discriminate levels of hormone exposure among cord blood samples.
Acknowledgements
This work was supported by a grant (R01CA90902) from the US National Cancer Institute, National Insti-tutes of Health.
References
1 Cauley JA, Gutai JP, Kuller LH, Powell JG. Reliability and interrelations among serum sex hormones in postmenopausal women. American Journal of Epidemiology 1991; 133:50–57. 2 Koenig KL, Toniolo P, Bruning PF, Bonfrer JM, Shore RE,
Reproducibility of hormone assays in umbilical cord blood 85
in women. Cancer Epidemiology, Biomarkers and Prevention
1993; 2:411–414.
3 Toniolo P, Koenig KL, Pasternack BS, Banerjee S, Rosenberg C, Shore RE, et al. Reliability of measurements of total, protein-bound, and unbound estradiol in serum. Cancer Epidemiology, Biomarkers and Prevention 1994; 3:47–50. 4 Hankinson SE, Manson JE, London SJ, Willett WC, Speizer
FE. Laboratory reproducibility of endogenous hormone levels in postmenopausal women. Cancer Epidemiology, Biomarkers and Prevention 1994; 3:51–56.
5 Potischman N, Falk RT, Laiming VA, Siiteri PK, Hoover RN. Reproducibility of laboratory assays for steroid hormones and sex hormone-binding globulin. Cancer Research 1994;
54:5363–5367.
6 Bolelli G, Muti P, Micheli A, Sciajno R, Franceschetti F, Krogh V, et al. Validity for epidemiological studies of long-term cryoconservation of steroid and protein hormones in serum and plasma. Cancer Epidemiology, Biomarkers and Prevention
1995; 4:509–513.
7 Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period.
Cancer Epidemiology, Biomarkers and Prevention 1995; 4:649– 654.
8 Gail MH, Fears TR, Hoover RN, Chandler DW, Donaldson JL, Hyer MB, et al. Reproducibility studies and
interlaboratory concordance for assays of serum hormone levels: estrone, estradiol, estrone sulfate, and progesterone.
Cancer Epidemiology, Biomarkers and Prevention 1996; 5:835– 844.
9 Muti P, Trevisan M, Micheli A, Krogh V, Bolelli G, Sciajno R, et al. Reliability of serum hormones in
premenopausal and postmenopausal women over a one-year period. Cancer Epidemiology, Biomarkers and Prevention 1996;
5:917–922.
10 McShane LM, Dorgan JF, Greenhut S, Damato JJ.
Reliability and validity of serum sex hormone measurements.
Cancer Epidemiology, Biomarkers and Prevention 1996; 5:923– 928.
11 Falk RT, Dorgan JF, Kahle L, Potischman N, Longcope C. Assay reproducibility of hormone measurements in postmenopausal women. Cancer Epidemiology, Biomarkers and Prevention 1997; 6:429–432.
12 Hilakivi-Clarke L, Cho E, Raygada M, Onojafe I, Clarke R, Lippman ME. Early life affects the risk of developing breast cancer. Annals of the New York Academy of Sciences 1995;
768:327–330.
13 Hilakivi-Clarke L, Clarke R, Onojate I, Raygada M, Cho E, Lippman M. A maternal diet high in n-6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring.
Proceedings of the National Academy of Sciences of the USA
1997; 94:9372–9377.
14 Ekbom A, Trichopoulos D, Adami H-O, Hsieh C-C, Lan S-J. Evidence of prenatal influences on breast cancer risk. Lancet
1992; 340:1015–1018.
15 Weiss HA, Potischman NA, Brinton LA, Brogan D, Coates RJ, Gammon MD, et al. Prenatal and perinatal risk factors for breast cancer in young women. Epidemiology 1997; 8:181–187.
16 Innes K, Byers T, Schymura M. Birth characteristics and subsequent risk for breast cancer in very young women.
American Journal of Epidemiology 2000; 152:1121–1128. 17 Vatten LJ, Maehle BO, Lund Nilsen TI, Tretli S, Hsieh CC,
Trichopoulos D, et al. Birth weight as a predictor of breast cancer: a case-control study in Norway. British Journal of Cancer 2002; 86:89–91.
18 Titus-Ernstoff L, Egan KM, Newcomb PA, Ding J,
Trentham-Dietz A, Greenberg ER, et al. Early life factors in relation to breast cancer risk in postmenopausal women.
Cancer Epidemiology, Biomarkers and Prevention 2002; 11:207– 210.
19 Mellemkjaer L, Olsen ML, Sorensen HT, Thulstrup AM, Olsen J, Olsen JH. Birth weight and risk of early-onset breast cancer (Denmark). Cancer Causes and Control 2003;
14:61–64.
20 Ahlgren M, Sorensen T, Wohlfahrt J, Haflidadottir A, Holst C, Melbye M. Birth weight and risk of breast cancer in a cohort of 106,504 women. International Journal of Cancer 2003;
107:997–1000.
21 Trichopoulos D. Hypothesis: does breast cancer originate in utero? Lancet 1990; 335:939–940.
22 Schernhammer ES. In-utero exposures and breast cancer risk. joint effect of estrogens and insulin-like growth factor? Cancer Causes and Control 2002; 13:505–508.
23 Shibata A, Harris DT, Billings PR. Concentrations of estrogens and IGFs in umbilical cord blood plasma: a comparison among Caucasian, Hispanic, and Asian-American females. Journal of Clinical Endocrinology and Metabolism 2002; 87:810–815.
24 Troisi R, Potischman N, Roberts J, Siiteri P, Daftary A, Sims C, et al. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States). Cancer Causes and Control 2003; 14:347– 355.
25 Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, et al. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. International Journal of Epidemiology 2003; 32:455– 460.
26 Maccoby EE, Doering CH, Jacklin CN, Kraemer H. Concentrations of sex hormones in umbilical-cord blood: their relation to sex and birth order of infants. Child Development 1979; 50:632–642.
27 Shibata A, Lee MM, Meyer PB. Laboratory assay
reproducibility of serum estrogens in umbilical cord blood samples. Cancer Epidemiology, Biomarkers and Prevention 1999;
8:147–151.
28 Ballen KK, Wilson M, Wuu J, Credona AM, Hsieh C-C, Stewart FM, et al. Bigger is better: maternal and neonatal predictors of hematopoietic potential of umbilical cord blood units. Bone Marrow Transplantation 2001; 27:7–14.
29 Ottaviano PJ, DiSalvo AF. Quality Control in the Clinical Laboratory: A Procedural Text. Baltimore, MD: University Park Press, 1977; pp. 17–29.
30 Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. Gaithersburg: Aspen Publishers, Inc., 2000; pp. 343–404. 31 SAS Institute, Inc. SAS/STAT User’s Guide, Version 6, 4th edn,
86 I. Baik et al.
32 Rao CR. Linear Statistical Inference and Its Applications, 2nd edn. New York: John Wiley & Sons, Inc., 1973.
33 Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Statistics in Medicine 1989; 8:1051–1069.
34 Knuiman MW, Divitini ML, Buzas JS, Fitzgerald PE. Adjustment for regression dilution in epidemiological regression analyses. Annals of Epidemiology 1998; 8:56–63.