1556-6811/09/$08.00⫹0 doi:10.1128/CVI.00424-08
T-Cell mRNA Expression in Response to
Mycobacterium bovis
BCG
Vaccination and
Mycobacterium bovis
Infection of White-Tailed Deer
䌤
Tyler C. Thacker,* Mitchell V. Palmer, and W. Ray Waters
U.S. Department of Agriculture, Agricultural Research Service, Bacterial Diseases of Livestock Research Unit, National Animal Disease Center, 2300 Dayton Ave., Ames, Iowa 50010
Received 15 November 2008/Returned for modification 10 December 2008/Accepted 1 June 2009
Understanding immune responses of white-tailed deer (WTD) to infection withMycobacterium bovisprovides
insight into mechanisms of pathogen control and may provide clues to development of effective vaccine
strategies. WTD were vaccinated with eitherM.bovisBCG strain Pasteur or BCG strain Danish. Both vaccinees
and unvaccinated controls were subsequently inoculated with virulentM. bovisvia the intratonsillar route.
Real-time PCR was used to assess T-cell mRNA expression in peripheral blood leukocytes (PBL) from animals following vaccination and infection. Recall T-cell responses were measured by assessing the relative expression
of gamma interferon (IFN-␥), T-cell-specific T-box transcription factor (Tbet), interleukin 12p40 (IL-12p40),
IL-12p35, IL-23p19, FoxP3, IL-17, and GATA3 in PBL stimulated in vitro with purified protein derivative
(PPD) ofM. bovis or a recombinant fusion protein, ESAT6-CFP10. Animals vaccinated with BCG Danish
expressed more IFN-␥and Tbet than either BCG Pasteur-vaccinated animals or unvaccinated controls. BCG
Pasteur-vaccinated animals expressed more GATA3 than either group. After infection, unvaccinated controls expressed more Tbet and IL-12p40 than vaccinated animals. BCG Pasteur-vaccinated animals expressed more GATA3 than either the unvaccinated controls or the BCG Danish-vaccinated animals after infection. Animals were divided into pathology groups to correlate gene expression with severity of pathology. Animals in the
visible lesion group expressed more Tbet and IFN-␥than animals that were culture negative, while Tbet and
IFN-␥expression in the culture-positive, no-visible-lesion group was intermediate. GATA3 expression inversely
correlated with pathology. Overall, expression of immune response genes correlated more closely with pathol-ogy than vaccination treatment.
A self-sustaining outbreak of Mycobacterium bovisin free-ranging white-tailed deer ([WTD]Odocoileus virginianus) has occurred in Michigan (36, 37). Epidemiological and strain typ-ing evidence suggests that infected WTD serve as a reservoir since interspecies transmission from deer to cattle occurs (21, 25, 29). In Minnesota, infected WTD have been found adjacent to infected cattle (http://www.bah.state.mn.us/tb/). It is not yet known if the 18 infected deer (from 2005 to 2008) represent a self-sustaining outbreak. Control of these wildlife reservoirs may be critical to preventing continued infection of domestic cattle. In Michigan, efforts to control tuberculosis (TB) in free-ranging WTD through removal of WTD and through changes in management practices, while providing some ben-efit, have not yet proven effective in eliminating the reservoir (22). Similar experiences have occurred in other areas of the world where a wildlife reservoir exists (6, 7). Development of an effective vaccine may provide a significant tool for eradica-tion ofM.bovisfrom the free-ranging WTD population.
Immunological responses of WTD and other ruminants to
M. bovisinfection appear to be complex. The adaptive immune
response is believed to be primarily responsible for immunity
to M. bovis infection. Specific T-cell responses have been
roughly divided into four types: T-helper type 1 (TH1), TH2, TH17, and regulatory T cells (Treg). Each of these responses
has been reported to play a role in M. bovis immunity or pathology. TH1 responses, characterized by gamma interferon (IFN-␥) expression, are required for effective immune re-sponses to mycobacteria (4, 9). However, IFN-␥ expression does not correlate with protection in mice (8, 19), deer (42), or cattle (41, 44). Two closely related cytokines, interleukin-12 (IL-12) and IL-23, mediate TH1 and TH17 responses, respec-tively (13). The proinflammatory TH17 cells produce IL-17 in response to antigens; these cells are implicated in inflamma-tory diseases such as experimental autoimmune encephalomy-elitis (15) and have been implicated in playing a role in TB (13). In mice, IL-17 is not required for initial clearance ofM.
bovisBCG but is required for protection after challenge with
M. tuberculosis(12), suggesting that IL-17 may be important in
long-term immunity to mycobacteria.
TH2 responses are implicated in poor prognosis relative to TB (31, 33, 38, 47), presumably by interfering with TH1-me-diated responses. In the murine model, blocking IL-4 in vivo results in decreased bacterial burden, suggesting that TH2 responses are detrimental to mycobacterial control (35). A similar detrimental role for IL-4 has been suggested from stud-ies in humans where IL-4 and IL-13 mRNA expression corre-late with disease severity (32, 38). In addition to TH2, Treg responses are implicated in limiting protective immunity (5, 10, 16). Treg frequency is increased in peripheral blood and sites of infection in TB patients and correlates with disease severity (10, 16).
Vaccination of WTD with theM. bovisBCG strain Danish (20) or BCG strain Pasteur (23) has been shown to be effica-cious as measured by a reduction in pathology (24). To
deter-* Corresponding author. Mailing address: National Animal Disease Center, Bacterial Diseases of Livestock Research Unit, 2300 Dayton Ave., Ames, IA 50010. Phone: (515) 663-7294. Fax: (515) 663-7458. E-mail: tyler.thacker@ars.usda.gov.
䌤Published ahead of print on 10 June 2009.
1139
on August 17, 2020 by guest
http://cvi.asm.org/
mine T-cell-mediated responses induced by vaccination with these BCG strains and to correlate these responses to protec-tion/pathology, WTD were vaccinated with either BCG Danish or BCG Pasteur and subsequently infected with virulent M.
bovis. Sixteen weeks after vaccination and then 16 weeks after
infection, gene expression was measured in isolated peripheral blood leukocytes (PBL) stimulated with either purified protein derivative (PPD) ofM. bovisor the recombinant fusion protein ESAT6 (early secreted antigenic target 6-kDa protein)-CFP10 (culture filtrate 10-kDa protein). ESAT6-CFP10 was evaluated because it is one of the dominant T-cell antigen proteins pro-duced byM. bovis(1, 30, 40) and has been used to increase diagnostic test specificity (45, 46). TH1 responses were evalu-ated using the T-cell-specific T-box transcription factor (Tbet), IFN-␥, and IL-12p35. TH2 responses were evaluated by mea-suring transcription of GATA binding protein 3 transcription factor (GATA3), IL-4, and IL-10. TH17 responses were eval-uated using IL-17, and IL-23p19 mRNA and Treg responses were evaluated using the transcription factor Forkhead box P3 (FoxP3).
MATERIALS AND METHODS
Animals, vaccination, and challenge.Thirty-five WTD (⬃1 year old) were obtained from a captive breeding herd (TB and paratuberculosis free) at the National Animal Disease Center (Ames, IA). All deer were housed and cared for according to institutional guidelines, and procedures were approved by the In-stitutional Animal Care and Use Committee prior to the beginning of the experiment. Deer were randomly assigned to one of three groups: a group receiving one subcutaneous dose of 107CFU ofM. bovisBCG Pasteur (n⫽12),
a group receiving one subcutaneous dose of 107
CFU ofM. bovisBCG Danish (n⫽11), or a group of unvaccinated deer (n⫽12). After 120 days deer received intratonsillar inoculations of approximately 495 CFU ofM. bovisstrain 95-1315 into each tonsillar crypt, for a total dose of 990 CFU, as described previously (28).
Strain 95-1315 used for challenge was originally isolated from a free-ranging, naturally infected WTD in Michigan. For inoculation, deer were anesthetized by intramuscular injection of a combination of xylazine (2 mg/kg of body weight) (Mobay Corporation, Shawnee, KS) and ketamine (6 mg/kg) (Fort Dodge Lab-oratories, Fort Dodge, IA). After inoculation, the effects of xylazine were re-versed by intravenous injection of tolazoline (4 mg/kg) (Lloyd Laboratories, Shanandoah, IA). Vaccinated and unvaccinated deer were housed together in an outdoor paddock prior to challenge with virulentM. bovis, at which time they were moved to an appropriate biosecurity level 3 animal facility. Deer were fed a commercial pelleted feed with free access to water.
TheM. bovisBCG strains as well as the challenge strainM. bovis95-1315 was grown in Middlebrook’s 7H9 medium supplemented with 10% oleic acid-albu-min-dextrose complex (Difco, Detroit, MI) plus 0.05% Tween 80 (Sigma Chem-ical Co., St. Louis, MO), as described previously (2). Mid-log-phase growth bacilli were pelleted by centrifugation at 750⫻g, washed twice with phosphate-buffered saline (0.01 M; pH 7.2), and diluted to the appropriate cell density in 2 ml of phosphate-buffered saline. Bacilli were enumerated by serial dilution plate counting on Middlebrook 7H11 selective medium (Becton Dickinson, Cock-eysville, MD).
Necropsy and tissue sampling.At 130 days postchallenge with virulentM. bovis, all deer were euthanized by intravenous sodium pentobarbital. At nec-ropsy, the following tissues or fluids were collected and processed for isolation of
M. bovisand microscopic analysis as described previously (26): palatine tonsil, lung, liver, and the mandibular, parotid, medial retropharyngeal, tracheobron-chial, mediastinal, hepatic, mesenteric, and prefemoral lymph nodes. Tissues were processed for isolation ofM. bovisas previously described (27). Isolates of
M. boviswere identified by colony morphology, growth, and biochemical char-acteristics as well as by PCR.
Leukocyte preparation and culture.Total PBL were prepared from the buffy coat fraction of blood collected in the anticoagulant acid-citrate-dextrose at 16 weeks after vaccination (prior to infection) and 16 weeks after infection (32 weeks after vaccination). Contaminating red blood cells were removed by hypo-tonic lysis as described previously (11, 34). PBL were seeded into 96-well round-bottom microtiter plates (Falcon, Becton-Dickinson; Lincoln Park, NJ) at 1⫻
106
cells in a total volume of 200l of complete RPMI medium (RPMI 1640 medium with 2 mML-glutamine, 25 mM HEPES buffer, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 1% nonessential amino acids [Sigma, St. Louis, MO], 2% essential amino acids [Sigma], 1% sodium pyruvate [Sigma], 50M 2-mer-captoethanol [Sigma], and 10% [vol/vol] fetal bovine serum). Wells contained medium alone (nil stimulated) or either 10g/mlM. bovisPPD or 10g/ml ESAT6-CFP10. PPD was obtained from CSL Animal Health, Parkville, Victoria, Australia, and ESAT6-CFP10 was kindly provided by F. Chris Minion. Cultures were incubated at 37°C in a 5% CO2atmosphere for 16 h.
Isolation and reverse transcription of leukocyte RNA.Isolation and reverse transcription of PBL RNA were performed as previously described (42). Briefly, cells were harvested by centrifugation, lysed with 150l of buffer RLT (Qiagen, Valencia, CA), and stored at⫺80°C. RNA was isolated using an RNeasy Mini Kit (Qiagen) according to the manufacturer’s directions and eluted from the column with 50l of RNase-free water (Ambion, Austin, TX). Contaminating DNA was enzymatically removed by treating RNA with DNA-free (Ambion). Twenty microliters of RNA was reverse transcribed in a 50-l reaction mixture using SuperScript II (Invitrogen, Carlsbad, CA) with 0.5g of oligo(dT)12-18and
40 units of RNaseOut (Invitrogen), according to the manufacturer’s directions. Samples were heated to 70°C for 5 min and then reverse transcribed at 42°C for 60 min. The resulting cDNA was stored at⫺80°C until used in real-time PCRs. Analysis of cytokine gene expression by real-time PCR.Real-time PCR was performed using SYBR green Master Mix (Applied Biosystems, Foster City, CA) according to the manufacturer’s directions. Briefly, 2.5l of cDNA was added to a 25-l reaction mixture with 1M of each primer. Primers used were designed using bovine sequences with Primer3Plus (43) and then sequenced to ensure correct design. The following primer pairs were used: FoxP3 Forward, TACGG GGCTCTTCTCTCTCA; FoxP3 Reverse, ACAGTCGAAAGGGTGCTGTC; GATA-3 Forward, AACCGGGCATTACCTGTGTA; GATA-3 Reverse, AGG ACGTACCTGCCCTTCTT; IL-12p35 Forward, TGACAACCCTGTGCCTT AAA; IL-12p35 Reverse, CCTGCATCAGCTCAGCAATA; IL-23p19 Forward, TCACAGGGGAGCCTTCTCTA; IL-23p19 Reverse, AGTTCCCTGAGGCCC AGTAT; IL-17 Forward, CACAGCATGTGAGGGTCAAC; IL-17 Reverse, GGTGGAGCGCTTGTGATAAT; T-bet Forward, CCTGGACCCAACTGTC AACT; T-bet Reverse, GGTAGAAACGGCTGGAGATG. Primers for IFN-␥, IL-12p40, IL-4, and IL-10 were as described previously (42). All reactions were performed in triplicate, and data were analyzed with the 2⫺⌬⌬Ct
method as
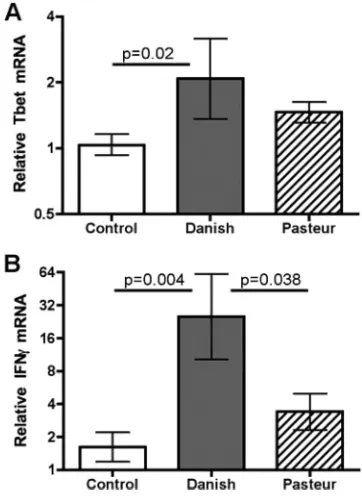
FIG. 1. Relative cytokine gene expression in vaccinated and control animals 16 weeks after vaccination. Gene expression was measured in PBL that were stimulated with PPD. Data are presented as means⫾ standard errors of the means relative to prevaccination. Statistical analysis was performed as described in Materials and Methods.
1140 THACKER ET AL. CLIN. VACCINEIMMUNOL.
on August 17, 2020 by guest
http://cvi.asm.org/
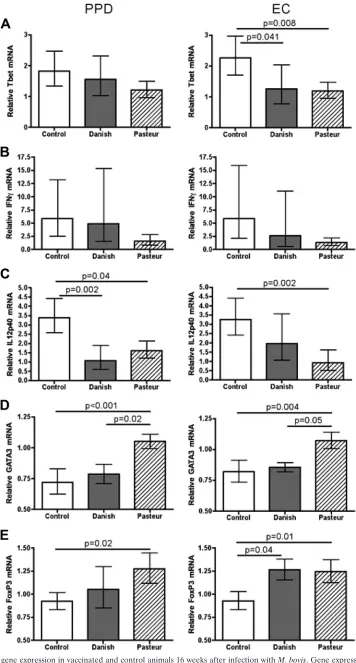
FIG. 2. Cytokine gene expression in vaccinated and control animals 16 weeks after infection withM. bovis. Gene expression was measured in PBL stimulated with either PPD or ESAT6-CFP10 (EC). Data are presented as means⫾standard errors of the means relative to prevaccination. Statistical analysis was performed as described in Materials and Methods.
1141
on August 17, 2020 by guest
described previously (18).-Actin served as the internal control to normalize RNA content between samples. The nil-stimulated sample was used as the calibrator. The use of-actin as the internal control was validated as suggested by Livak and Schmittgen (18). The data are expressed relative to samples col-lected prior to vaccination.
Statistical analysis.All statistical analyses were performed with SAS software, version 9.1.3 (SAS Institute, Inc., Cary, NC). A mixed model for repeated measures (PROC MIXED) using the spatial power law for unequally spaced time points as the repeated effect (17) was used. For each stimulus and gene, the outcome variable (2⫺⌬⌬Ct) was log transformed. The model accounted for the
effects of vaccination/pathology and time along with the interaction of vaccina-tion/pathology and time. Prior to either analysis, the following covariance struc-tures were tested, and the structure with the lowest scores was used in the final analysis: ⫺2 REML (residual maximum likelihood) log likelihood, Akaike’s information criterion, and the Schwarz’s Bayesian information criterion. A
Pvalue of less than 0.05 was considered significant.
Correlations were calculated using the PROC CORR function (SAS). A Pear-son product moment correlation was calculated for cytokine-to-cytokine corre-lations. Correlation of cytokine gene expression with pathology was performed using Spearman’s correlation using the relative gene expression verses pathology. The pathology groups were assigned the following ordinal values: culture nega-tive (CN group), 1; no visible lesions (NVL group), 2; and visible lesions (VL group), 3. Effects with aPvalue of less than 0.05 and anRvalue of greater than 0.5 were considered significant.
RESULTS
Vaccine-induced immunological responses. Sixteen weeks
after vaccination and prior to infection, PPD-specific immune responses were evaluated. Animals vaccinated with BCG Dan-ish expressed approximately twofold more Tbet mRNA in re-sponse to PPD than did the unvaccinated controls (Fig. 1A), whereas the BCG Pasteur-vaccinated animals were not statis-tically different from controls. IFN-␥ expression followed a pattern similar to that of Tbet expression (Fig. 1B). Animals vaccinated with BCG Danish expressed 15-fold more IFN-␥ mRNA than controls expressed and 7-fold more than the BCG Pasteur-vaccinated animals. Vaccination did not induce signif-icant differential expression of GATA3, IL-4, IL-10, IL-12p35, IL-12p40, IL-17, IL-23p19, and FoxP3 in response to PPD stimulation (data not shown). There was no significant gene expression in response to ESAT6-CFP10 stimulation in con-junction with BCG vaccination (data not shown). These data are consistent with the absence of the genes encoding ESAT6 or CFP10 in BCG Danish or BCG Pasteur.
Gene expression in vaccinees after infection.Sixteen weeks
after infection, PPD- and ESAT6-CFP10-specific immune re-sponses were evaluated. When recall rere-sponses to ESAT6-CFP10 were evaluated, BCG Danish-vaccinated deer ex-pressed 1.9-fold less Tbet mRNA than unvaccinated controls (Fig. 2A), and FoxP3 gene expression was 1.3-fold greater (Fig. 2E). No significant differences between IFN-␥, 12p40, IL-17, IL-4, IL-10, or GATA3 gene expression in response to ESAT6-CFP10 stimulation were detected between BCG Dan-ish vaccinees and unvaccinated controls (Fig. 2 and data not shown). PPD stimulation resulted in decreased IL-12p40 ex-pression in the PBL from BCG Danish vaccinees compared to controls. Tbet, IFN-␥, GATA3, or FoxP3 gene expression lev-els were not different between the BCG Danish vaccinees and unvaccinated controls (Fig. 2).
PBL from BCG Pasteur-vaccinated animals expressed sig-nificantly less Tbet and IL-12p40 mRNA than controls when cells were stimulated with ESAT6-CFP10, whereas GATA3 and FoxP3 mRNA increased (Fig. 2). IFN-␥, IL-17, IL-4, or
IL-10 recall responses to ESAT6-CFP10 were not significantly different between these two groups. When PPD was used as the antigen, PBL from BCG Pasteur vaccinees expressed sig-nificantly less IL-12p40 and sigsig-nificantly more GATA3 and FoxP3 (Fig. 2). IFN-␥, Tbet, IL-17, IL-4, and IL-10 mRNA expression levels were not significantly different between BCG Pasteur-vaccinated deer and controls when cells were stimu-lated with PPD (Fig. 2 and data not shown).
Gene expression was similar between BCG Danish- and BCG Pasteur-vaccinated animals as determined by measure-ment of Tbet, IFN-␥, IL-12p40, IL-17, IL-4, IL-10, and FoxP3 (Fig. 2 and data not shown). BCG Pasteur-vaccinated animals expressed significantly more GATA3 mRNA than either BCG Danish-vaccinated animals or the unvaccinated con-trols (Fig. 2D).
Across all time points and conditions IFN-␥gene expression correlated with Tbet expression (for PPD,R⫽0.86 andP⬍
0.0001; for ESAT6-CFP10,R⫽0.79 andP⬍0.0001). Neither vaccination nor infection induced significant 12p35 or IL-23p19 mRNA expression in stimulated PBL.
Correlation of cytokine gene expression with pathology.M.
bovisinfection of WTD produces variable pathology. To assess
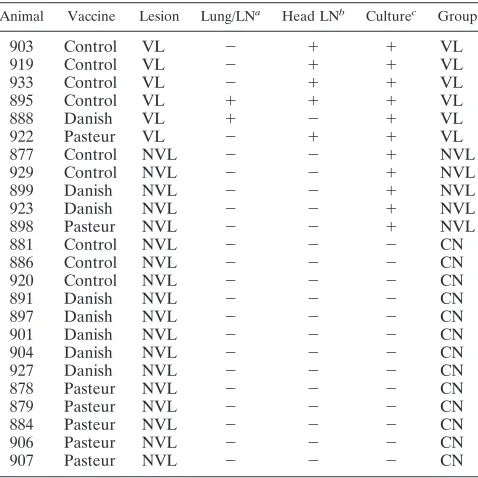
the association between gene expression and pathology, ani-mals were divided into three pathology groups, irrespective of vaccine, based on pathology and culture results (Table 1). Animals with visible lesions at necropsy and from which M.
boviswas cultured were included in the VL group (n⫽6).M.
bovis culture-positive animals with no gross lesions were
as-signed to the NVL group (n ⫽5). M. bovisculture-negative animals without visible lesions were assigned to the CN group (n⫽ 13). The low numbers of vaccinated animals in the VL and NVL pathology groups prevented the analysis of
vaccine-TABLE 1. Pathology and culture results
Animal Vaccine Lesion Lung/LNa Head LNb Culturec Group
903 Control VL ⫺ ⫹ ⫹ VL
919 Control VL ⫺ ⫹ ⫹ VL
933 Control VL ⫺ ⫹ ⫹ VL
895 Control VL ⫹ ⫹ ⫹ VL
888 Danish VL ⫹ ⫺ ⫹ VL
922 Pasteur VL ⫺ ⫹ ⫹ VL
877 Control NVL ⫺ ⫺ ⫹ NVL
929 Control NVL ⫺ ⫺ ⫹ NVL
899 Danish NVL ⫺ ⫺ ⫹ NVL
923 Danish NVL ⫺ ⫺ ⫹ NVL
898 Pasteur NVL ⫺ ⫺ ⫹ NVL
881 Control NVL ⫺ ⫺ ⫺ CN
886 Control NVL ⫺ ⫺ ⫺ CN
920 Control NVL ⫺ ⫺ ⫺ CN
891 Danish NVL ⫺ ⫺ ⫺ CN
897 Danish NVL ⫺ ⫺ ⫺ CN
901 Danish NVL ⫺ ⫺ ⫺ CN
904 Danish NVL ⫺ ⫺ ⫺ CN
927 Danish NVL ⫺ ⫺ ⫺ CN
878 Pasteur NVL ⫺ ⫺ ⫺ CN
879 Pasteur NVL ⫺ ⫺ ⫺ CN
884 Pasteur NVL ⫺ ⫺ ⫺ CN
906 Pasteur NVL ⫺ ⫺ ⫺ CN
907 Pasteur NVL ⫺ ⫺ ⫺ CN
aVisible lesions in the lungs and/or associated lymph nodes. bVisible lesion in the lymph nodes of the head.
cIsolation ofM. bovisfrom one or more tissues.
1142 THACKER ET AL. CLIN. VACCINEIMMUNOL.
on August 17, 2020 by guest
http://cvi.asm.org/
specific effects on gene expression and pathology; therefore, the association of gene expression with pathology was per-formed irrespective of vaccination.
Sixteen weeks after challenge with virulent M. bovis, Tbet gene expression in response to PPD stimulation was approxi-mately four- and threefold greater in PBL from the VL group than in the CN and NVL groups, respectively, while the NVL group expressed 1.5-fold more than the CN group (Fig. 3A). Recall responses to ESAT6-CFP10 resulted in approximately fivefold greater Tbet expression in the VL group than in either the NVL or CN group.
IFN-␥gene expression was greatest in the VL group, with no significant difference between the NVL and CN groups (Fig. 3B). IFN-␥expression in PBL from the VL group was 86-fold greater than in the CN group and 26-fold greater than that in the NVL group when cells were stimulated with PPD.
ESAT6-CFP10 stimulation of PBL from the VL group resulted in IFN-␥mRNA expression at levels 193-fold greater than in the CN group and 110-fold greater than in the NVL group. IFN-␥ mRNA expression was not statistically different between the NVL and CN groups, regardless of antigenic stimulus. IFN-␥ mRNA expression correlated with pathology (for PPD,R ⫽
0.68989 andP⫽0.0008; for ESAT6-CFP10,R⫽0.79805 and
P⬍0.0001). Tbet expression correlated with IFN-␥(r⫽0.86 andP⬍0.0001) expression.
IL-17 expression in the VL group was approximately 4-and 25-fold greater than expression in the NVL 4-and CN groups, respectively, when cells were stimulated with ESAT6-CFP10 (Fig. 3C). There were no statistically signif-icant differences in IL-17 gene expression detected in the PPD-stimulated cells primarily due to one animal with high expression levels in the NVL group; however, IL-17
expres-FIG. 3. T-cell mRNA expression in different pathology groups following vaccination and infection. Animals were grouped by pathology. Tbet (A), IFN-␥(B), IL-17 (C), FoxP3 (D), and GATA3 (E) were measured in PBL stimulated with either PPD or ESAT6-CFP10 (EC) 16 weeks after infection withM. bovis. Data are presented as means⫾standard errors of the means relative to prevaccintion.
on August 17, 2020 by guest
http://cvi.asm.org/
sion correlated with pathology (for PPD,R ⫽0.57997 and
P ⫽ 0.0147; for ESAT6-CFP10, R ⫽ 0.81482 and P ⫽
0.0002). Expression of IL-23, a cytokine that is closely re-lated to IL-17, correre-lated with IL-17 expression (r ⫽ 0.56 andP⬍0.03; data not shown).
FoxP3, a transcription factor responsible for Treg differen-tiation and function, was not differentially regulated between the groups in this study (Fig. 3D). GATA3 expression inversely correlated with pathology (Fig. 3E) (R ⫽ ⫺0.67834; P ⫽
0.0020). When cells were stimulated with ESAT6-CFP10, the VL group expressed approximately 1.5-fold fewer GATA3 transcripts than the CN group, and the NVL group was inter-mediate. When cells were stimulated with PPD, the NVL and VL groups expressed similar levels of GATA3, yet each ex-pressed less than the CN group (Fig. 3D).
DISCUSSION
Differential immune responses as measured by mRNA ex-pression were elicited by vaccination with BCG Danish versus BCG Pasteur. BCG Danish vaccination induced stronger TH1 responses, as indicated by Tbet and IFN-␥expression. Unex-pectedly, BCG Pasteur vaccination elicited greater GATA3 expression than vaccination with BCG Danish. After infection, BCG Danish-vaccinated animals did not have lesions in head-or lung-associated lymph nodes; however, they did have min-imal lung lesions (24). In contrast, BCG Pasteur-vaccinated animals had lesions in head- and lung-associated lymph nodes but none in the lungs (24). Differential gene expression in peripheral blood may explain, in part, the observed difference in lesion location. BCG Danish induced a stronger IFN-␥ re-sponse (Fig. 1) in peripheral leukocytes that may be reflective of immune competence in the lymph nodes that prevents es-tablishment of infection at that site. BCG Pasteur may gener-ate a more tissue-oriented immune response since the IFN-␥ response was low in peripheral blood (Fig. 1) and since there were no lesions in the lungs.
TH1 immune responses after infection were generally greater in the unvaccinated group than in either vaccine group. The large variation observed, particularly with IFN-␥ expres-sion, obscured vaccine effects (Fig. 2B). This large variation may be explained by the failure of the vaccine in some animals to limit pathology. When gene expression is considered irre-spective of the vaccine group, the variation is considerably smaller (Table 1; Fig. 3B). The correlation of IFN-␥expression to pathology is consistent with previously published data from WTD (42) and cattle (41, 44). IL-17 expression was similar to that of the IFN-␥ (Fig. 3). These data are consistent with previous reports that vaccination of mice produces similar numbers of IFN-␥- and IL-17-producing cells in the lungs (12). In addition, it has been reported that IL-17 was not required for the primary response to vaccination; however, it was re-quired for the recall response when the mice were infected with virulentM. tuberculosis(12, 48). The contribution of IL-17 to pathology is not clear; however, the absence of IL-23 and IL-17 in mice results in increased lung inflammation (14) after TB infection. Here, we report that IL-17 expression correlates with pathology, suggesting that IL-17 may contribute to overall immunopathology or is indicative of uncontrolled infection (i.e., continuous antigenic stimulation).
GATA3 gene expression is associated with TH2 differenti-ation (49) and is the transcription factor that is believed to be the master regulator of TH2 cells (3). Regardless of the vac-cine group, GATA3 expression was least in the VL group and inversely correlated with pathology. These data suggest that, over the time frame examined in this study, increased TH2 responses are not indicative of increased pathology (42) and may correlate with bacterial control. InM. tuberculosis-infected humans, GATA3 expression was 3.8-fold greater in patients that had fast responses to anti-TB therapy than in those in the slow-response group (39). Among the vaccinees, PBL from the BCG Pasteur-vaccinated animals expressed more GATA3 af-ter infection (Fig. 2D). The relevance of induction of GATA3 by BCG Pasteur is not clear; however, these animals did not have lesions in the lung (24).
In the current study, there was no clear correlation between gene expression and protection; however, the correlation of IFN-␥ with pathology was confirmed, and GATA3 inverse correlation with pathology is established. Here, we report that immune responses in the peripheral blood did not identify a mechanism for the differences observed in efficacy, at least for the genes measured in this experiment. Measurement of im-mune responses at foci of infection may be required to deter-mine the immunological responses that correlate with the dif-ferences in vaccine efficacy.
REFERENCES
1.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai.1995. Recall of long-lived immunity toMycobacterium tuberculosisinfection in mice. J. Im-munol.154:3359–3372.
2.Bolin, C. A., D. L. Whipple, K. V. Khanna, J. M. Risdahl, P. K. Peterson, and T. W. Molitor.1997. Infection of swine withMycobacterium bovisas a model of human tuberculosis. J. Infect. Dis.176:1559–1566.
3.Bowen, H., A. Kelly, T. Lee, and P. Lavender.2008. Control of cytokine gene transcription in Th1 and Th2 cells. Clin. Exp. Allergy38:1422–1431. 4.Casanova, J. L., and L. Abel.2002. Genetic dissection of immunity to
my-cobacteria: the human model. Annu. Rev. Immunol.20:581–620. 5.Chen, X., B. Zhou, M. Li, Q. Deng, X. Wu, X. Le, C. Wu, N. Larmonier, W.
Zhang, H. Zhang, H. Wang, and E. Katsanis.2007. CD4⫹CD25⫹FoxP3⫹ regulatory T cells suppressMycobacterium tuberculosisimmunity in patients with active disease. Clin. Immunol.123:50–59.
6.Corner, L. A., M. A. Stevenson, D. M. Collins, and R. S. Morris.2003. The re-emergence ofMycobacterium bovisinfection in brushtail possums ( Tricho-surus vulpecula) after localised possum eradication. N. Z. Vet. J.51:73–80. 7.Donnelly, C. A., R. Woodroffe, D. R. Cox, F. J. Bourne, C. L. Cheeseman, R. S. Clifton-Hadley, G. Wei, G. Gettinby, P. Gilks, H. Jenkins, W. T. Johnston, A. M. Le Fevre, J. P. McInerney, and W. I. Morrison.2006. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature439:843–846.
8.Elias, D., H. Akuffo, and S. Britton.2005. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobac-terium tuberculosis. Trans. R. Soc. Trop. Med. Hyg.99:363–368.
9.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom.1993. An essential role for interferon␥in resistance to Mycobacte-rium tuberculosisinfection. J. Exp. Med.178:2249–2254.
10.Guyot-Revol, V., J. A. Innes, S. Hackforth, T. Hinks, and A. Lalvani.2006. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med.173:803–810.
11.Kehrli, M. E., Jr., B. J. Nonnecke, and J. A. Roth.1989. Alterations in bovine lymphocyte function during the periparturient period. Am. J. Vet. Res. 50:215–220.
12.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper.2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4⫹T cell responses after vacci-nation and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377.
13.Khader, S. A., and A. M. Cooper.2008. IL-23 and IL-17 in tuberculosis. Cytokine41:79–83.
14.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. deSauvage, and A. M. Cooper.2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17
1144 THACKER ET AL. CLIN. VACCINEIMMUNOL.
on August 17, 2020 by guest
http://cvi.asm.org/
response during tuberculosis but is dispensable for protection and antigen-specific IFN-␥responses if IL-12p70 is available. J. Immunol.175:788–795. 15.Komiyama, Y., S. Nakae, T. Matsuki, A. Nambu, H. Ishigame, S. Kakuta, K. Sudo, and Y. Iwakura.2006. IL-17 plays an important role in the develop-ment of experidevelop-mental autoimmune encephalomyelitis. J. Immunol.177:566– 573.
16.Li, L., S. H. Lao, and C. Y. Wu.2007. Increased frequency of CD4⫹CD25high
Treg cells inhibit BCG-specific induction of IFN-␥by CD4⫹T cells from TB patients. Tuberculosis87:526–534.
17.Littell, R. C., G. A. Milliken, W. W. Stroup, and R. D. Wolfinger.1996. SAS system for mixed models. SAS Institute Inc., Cary, NC.
18.Livak, K. J., and T. D. Schmittgen.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2⫺⌬⌬CT
method. Methods 25:402–408.
19.Majlessi, L., M. Simsova, Z. Jarvis, P. Brodin, M. J. Rojas, C. Bauche, C. Nouze, D. Ladant, S. T. Cole, P. Sebo, and C. Leclerc.2006. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immuni-zation does not enhance protection against tuberculosis. Infect. Immun. 74:2128–2137.
20.Nol, P., M. V. Palmer, W. R. Waters, F. E. Aldwell, B. M. Buddle, J. M. Triantis, L. M. Linke, G. E. Phillips, T. C. Thacker, J. C. Rhyan, M. R. Dunbar, and M. D. Salman.2008. Efficacy of oral and parenteral routes of
Mycobacterium bovisbacille calmette-guerin vaccination against experimen-tal bovine tuberculosis in white-tailed deer (Odocoileus virginianus): a feasi-bility study. J. Wildl. Dis.44:247–259.
21.O’Brien, D. J., S. M. Schmitt, J. S. Fierke, S. A. Hogle, S. R. Winterstein, T. M. Cooley, W. E. Moritz, K. L. Diegel, S. D. Fitzgerald, D. E. Berry, and J. B. Kaneene.2002. Epidemiology ofMycobacterium bovisin free-ranging white-tailed deer, Michigan, USA, 1995–2000. Prev. Vet. Med.54:47–63. 22.O’B rien, D. J., S. M. Schmitt, S. D. Fitzgerald, D. E. Berry, and G. J.
Hickling.2006. Managing the wildlife reservoir ofMycobacterium bovis: the Michigan, USA, experience. Vet. Microbiol.112:313–323.
23.Palmer, M. V., T. C. Thacker, and W. R. Waters. 2007. Vaccination of white-tailed deer (Odocoileus virginianus) withMycobacterium bovisbacillus Calmette Guerin. Vaccine25:6589–6597.
24.Palmer, M. V., T. C. Thacker, and W. R. Waters.2008. Vaccination with
Mycobacterium bovisBCG strains Danish and Pasteur in white-tailed deer (Odocoileus virginianus) experimentally challenged with Mycobacterium bovis. Zoonoses Public Health56:243–251.
25.Palmer, M. V., W. R. Waters, and D. L. Whipple.2004. Investigation of the transmission ofMycobacterium bovisfrom deer to cattle through indirect contact. Am. J. Vet. Res.65:1483.
26.Palmer, M. V., W. R. Waters, and D. L. Whipple.2002. Lesion development in white-tailed deer (Odocoileus virginianus) experimentally infected with
Mycobacterium bovis. Vet. Pathol.39:334–340.
27.Palmer, M. V., W. R. Waters, and D. L. Whipple.2002. Susceptibility of raccoons (Procyon lotor) to infection withMycobacterium bovis. J. Wildl. Dis. 38:266–274.
28.Palmer, M. V., D. L. Whipple, and S. C. Olsen.1999. Development of a model of natural infection withMycobacterium bovisin white-tailed deer. J. Wildl. Dis.35:450–457.
29.Payeur, J. B., S. Church, L. Mosher, B. Robinson-Dunn, S. Schmitt, and D. Whipple.2002. Bovine tuberculosis in Michigan wildlife. Ann. N. Y. Acad. Sci.969:259–261.
30.Pollock, J. M., and P. Andersen.1997. Predominant recognition of the ESAT-6 protein in the first phase of interferon withMycobacterium bovisin cattle. Infect. Immun.65:2587–2592.
31.Ritacco, V., B. Lopez, I. N. De Kantor, L. Barrera, F. Errico, and A. Nader. 1991. Reciprocal cellular and humoral immune responses in bovine tuber-culosis. Res. Vet. Sci.50:365–367.
32.Rook, G. A.2007. Th2 cytokines in susceptibility to tuberculosis. Curr. Mol. Med.7:327–337.
33.Rook, G. A., R. Hernandez-Pando, K. Dheda, and G. Teng Seah.2004. IL-4
in tuberculosis: implications for vaccine design. Trends Immunol.25:483– 488.
34.Roth, J. A., and M. L. Kaeberle.1981. Evaluation of bovine polymorphonu-clear leukocyte function. Vet. Immunol. Immunopathol.2:157–174. 35.Roy, E., J. Brennan, S. Jolles, and D. B. Lowrie.2008. Beneficial effect of
anti-interleukin-4 antibody when administered in a murine model of tuber-culosis infection. Tubertuber-culosis88:197–202.
36.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis.33:749.
37.Schmitt, S. M., D. J. O’Brien, C. S. Bruning-Fann, and S. D. Fitzgerald. 2002. Bovine tuberculosis in Michigan wildlife and livestock. Ann. N. Y. Acad. Sci.969:262–268.
38.Seah, G. T., G. M. Scott, and G. A. Rook.2000. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tubercu-losis. J. Infect. Dis.181:385–389.
39.Siawaya, J. F., N. B. Bapela, K. Ronacher, N. Beyers, P. van Helden, and G. Walzl. 2008. Differential expression of interleukin-4 (IL-4) and IL-4␦2 mRNA, but not transforming growth factor beta (TGF-), TGF-RII, Foxp3, gamma interferon, T-bet, or GATA-3 mRNA, in patients with fast and slow responses to antituberculosis treatment. Clin. Vaccine Immunol. 15:1165–1170.
40.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen.1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted byMycobacterium tuberculosis. Infect. Immun.63:1710–1717. 41.Thacker, T. C., M. V. Palmer, and W. R. Waters.2007. Associations between
cytokine gene expression and pathology inMycobacterium bovis infected cattle. Vet. Immunol. Immunopathol.119:204–213.
42.Thacker, T. C., M. V. Palmer, and W. R. Waters.2006. Correlation of cytokine gene expression with pathology in white-tailed deer (Odocoileus virginianus) infected withMycobacterium bovis. Clin. Vaccine Immunol.13: 640–647.
43.Untergasser, A., H. Nijveen, X. Rao, T. Bisseling, R. Geurts, and J. A. Leunissen.2007. Primer3Plus, an enhanced web interface to Primer3. Nu-cleic Acids Res.35:W71–W74.
44.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Sim-mons, and R. G. Hewinson.2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle followingMycobacterium bovis
BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026.
45.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson.2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol.8:571–578.
46.Waters, W. R., B. J. Nonnecke, M. V. Palmer, S. Robbe-Austermann, J. P. Bannantine, J. R. Stabel, D. L. Whipple, J. B. Payeur, D. M. Estes, J. E. Pitzer, and F. C. Minion.2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle byMycobacterium bovisand byM. aviumsubsp.aviumandM. aviumsubsp.paratuberculosis. Clin. Diagn. Lab. Immunol.11:729–735.
47.Welsh, M. D., R. T. Cunningham, D. M. Corbett, R. M. Girvin, J. McNair, R. A. Skuce, D. G. Bryson, and J. M. Pollock.2005. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology114:101.
48.Wozniak, T. M., A. A. Ryan, and W. J. Britton.2006. Interleukin-23 restores immunity toMycobacterium tuberculosisinfection in IL-12p40-deficient mice and is not required for the development of IL-17-secreting T cell responses. J. Immunol.177:8684–8692.
49.Zheng, W., and R. A. Flavell.1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89:587–596.