ABSTRACT
ENNIS, GILDA EDWARDS. Glucose Attenuation of the Associative Memory Deficit in Older Adults. (Under the direction of Drs. Shevaun D. Neupert and Thomas M. Hess.)
This study examined if glucose would attenuate the associative memory deficit in non-diabetic older adults and investigated whether memory enhancement would be dependent upon glucose regulation. Working memory was also assessed to examine the counter-hypothesis that glucose may enhance performance on any demanding memory test and that effects are not specific to hippocampal-mediated functions. Older and younger adults drank a glucose (50 g) sweetened beverage in one session and an artificially sweetened beverage in another session in a counterbalanced order. Contrary to expectations,
administration of 50 g glucose did not attenuate an associative deficit or improve associative memory in older adults. Glucose may not have ameliorated the associative memory deficit in this study because the memory task may not have been demanding enough and the dose of glucose given may have been incorrect. Processing speed was found to moderate the relationship between age and associative memory; older adults with slow processing speed had worse associative memory relative to older adults with faster processing speed. Absence of glucose memory enhancement may also have been partly due to the inadequacy of glucose to facilitate a global aging process. Glucose regulation did not moderate the effect of glucose upon memory in older adults. A glucose regulatory index (i.e., glucose measured 60 min following glucose ingestion – fasting glucose) was not related to associative memory; another measure of glucose regulation – fasting glucose – was negatively related to
Glucose Attenuation of the Associative Memory Deficit in Older Adults
by Gilda E. Ennis
A dissertation submitted to the Graduate Faculty of North Carolina State University
in partial fulfillment of the requirements for the Degree of
Doctor of Philosophy
Psychology
Raleigh, North Carolina
2012
APPROVED BY:
_________________________________ ________________________________
Daniel Grühn, Ph.D. Lynne Baker-Ward, Ph.D.
________________________________ _________________________________
Thomas M. Hess, Ph.D. Shevaun D. Neupert, Ph.D.
BIOGRAPHY
After receiving a B.S. degree in Medical Technology in 1985 at the University of North Carolina at Chapel Hill, I worked as a medical technologist in a variety of hospital laboratories. I served as a supervisor in the Clinical Chemistry Laboratory at Duke University Medical Center and in the Family Practice Center at The Ohio State University. For 14 years I was an instructor within the Medical Laboratory Technology program at Wake Technical Community College. Upon deciding to broaden my educational experience beyond medical technology, I enrolled in the Liberal Studies Program at North Carolina State University and obtained an M.A. degree in 2005. After completing that degree, I resigned from my
ACKNOWLEDGMENTS
I would first like to acknowledge and thank my advisor and doctoral committee co-chair, Dr. Shevaun Neupert, for providing the mentorship, guidance, and support to finish my doctoral degree. I am also grateful for my doctoral committee co-chair, Dr. Thomas Hess, who was always available to provide the advice and support needed to help me successfully initiate, plan, and complete my dissertation research.
I would also like to thank my committee members, Drs. Lynn Baker-Ward and Daniel Grühn, for their valued suggestions and feedback.
My dissertation research would not have been possible without the assistance of several other people. These include Carla Strickland, who helped with the initial stages of my project, Mary Luong, who designed a program for testing item and associative memory, Jim Yuill, Ph.D., who designed a program for checking word relatedness, Logan Collins, who scheduled participants and helped with data management, and Katie Bigelow, who also assisted with data management. I am thankful for the invaluable contributions made by each of these people.
TABLE OF CONTENTS
LIST OF TABLES……… LIST OF FIGURES………... INTRODUCTION………. LITERATURE REVIEW……….
Glucose Transport and Diffusion in the Bran………... Age-related Differences in Hippocampal Extracellular Fluid Glucose during Demanding Memory Tasks: Implications for Memory and the Role of Glucose Replacement in Older Adults……….. Glucose Memory Facilitation in Older Adults……….. The Role of Task Demand in Glucose Memory Facilitation……… Glucose Memory Facilitation in Young Adults……… The Role of Glucose Regulation in Glucose Memory Facilitation…………... HYPOTHESES………. METHOD………. Participants………... Treatment……….. Measures and Equipment……….. Item and associative recognition memory test………. Working memory……….. Glucose (mg/dL)………... Glucose regulatory index……….. Drink questionnaire……….. Cognitive ability tests………... Procedure……….. Item and associative recognition memory testing……… Working memory testing……….. RESULTS………. Preliminary Analyses……… Participant characteristics………. Manipulation check……….. Counterbalancing check………... Hypothesis 1: Glucose Will Attenuate the Age-Related Associative
Deficit……… Hypothesis 2: Glucose Regulation Will Moderate Glucose Attenuation of the Age-Related Associative Deficit……… Hypothesis 3: Relative to the Saccharin Condition, Glucose will
LIST OF TABLES
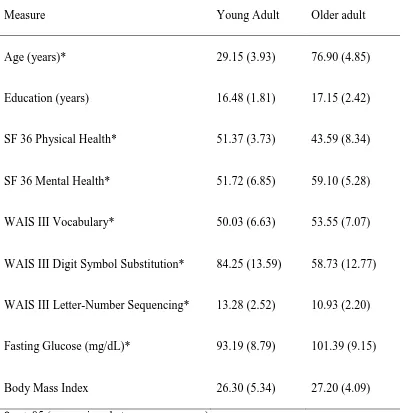
Table 1 Means and (Standard Deviations) of Participant Characteristics……. 57
Table 2 Means and (Standard Deviations) of Item and Associative Corrected Recognition Scores (Hits – False Alarms) in the
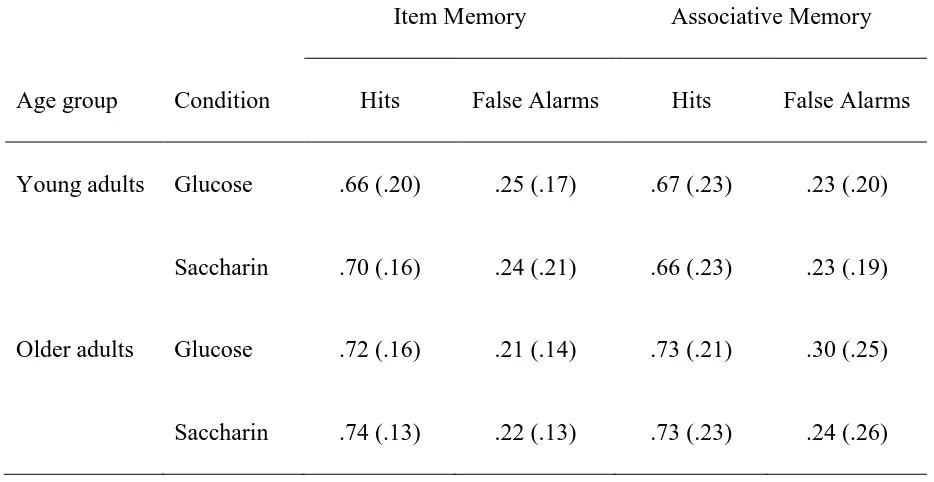
Saccharin Condition……….. 58 Table 3 Means and (Standard Deviations) of Proportion of Hits and False
Alarms in the Item and Associative Recognition Memory Test in
the Glucose and Saccharin Condition………... 59 Table 4 Means and (Standard Deviations) of Glucose Regulatory Index
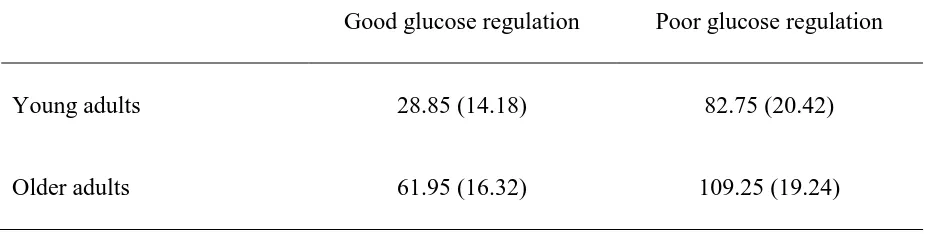
Values (mg/dL) in Glucose Regulation Groups……… 60
Table 5 Means and (Standard Deviations) of Operation Span Scores in
LIST OF FIGURES
Figure 1. Age group and condition differences in glucose values in young and older adults when fasting and at 30 min and 60 min following
administration of 50 g glucose... 62
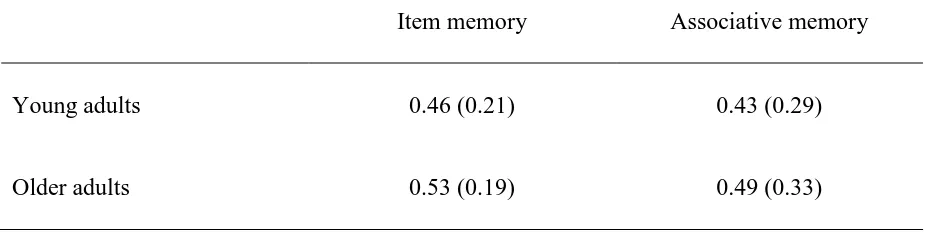
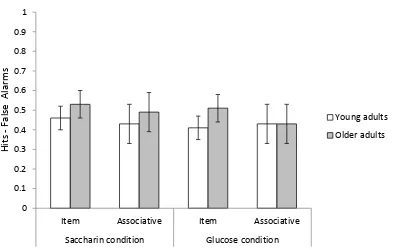
Figure 2. Age group and condition differences in item and associative
recognition memory... 63
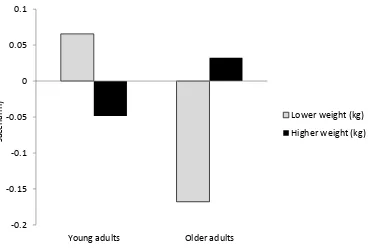
Figure 3. Predicted associative deficit difference scores (glucose – saccharin) graphed as a function of age group and weight (kg). Lower and higher weight represented less than and greater than one SD from
the mean, respectively... 64
Figure 4. Predicted associative memory difference scores (glucose – saccharin) graphed as a function of age group and weight (kg). Lower and higher weight represented less than and greater than one SD from
INTRODUCTION
Reflective of the multidirectionality characteristic of age-related changes in cognition (Baltes, 1987), older adults have been found to perform somewhat similarly to young adults on item recognition, but worse than young adults on associative recognition tests. When asked to remember word-pairs (e.g., pencil-chamber or stable-basket), older adults tend to recognize the individual words within the pair at a level similar to that of young adults, but are worse than young adults at distinguishing previously studied pairs from novel pairs (e.g., pencil-basket) formed from previously seen items (Chalfonte & Johnson, 1996; Naveh-Benjamin, 2000). Older adults are thought to be less able than young adults to bind together disparate pieces of information into a cohesive unit (Chalfonte & Johnson, 1996; Shing, Werkle-Bergner, Li, & Lindenberger, 2008). This cognitive inefficiency has been described as an associative memory deficit and is thought to partially explain overall age differences in episodic memory (Old & Naveh-Benjamin, 2008b), a concept central to Naveh-Benjamin’s (2000) associative deficit hypothesis.
orally administered glucose following an overnight fast (Parent et al., 2011). While the provision of glucose (either 25 or 50 g) has been found to improve hippocampal-mediated memory (e.g., paragraph recall), relative to performance while fasting, in both older and young adults (for review, see Smith, Riby, van Eekelen, & Foster, 2011), glucose
supplementation may have greater benefits for older adults since they experience greater depletion of hippocampal glucose relative to young adults during effortful memory testing (McNay & Gold, 2001). Glucose memory facilitation is thought to occur primarily when hippocampal-mediated tasks are demanding (McNay, Fries & Gold, 2000) and require additional energy to maintain neural excitability (McNay & Gold, 2002). Whether glucose specifically attenuates the associative memory deficit in older adults has not been previously tested; thus, one aim of my dissertation was to examine if glucose would reduce the
associative memory deficit in non-diabetic older adults.
Another aim was to investigate if glucose regulation would moderate glucose
episodic memory in older adults (Bruehl, Sweat, Hassenstab, Polyakov, & Convit, 2009) and has been associated with hippocampal volume reduction (Convit, Wolf, Tarshish, & de Leon, 2003). Modest increases in peripheral glucose might overcome the inefficient transfer of glucose across the blood-brain barrier and maintain the levels of hippocampal glucose needed for memory function in non-diabetic older adults with somewhat abnormal glucose
regulation (Lamport et al., 2009).
LITERATURE REVIEW Glucose Transport and Diffusion in the Brain
Glucose from the peripheral circulation is essential for meeting the brain’s metabolic energy requirements. It is almost the sole substrate for brain energy metabolism (Duelli & Kuschinsky, 2001). Glucose is able to cross through the blood-brain barrier (i.e., the tightly joined endothelial cells of the brain’s blood vessels) through a glucose transporter known as GLUT1 found within the endothelium of the barrier. GLUT1 is not dependent upon insulin for glucose uptake, unlike GLUT4 found within the myocardium, fat, and skeletal muscle (Duelli & Kuschinsky, 2001). GLUT1 is asymmetrically distributed within the endothelium, with a lower proportion found on endothelial membrane in contact with blood (i.e., the luminal side) compared to membrane facing the brain (i.e., the abluminal side). This unequal distribution facilitates the diffusion of glucose from the blood into the brain (for review, see Messier, 2004).
Cipp, & Haspel, 1991; Vorbrodt, Dobrogowska, Meeker, & Carp, 1999). Although the mechanisms are not known, abnormal glucose regulation may also contribute to decreases in glucose transport, perhaps due to lack of dilation of the brain’s blood vessels (Convit, 2005). Dysfunctional endothelial dilation in peripheral vessels is associated with diabetes, as well as the insulin resistance that precedes Type II diabetes (Laight, Carrier, & Änggård, 2000). Some hypothesize that dysfunctional vasodilation may also occur in the brain (Convit, 2005). Dilation of the brain’s blood vessels ensures that GLUT1 is exposed to blood enabling the transfer of glucose to the brain. If dilation is inadequate, insufficient numbers of GLUT1 will come into contact with blood and the transport of glucose across the blood-brain barrier will be impaired (Convit, 2005; Lamport et al., 2009).
memory impairment could result (Convit, 2005; Lamport et al., 2009; McNay & Gold, 2001; Messier, 2004).
Age-Related Differences in Hippocampal Extracellular Fluid Glucose during Demanding
Memory Tasks: Implications for Memory and the Role of Glucose Replacement in Older
Adults
Hippocampal extracellular fluid (ECF) glucose may decline during demanding memory tasks due to increased neural activity (McNay & Gold, 2002; McNay et al., 2000). Such localized deficits of glucose in the brain were traditionally considered not possible (McNay & Gold, 2002). McNay et al. (2000) challenged this assumption by finding that ECF glucose varied locally within the rat hippocampus, and not in other brain areas, depending upon memory demand and age. Harder memory tasks were related to greater decreases in hippocampal ECF glucose than less demanding ones (McNay et al., 2000), and decreases in glucose during demanding tasks were disproportionately greater in old compared to young adult rats (McNay & Gold, 2001).
Specifically, ECF glucose in old adult rats declined by up to 43% from baseline during complex maze testing and did not recover to basal levels during or after the
The greater depletion of hippocampal ECF glucose in older relative to younger adult animals was probably not caused by greater neural utilization of glucose since glucose metabolism in the brain and hippocampus generally declines with age (Gage, Kelly, & Bjornklund, 1984). It is more likely that increased depletion was due to inefficient glucose replacement (Messier, 2004). As described in the previous section, potential age-related impairments in the GLUT1 transfer of glucose across the blood-brain barrier and slowed diffusion of glucose through extracellular space may result in delayed replenishment of hippocampal ECF glucose consumed due to increased cognitive demand (McNay, 2005; Mooradian et al., 1991; Syková, 2001; Syková et al., 2002).
Old and young adult animals were injected in the periphery with glucose to determine if glucose would prevent hippocampal ECF glucose deficits during complex maze testing and influence performance (McNay & Gold, 2001). Following peripheral glucose administration, hippocampal ECF glucose remained at or above baseline levels in both age groups during memory testing. Glucose facilitated performance in young and old adults, who both
performed significantly better than same-aged untreated animals. Older adults benefited more from glucose than younger animals. Glucose eliminated age-related performance differences observed in the untreated animals so that there was no significant difference in the maze scores of glucose-treated old and young adult rats (McNay & Gold, 2001).
associative deficit in older adults by supplying additional energy to maintain cognitive operations.
Glucose may also enhance memory by increasing the production of neurotransmitters. The catabolism (or metabolic breakdown) of glucose following its administration may
provide precursor substances for the manufacture of neurotransmitters, such as glutamate and acetylcholine, thought to play a role in learning and memory (Korol & Gold, 1998). Through a process involving acetylcholine release, glucose may increase the functional connectivity between the hippocampus and other brain regions (Parent et al., 2011). Glucose has been shown to enhance functional connectivity between the hippocampus and other brain regions associated with successful episodic encoding in young adults (Parent et al., 2011). Age-related changes in the functional connectivity between the hippocampus and PFC are thought to provide at least partial explanation for the associative deficit observed in older adults (Mitchell & Johnson, 2009; Mitchell, Johnson, Raye, & D’Esposito, 2000). Whether glucose administration would improve functional connectivity and the associative deficit in older adults has not been investigated.
Glucose Memory Facilitation in Older Adults
order. Generally, cognitive testing began approximately 15 minutes after the consumption of glucose or artificial sweetener. In the majority of cases, modest increases in glucose
improved episodic memory in non-diabetic old adults compared to performance in a fasting state (Craft et al., 1994; Hall et al., 1989; Manning et al., 1990, 1997; Parsons & Gold, 1992; Riby et al., 2004, 2006). In those studies examining glucose’s effect upon cognitive domains other than episodic memory, glucose did not facilitate implicit (Manning et al., 1997; Craft et al., 1994), semantic (Riby et al., 2004, 2006), or working (Craft et al., 1994; Hall et al., 1989; Manning et al., 1990; Riby et al., 2004) memory. Results have been mixed in studies
examining the effects of glucose upon attention and executive function (Allen, Gross, Aloia, & Billingsley, 1996; Craft et al., 1994; Gagnon, Greenwood, & Bherer, 2010; Kaplan et al., 2000; Messier et al., 1997). Glucose enhanced attention in two studies (Gagnon et al., 2010; Messier et al., 1997), and had no effect in two others (Kaplan, 2000; Manning et al., 1990). In regards to glucose’s effect upon executive function, glucose improved verbal and design fluency in one study (Allen et al., 1996) and had no effect upon verbal fluency or cognitive control in another (Craft et al., 1994). Overall, results suggest that the facilitative effect of glucose is more clearly isolated to episodic memory than other cognitive domains in older adults. Perhaps in older adults glucose is more likely to facilitate cognition supported by hippocampal function.
attenuates the associative deficit in older adults. To identify the age-related associative deficit, recognition memory of younger and older adults for items and unrelated item pairs must be compared (Naveh-Benjamin, 2000). The associative deficit in older adults is found when the recognition performance of older relative to younger adults is much worse for associations (e.g., word-pairs) than for individual items (e.g. words only) (Old & Naveh-Benjamin, 2008a). Paragraph and word list recall do not effectively test the ability to make unique associations. The content of a paragraph recall task may contain semantic associations that can lend support to associative memory. Word list recall relies strongly on the initiation of memory search, which is thought to have “little to do with associative disadvantage” (Naveh-Benjamin, 2000, p. 1171). Cued recall may more effectively test the ability to make unique associations than paragraph or word list recall, but this task alone does not allow for comparison of age-related differences in item and associative memory, which is necessary for the detection of the associative memory deficit in older adults.
The Role of Task Demand in Glucose Memory Facilitation
Associative recognition is thought to be more demanding than item recognition because it involves the encoding and recollection of disparate pieces of information (Old & Naveh-Benjamin, 2008a). To recognize associations, participants must recollect, or consciously remember enough detail to determine if two unrelated items were studied together. In item recognition, recollection may not be necessary for task success; a sense of knowing without remembering specific details, a process referred to as familiarity, may be sufficient (Old & Naveh-Benjamin, 2008a; Yonelinas, 2002). Recollection is thought to place higher demands on self-initiated processing than the more automatic process of familiarity (Craik, 1986; Yonelinas, 2002).
Glucose Memory Facilitation in Young Adults
The issue of cognitive demand is especially relevant to glucose memory enhancement in young adults. Relative to older adults, young adults have better functioning in the
cognitive mechanics (Baltes, Staudinger, & Lindenberger, 1999). They also experience less depletion of hippocampal ECF glucose than older adults during demanding cognitive tasks (McNay & Gold, 2001). To appropriately test for glucose facilitation of episodic memory, young adults require tests of sufficient difficulty that prevent ceiling effects and allow adequate room for glucose enhancement (Korol & Gold, 1998; Smith et al., 2011). The episodic memory of young adults was improved by glucose when the young adults’ cognitive resources were taxed, such as when they encoded material during a divided attention
condition (i.e., while performing sequences of hand movements) (e.g., Sünram-Lea, Foster, Durlach, & Perez, 2002). In most studies, glucose memory facilitation has not been observed when material was encoded without distraction (for review, see Smith et al., 2011). One exception comes from research by Riby et al. (2006), who observed glucose facilitation of cued recall in young adults when encoding occurred without attentional distraction. In regards to my dissertation, young adults were included primarily as a reference group to identify the associative deficit in older adults. No specific hypotheses were made regarding the influence of glucose’s performance on this group.
The Role of Glucose Regulation in Glucose Memory Facilitation
calculating a glucose recovery index. This is done by subtracting baseline or fasting glucose from the value obtained 60 minutes following glucose ingestion (e.g., Craft et al., 1994). Low values represent good regulation and high values indicate poor regulation. If values for older adults are significantly higher than those for young adults, a median split is performed within each age group to determine good and poor glucose regulation (or control) groups (see Craft et al.). In one study where a median split was performed within each age group to determine glucose regulatory status, glucose improved the episodic memory (i.e., paragraph recall) of older adults with relatively good glucose control, but had no influence on the memory of older adults with relatively poor control (Craft et al., 1994). In young adults, glucose
facilitated the memory of those with relatively poor glucose regulation, but had no influence on those with good regulation. Young adults characterized as having good glucose control had the lowest mean recovery index, while older adults with relatively poor control had the highest. The memory of the group (whether young or old) whose mean glucose recovery was intermediate to the group who had the best and worst regulation was positively influenced by glucose. Effects were isolated only to men; the memory of women, regardless of their
glucose regulatory status, was not influenced by glucose in this study.
perhaps adds some support to the findings of sex differences by Craft et al. (1994); however, Messier et al. (2003, 2010) did not specifically test for sex differences. Methods for
determining glucose regulatory status were also dissimilar. Messier et al. calculated this index using glucose data obtained on a day prior to cognitive testing. Fasting glucose values were subtracted from levels measured at 60 minutes following the ingestion of 75 g (not 50 g) of glucose.
In another study of middle-aged and older adults, 50 g of glucose improved the primacy effect on a paragraph recall task in men characterized as having good glucose regulation (Messier et al., 1997). Glucose regulatory status was determined by subtracting baseline glucose from final glucose at 75 minutes. There were no younger adults in this study, so it is not known whether the men with “good” regulation had the same or worse glucose regulation as younger adults. There was a non-significant tendency for glucose improvement of total paragraph recall score in all participants, except for men with poor glucose regulation. The absence of significant effects in this case may have been due to insufficient power due to small sample size.
insulin secretion were more sensitive to the memory enhancing effects of glucose. Regression analyses performed separately on men and women yielded similar results,
suggesting the absence of sex group differences in contrast to the Craft et al. (1994) findings. Because there is evidence of sex differences in glucose regulation(Armoni, Rafaeloff,
Barzilai, Eitan, & Karnieli, 1987; Hale, Wright, & Nattrass, 1985), the current study explored whether sex would be a significant factor when analyzing glucose regulatory control as a moderator of the glucose memory facilitation effect.
inconsistent pattern of results, glucose memory facilitation appears to be dependent upon glucose regulation in older adults. The direction of this moderating effect was explored in the current study.
Advancing age is associated with a progressive decline in glucose regulation partly due to age-related decreases in insulin secretion (Lamberts, 2008). Increased insulin resistance also contributes to poor glucose control in older adulthood, but is not a consequence of aging per se, but rather to physical inactivity, poor diet, and increased
abdominal fat mass (Szoke et al., 2008). Approximately 40% of the population over 60 years of age has either Type II diabetes or impaired glucose tolerance (Szoke et al., 2008). If abnormalities in glucose regulation predispose individuals to memory facilitation by glucose, then it would seem more probable that non-diabetic older rather than young adults would receive this benefit. The episodic memory of diabetic adults is not enhanced from acute increases in glucose; transitory dysfunction is more likely (Greenwood & Winocour, 2005). The mechanisms responsible for glucose facilitation of memory in non-diabetic adults with abnormal glucose regulation are currently not known (Lamport et al., 2009). Some have proposed that impaired glucose regulation due to increased insulin resistance is associated with endothelial dysfunction, specifically lack of endothelial dilation, in the brain’s blood vessels (Convit, 2005). Under normal circumstances endothelial dilation ensures that GLUT1 is exposed to blood. Inadequate endothelial dilation is thought to prevent blood from coming into contact with enough GLUT1 to enable the transport of sufficient glucose into
consequently experience greater depletion of hippocampal ECF glucose during cognitive demand than those with normal regulation. Increasing glucose above normal levels, as would be achieved through oral administration of glucose, might facilitate the transport of enough glucose through available GLUT1 to prevent ECF glucose depletion and perhaps improve memory (Lamport et al, 2009).
Previous studies of the glucose memory facilitation effect have not focused on whether glucose would specifically ameliorate the associative deficit in older adults, so it is not clear whether glucose enhancement of episodic memory in older adults is due to
improvement of the associative deficit or another process supporting episodic memory. It is also not known if glucose attenuation of the associative deficit would be moderated by glucose regulatory control. Considering that non-diabetic adults with abnormal glucose regulation are more likely to receive a memory benefit from glucose than those with normal control (Lamport et al., 2009), it would be important to address this issue. The purpose of this study was to investigate whether glucose would attenuate the age-related associative deficit in non-diabetic older adults and to explore whether this effect would be dependent upon glucose regulation. Working memory was also assessed to investigate the alternative
hypothesis that glucose would enhance performance on any demanding memory test and that effects would not be specific to hippocampal-mediated functions.
HYPOTHESES
1. Glucose will attenuate the age-related associative deficit observed in the saccharin condition by improving associative recognition memory in older adults. Both older and young adults will have similar associative recognition memory in the glucose condition.
2. Glucose regulation will moderate glucose attenuation of the age-related associative deficit. The direction of this effect (whether glucose facilitation of memory would depend upon relatively good or poor regulation) was explored. Whether the moderating effect of glucose regulation would depend upon sex was also explored.
3. Counter-hypothesis: Relative to the saccharin condition, glucose will differentially improve the working memory performance of older adults, reflecting a general rather than a specific enhancement effect.
METHOD Participants
had systolic blood pressure indicative of hypertension (i.e., > 160 mm Hg; Chobanian et al., 2003) was also excluded. Three older adults and one younger adult were excluded because of technical difficulties (e.g., incorrect usage of computer keyboard) during testing. The final sample consisted of 40 young (n =20 female; range 21 – 36 years) and 40 older (n = 20 female; range 69 – 89 years) adults. Participant characteristics are displayed in Table 1. Treatment
The glucose drink contained 50 g glucose (Now® Foods, Bloomingdale, IL) dissolved into 300 mL of water flavored with 10 mL of ReaLemon lemon juice (Dr Pepper Snapple Group, Inc., Plano, TX). A glucose dose of 50 g was selected since this dose has been shown more often to improve memory in older adults (Messier, 2004). The artificially sweetened beverage contained 5 mL of water soluble saccharin (Smoky Mountain Sweetener®, Knoxville, TN) dissolved into 300 mL of water flavored with 10 mL of ReaLemon lemon juice. Four drops of saccharin were added to the glucose drink to attempt to equate the flavor of the two drinks. Formulations similar to the glucose and saccharin drinks described here have been used in previous research and these two drinks have been found to taste similarly (Gagnon, Greenwood, & Bherer, 2010).
Measures and Equipment
imagery rating and 700 the highest (M = 570.23, SD = 36.9, range = 498 - 670). Words also had relatively low written frequency of occurrence as given in the norms of Kučera and Francis (1967) where maximum frequency is 69,971 and the minimum is 0 (M = 47.52, SD =
55.34,range = 10 – 382). There were no significant differences between the two lists in word concreteness, t(214) = .88, p = .38, and frequency ratings, t(214) = -.30, p = .76 (List 1: Mconcreteness = 572.44, Mfrequency = 45.38; List 2: Mconcreteness = 568.02, Mfrequency = 47.66).
Words within each list were not semantically related according to the Free Association Norms database (Nelson, McEvoy, & Schreiber, 1998). Eighty-eight words from each list were used to form 44 word-pairs (see Appendix A for examples of word-pairs used). Twenty words from each list were selected as distractors to be used in item recognition testing. This ensured that distractor words had the same characteristics as target words. The formation of word-pairs and their order of presentation were randomized for each participant. Testing for word and word-pair recognition followed the presentation of word-pairs.
An additional list of 12 words with similar characteristics as those in the main lists was used for practice testing. Ten words formed five practice word-pairs, and the remaining two words were used as item distractors.
Glucose (mg/dL). Glucose was measured using the Accu-Chek® Aviva monitor (Roche Diagnostics, Indianapolis, IN). This unit was designed for the self-monitoring of glucose by patients with diabetes, and has demonstrated validity for this application (Savoca, Jaworek, & Huber, 2006) and good reliability (Lyon et al., 2009). Self-monitoring glucose systems have been used in studies of the glucose memory facilitation effect (Hall et al., 1989; Manning et al., 1997), and the Aviva monitor has been used in previous research (Napoli et al., 2011).
Glucose regulatory index. An index was calculated by subtracting baseline glucose from values obtained at 60 min following glucose ingestion to determine how well glucose was metabolized. Low values represented good regulation while high values indicated worse glucose regulation. To identify good and poor glucose control groups, a median split was performed within each age group since glucose regulatory index values differed significantly by age (see results section; Craft et al., 1994; Messier et al., 1997).
Drink questionnaire. Participants were asked to select one of the following to indicate how they thought the beverage was sweetened: 1 – artificial sweetener, 2 – sugar, 3 – other.
Cognitive ability tests. The vocabulary subtest from the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997) was used to assess verbal ability. The WAIS-III digit-symbol-substitution and letter-number-sequencing subtests were used to assess perceptual speed and working memory, respectively.
Procedure
glucose session and the drink questionnaire was given at the end of the second session. Participants were de-briefed at the end of their second session. Participants were given $20 for attending the first session and $30 for attending the second.
Item and associative recognition memory testing. Two equivalent forms of the recognition memory test were counterbalanced across age groups and glucose and saccharin conditions. Order of presentation of test phase (i.e., item vs. associative) was also
counterbalanced in the same manner. The procedure for administering recognition memory testing was based on previous research (Naveh-Benjamin, 2000). Participants were instructed to study word-pairs presented individually on a computer screen in preparation for two subsequent exercises where words and word-pairs previously seen would need to be distinguished from non-target stimuli. Participants were told to press the “Y” key if they recognized a word or word-pair and the “N” key if they did not. A practice test was given to ensure that participants understood test instructions. Based on pilot testing of five younger and eight older adults, the presentation rate was set at 4 s for older and 2 s for younger adults. Slower presentation rates of word-pairs in item and associative recognition memory testing has been used in previous research (e.g., Naveh-Benjamin, Brav, & Levy, 2007). Following study of the 44 word-pairs, participants counted forward from zero by twos for 90s. In order to control for primacy and recency effects, words from the first and last two word-pairs were not included in testing. Item and associative recognition testing was self-paced. In the
associative testing - from distractor words. Memory accuracy was determined by subtracting the proportion of false alarms from the proportion of hits.
Working memory testing. Two versions of the operation span were counterbalanced across age groups and glucose and saccharin conditions. Equation word-strings were presented on a computer screen. The set size of the equation word-strings varied randomly from two to five. A working memory score was calculated by adding the number of words recalled from perfectly recalled trials.
RESULTS Preliminary Analyses
Participant characteristics. One-way multivariate analyses of variance (MANOVA) were conducted to determine if there were significant age group differences in cognitive ability (i.e., education, vocabulary, processing speed, and working memory) and health (i.e., physical and mental health, fasting glucose, and body mass index). Since fasting glucose was strongly correlated across both test sessions, r(78) = 0.89, p < .001, the fasting glucose results from both testing times were averaged before inclusion as a health measure.
Significant age group differences were found in cognitive ability, F(4,75) = 24.94 , p < .001, ηp2 = .57 (see Table 1). Follow-up univariate analyses of variance (ANOVA) revealed that
older adults scored lower than young adults on measures of processing speed, F(1,78) = 74.90, p < .001, ηp2 = .49, and working memory, F(1,78) = 19.73, p < .001, ηp2 = .20,and
higher than young adults on vocabulary, F(1,78) = 5.29, p = .02, ηp2 = .06. These differences
(Bialystok & Craik, 2006). There were no age group differences in education, F(1,78) = 1.99, p = .16, ηp2 = .03.
Significant age group differences were also found in measures of health, F(4,75) = 16.84, p < .001, ηp2 = .47 (see Table 1). Follow-up ANOVAs found that older adults reported
worse physical health, F(1,78) = 29.78, p < .001, ηp2 = .28, and better mental health, F(1,78)
= 29.84, p < .001, ηp2 = .28, than younger adults. Older adults had higher fasting glucose than
young adults, F(1,78) = 16.71, p < .001, ηp2 = .18, and there were no significant age group
differences in body mass index (BMI), F(1,78) = 0.72, p = .40, ηp2 = .009. Variations in BMI
were of interest since the effect of glucose upon memory has been found to vary depending upon glucose dose (i.e., glucose mg/ kg of body weight) (Messier, Pierre, Desrochers, & Gravel, 1998). A Chi-Square test was then performed to determine whether more older relative to younger adults had a fasting glucose ≥ 100 mg/dL, which is the cut-off for determining the prediabetic condition known as impaired fasting glucose (Rao, Disraeli, & McGregor, 2004). Significantly more older (N = 23) than young (N = 8) adults had a fasting glucose ≥ 100 mg/dL, χ2
(1) = 11.85, p < .001. This finding is consistent with the greater prevalence of impaired fasting glucose in older relative to young adults in the adult population (Cowie et al., 2009).
saccharin condition in both age groups, F(2,78) = 327.51, p < .001, ηp2 = .81. There was also
a significant Age X Time X Condition interaction, F(2,78) = 13.86, p < .001, ηp2 = .15 (see
Figure 1). Older adults had significantly higher glucose levels than young adults at each measurement occasion across the saccharin condition and higher fasting and 60 min values in the glucose condition. There were no significant age differences in the 30 min measure in the glucose condition. The higher value at 60 min in the glucose condition found in the older adults is consistent with typical age-related declines in glucose tolerance that have been previously described in the literature (Cowie et al., 2009; Lamberts, 2008; Szoke et al., 2008).
To examine whether expectancy effects regarding the influence of the glucose drink on memory were minimized (see Green et al., 2001), I determined the percentage of
participants who were able to guess correctly that the glucose beverage was sweetened with sugar. Fifty-six percent of participants in the first session and 54% in the second session thought the glucose beverage was sweetened with sugar. There were no age group
differences in the ability to guess the content of the glucose drink (first session: χ2(1) = 3.08, p = .08; second session: χ2(1) = 0.63, p = 0.43). Since the percentage of participants
accurately guessing the nature of the glucose beverage was essentially at chance level, expectancy effects were most likely minimized.
age and condition upon item and associative memory. Item and associative corrected
recognition scores (hits – false alarms) were tested as the dependent variable in each analysis. It should be noted that testing the effect of treatment order also tests for practice effects. For example, comparisons of performance when glucose (or saccharin) was administered first versus second incorporate the impact of practice. Thus, significant practice effects would be reflected in interactions involving treatment order, condition (glucose vs. saccharin), and test (item vs. associative). The interaction of treatment order, condition, and test was not
significant, F(1,76) = 1.53, p = .22, ηp2 = .02, and the interaction of age, treatment order,
condition, and test was also not significant, F(1,76) = .14, p = .71, ηp2 = .002. This indicated
that the order of glucose treatment did not influence the relationship between age and condition on item and associative memory and that the memory performance of young and older adults did not significantly vary between session one and two. The interaction of test form (form A tested first vs. second), condition, and test was not significant, F(1,76) = .20, p = .66, ηp2 = .003, and the interaction of age, test form, condition, and test was also not
significant, F(1,76) = .17, p = .68, ηp2 = .002. Finally, the interaction of test phase (item
memory tested first vs. second), condition, and test was not significant, F(1,76) = .27, p = .61, ηp2 = .004, and the interaction of age, test phase, condition, and test was also not
significant, F(1,76) = .31, p = .58, ηp2 = .004. Thus, order of treatment, test form, and test
phase did not affect the relationship between age and condition upon item and associative memory, and significant practice effects were not found.
test. The interaction of treatment order and condition was significant, F(1,76) = 7.89, p = .006, ηp2 = .10. Participants who received glucose first had better working memory during
their second testing session (i.e. during the saccharin condition) (Mfirst session = 13.74, SD =
6.20; Msecond session = 16.36, SD = 7.56). This same pattern was not found for individuals who
received saccharin first; there were no significant differences in working memory scores between the first and second session in those participants who received saccharin first (Mfirst
session = 14.22, SD = 8.92; Msecond session = 15.22, SD = 7.33). This indicated that only
participants who received glucose first experienced practice effects. The interaction of treatment order and condition was not moderated by age, however, F(1,76) = 1.04, p = .31, ηp2 = .01, indicating that the order of glucose treatment did not affect the relationship
between age and condition upon working memory and that the memory performance of the young and older adult group did not significantly vary from session one to session two. The interaction of test form and condition was not significant, F(1,76) = .03, p = .87, ηp2 < .001,
and the interaction of age, test form, and condition was also not significant, F(1,76) = 1.96, p = .17, ηp2 = .03, thus test form did not affect the relationship between age and condition upon
working memory. Taken together, these analyses demonstrate that the counterbalancing procedures did not influence the relationship between age, condition, and working memory. Hypothesis 1: Glucose Will Attenuate the Age-Related Associative Deficit
memory compared to young adults? Results indicated that the Age X Test interaction was not significant, F(1,78) = .003, p = .96, ηp2 < .001. Older adults’ corrected recognition scores for
item and associative memory were similar to those of young adults (see Table 2 for corrected recognition scores and Table 3 for proportion of hits and false alarms in both young and older adults). This finding was unexpected considering the existing literature reporting the
presence of an associative deficit in older adults (Old & Naveh-Benjamin, 2008a). Since the rate of presentation of word-pairs was slightly slower for older than young adults (4 s vs. 2 s), older adults might have been able to compensate for any existing deficits in associative memory depending upon their processing speed. Older adults with relatively good processing speed may have been at an advantage compared to older adults with poor processing speed. To examine this assumption, I explored whether processing speed had a greater influence on the performance of older adults relative to young adults on the item and associative
recognition test. A processing speed group (slow vs. fast) was determined by performing a median split within each age group on digit symbol test scores. The slow and fast processing speed means within each age group were as follows: a) older adults: Mslow = 48.21, Mfast =
68.24; and b) young adults: Mslow = 73.90, Mfast = 94.60. An Age (young vs. old) X
Processing Speed (slow vs. fast) X Test (item vs. associative) repeated measures ANOVA performed on corrected recognition scores from the saccharin condition revealed that the interaction of age and processing speed was significant, F(1,76) = 4.94, p = .03, ηp2 = .06.
Further examination within age groups revealed that the effect of processing speed was close to significance in older adults, F(1,38) = 4.07, p = .05, ηp2 = .10, and not significant in young
speed group, older adults in the slow group had lower associative memory scores (Slow speed: M =.36, SD = .45; Fast speed: M = .60, SD = .42), but similar item memory scores (Slow speed: M = .50, SD = .29; Fast speed: M = .55, SD = .27). Perhaps older adults with faster processing speed would have demonstrated worse associative memory if the word-pairs had been presented at 2 s instead of 4 s.
An Age (young vs. old) X Condition (glucose vs. saccharin) X Test (item vs. associative) mixed ANOVA performed on corrected recognition scores indicated that the interaction of age, condition, and test was not significant, F(1,78) = 1.82, p = .18, ηp2 = .02.
Contrary to expectations, administration of glucose did not improve associative memory in older adults relative to their performance in the saccharin condition (see Figure 2).
Considering that older adults with slow processing speed relative to older adults with faster processing speed had worse associative memory in the saccharin condition, I explored whether glucose improved associative memory in these older adults. A mixed ANOVA performed on the corrected recognition scores of older adults revealed that a Processing Speed X Condition X Test interaction was not significant, F(1,38) = 0.92, p = .35, ηp2 = .02;
thus glucose did not improve associative memory in older adults with slow (or fast) processing speed.
subtracting associative scores from item memory scores. Next, an associative deficit difference score was calculated by subtracting the associative deficit score in the saccharin condition from the deficit score in the glucose condition. Values below zero indicated the degree of reduction in the associative deficit from the saccharin to the glucose condition. There were no significant age group differences in body weight, F(1,78) = .08, p = .78, ηp2 =
.001: a) older adults: M = 79.44 kg, SD = 17.04; and b) young adults: M = 78.35 kg, SD = 17.92. One older adult whose weight was slightly greater than three standard deviations above the mean was excluded from subsequent analyses involving weight. Body weight was centered and entered into a regression analysis along with age group and an Age Group X Body Weight interaction term. The interaction of Age Group X Body Weight was marginally related to the associative deficit difference score, B = -.009, t(75) = - 1.86, p = .07. Age group, B = .10, t(75) = 1.46, p = .15, and body weight, B = .002, t(75) = .58, p = .57, were not significantly associated with this score, R2total model = .08, p = .11. To explore the Age Group
associative deficit from the saccharin to the glucose condition could be due to either an increase in item memory or a decrease in associative memory across conditions, I next examined whether relationships were isolated to item or associative memory. An associative memory difference score was calculated by subtracting associative memory in the saccharin condition from associative memory in the glucose condition. An item memory difference score was calculated in the same manner. Values above zero indicated improvement in memory from the saccharin to the glucose condition. Body weight did not moderate the relationship between age group and the item memory difference score, B = .001, t(75) = .39, p = .71, and neither age, B = .03, t(75) = .64, p = .52, nor body weight, B = -.002, t(75) = -1.08, p = .29, were associated with this score, R2total model = .02, p = 0.64. In regards to the
associative memory difference score, age group, B = -0.08, t(75) = -1.25, p = .21, and body weight, B = -.004, t(75) = -1.48, p = .14, were not significant predictors, but the Age Group X Body Weight term was significant, B = .01, t(75) = 2.49, p = .02, R2total model= 0.09, p = .06.
to a decrease in associative memory across these same conditions. These results suggest that 50 g of glucose may have slightly suppressed the memory of lower weight older adults. Hypothesis 2: Glucose Regulation Will Moderate Glucose Attenuation of the Age-Related
Associative Deficit
A one-way ANOVA examining age group differences in glucose regulatory index values was significant, F(1,78) = 18.40, p < .001, ηp2 = .19, indicating that older adults had
worse glucose regulation than young adults: a ) older adults: M = 85.60 mg/dL, SD = 29.73; and b ) young adults: M = 55.80 mg/dL, SD = 32.35. A median split was then performed within each age group to determine glucose regulation groups (see Table 4 for descriptive statistics of older and young adults with relatively good and poor glucose regulation). To examine if glucose regulation moderated the relationship between glucose and memory in older adults, an Age (young vs. old) X Glucose Regulation (good vs. poor) X Condition (glucose vs. saccharin) X Test (item vs. associative) mixed ANOVA was performed on corrected recognition scores. The Age X Glucose Regulation X Condition X Test interaction was not significant, F(1,76) = 1.54, p = .22, ηp2 = .02. Glucose regulation did not have a
moderating influence on the change in corrected recognition scores from the saccharin to glucose condition in either older or young adults. I also explored whether sex and glucose regulation together would moderate glucose facilitation of memory in older adults in congruence with previous research (e.g., Craft et al., 1994). The interaction between age, glucose regulation, sex, condition, and test was not significant, F(1,72) = 0.04, p = .85, ηp2 <
Since glucose regulation did not act as a significant moderator in my study, I explored whether individual differences in glucose regulatory index values were related to an
Hypothesis 3: Relative to the Saccharin Condition, Glucose Will Differentially Improve the
Working Memory Performance of Older Adults.
A one-way AVOVA using operation span scores from the saccharin condition was analyzed to determine if older adults had worse working memory than young adults. As expected, there were significant age group differences in working memory, F(1,78) = 11.10, p = .001, ηp2 = .13, with older adults performing worse than young adults (see Table 4). Next,
an Age (young vs. old) X Condition (glucose vs. saccharin) repeated measures ANOVA was performed on working memory scores to test the counter-hypothesis that glucose will
enhance performance on any demanding memory test and that the effects are not specific to hippocampal-mediated functions. The Age X Condition interaction was not significant, F(1,78) = 0.43, p = .52, ηp2 = 005. Glucose did not improve the working memory of older (or
younger) adults observed in the saccharin condition (see Table 5).
Because individual differences in weight appeared to influence change in associative memory from the saccharin to the glucose condition in older adults, I examined whether similar effects would be found using a working memory difference score (glucose score minus saccharin score) as an outcome variable. Significant differences were not found for age group, B = 0.61, t(75) = .45, p = .65, weight, B = -.035, t(75) = -.65, p = .52, or an Age Group X Body Weight interaction, B = .014, t(75) = .16, p = .87, R2total model = .009, p = .87.
DISCUSSION
This study examined if glucose would ameliorate the associative memory deficit in non-diabetic older adults and investigated whether memory enhancement would be
dependent upon glucose regulation. Older and younger adults drank a glucose (50 g)
sweetened beverage in one session and an artificially sweetened beverage in another session in a counterbalanced order. Contrary to expectations, administration of 50 g glucose did not attenuate an associative deficit or improve associative memory in older adults. In fact, the older adult participants did not demonstrate an associative deficit, and performed similarly to younger adults on both the item and associative recognition tests. Essentially the older adults appeared not to require ‘treatment’ of associative memory.
2000). Consistent with previous research (e.g. Naveh-Benjamin et al., 2007), the presentation rate for word-pairs in my study was slightly slower for older relative to young adults (4 s vs. 2 s), but this may in fact have reduced the difficulty of the task. Having more time to study word-pairs may have allowed the older adult participants to form strategies to assist in encoding the words and word-pairs. Previous research has found that strategy use during encoding and retrieval can attenuate the associative memory deficit in older adults (Naveh-Benjamin, et al., 2007). Older adults who had slower processing speed had lower associative, but similar item memory scores compared to older adults with faster processing speed, a difference across processing speed levels that was not found in the young adult group. This does suggest that the task may have been too easy for those older adults with faster
processing speed, perhaps allowing them to compensate for any existing deficits in associative memory. Increasing the speed of word-pair presentation would have made the item and associative recognition test more demanding for these individuals. The associative recognition test did not seem too easy for older adults with slower processing speed. Despite the task being more demanding for these older adults, glucose did not facilitate their
amelioration of associative memory may depend at least partly upon the efficacy of glucose in treating a global aging process, as opposed to the specific localized effect originally proposed.
Another reason for lack of memory facilitation may have related to the dose of glucose given. Although 50 g of glucose has been shown to facilitate the episodic memory of older adults in some studies (Craft et al., 1994; Manning et al., 1997), others have found that 25 g is effective (Parsons & Gold, 1992; Riby et al., 2004). In a dose response study where 10 g, 25 g, and 50 g of glucose were administered to older adults, only the 25 g dose proved to be effective in improving episodic memory, whereas the 50 g dose resulted in a non-significant decrease in memory scores compared to performance in the saccharin condition (Parsons & Gold, 1992). The relationship between body weight and condition differences to memory in the present study tentatively suggests that the 50 g dose given may not have been optimal. Although results were not significant, older adults with lower body weight appeared to experience an increase in the associative deficit from the saccharin to the glucose
condition, while older adults with higher body weight seemed to experience a slight reduction in the associative deficit between these same conditions. The performance
received varied depending upon weight: adults who weighed 45 kg (i.e., - 2 SDs) received a high dosage (i.e., 1,111 mg/kg), while those who weighed 113 kg (i.e., + 2 SDs) received a relatively low dosage (i.e., 442 mg/kg). The effectiveness of a range of glucose dosages (i.e., 10, 100, 300, 500, 800, and 1000 mg/kg) upon word recall has been examined in young adult females (Messier et al., 1998). In that study, the 300 mg/kg dosage was more effective than water, saccharin, and the remaining dosages in enhancing the primacy effect. A dosage of 300 mg/kg equates to a dose of 24 g when utilizing the mean body weight of participants in my study (i.e., 79 kg). Thus, the dose of 50 g glucose given may have been too high.
Glucose regulation has been found to moderate glucose facilitation of episodic memory in past research (e.g., Craft et al., 1994; Kaplan et al., 2000), perhaps because the administration of additional glucose overcomes impediments in the transport of glucose across the blood brain barrier that may be present in those with somewhat abnormal glucose regulation (Convit, 2005; Lamport et al., 2009). Glucose regulation as measured by a glucose regulatory index (60 min glucose – fasting glucose) in my study did not moderate change in memory from the saccharin to the glucose condition. Older adults had worse glucose
such as the 2-hour oral glucose tolerance test2 used to diagnose the prediabetic condition known as impaired glucose tolerance (IGT) (Rao et al., 2004). Standardization of measures is needed so that any moderating effect of glucose regulation upon glucose memory facilitation can be compared across studies.
as a measure of glucose regulation in my study, fasting glucose did not moderate the
relationship between age, condition, and item and associative memory. While fasting glucose may contribute to associative memory impairment in older adulthood, it is possible that pathophysiologic mechanisms linking high fasting glucose to poor associative memory may not be influenced by glucose administration.
needed in future work to clarify whether glucose facilitation of episodic memory is dependent upon the development of one of these impairments in glucose regulation. If glucose facilitates episodic memory in older adults who have (or are developing) a particular form of prediabetes, then this might suggest pathophysiologic mechanisms relating to
episodic memory impairment that are amenable to intervention.
Gender did not interact with glucose regulation to influence the effect of glucose upon memory in older adults. Some studies have found that glucose improvement of episodic memory is isolated only to older and younger adult men, with facilitation being dependent upon relatively good glucose regulation in the former and relatively poor regulation in the latter (e.g., Craft et al., 1994). Other research has not found that gender has a moderating effect. Because estrogen level may influence glucose regulation (Lunt & Brown, 1996), it would be important to know the level of estrogen in female participants to disentangle these mixed effects.
memory complicates interpretation of this null effect. Body weight was not related to changes in working memory scores between the saccharin and glucose condition, unlike the findings for associative memory. This may suggest that glucose, across a range of dosages (i.e., 442 mg/kg to 1,111 mg/kg), has no influence on working memory in older adults, and that any effects associated with glucose are more specific and do not just relate to task difficulty.
In summary, no evidence was obtained supporting the hypothesis that oral
administration of 50 g glucose would attenuate the associative deficit in older adults. Lack of memory enhancement, at least in older adults with faster processing speed, may have been related to the use of an item and associative recognition task that was insufficiently
confirm that the effect of glucose is domain-specific, isolated to hippocampal-mediated memory in older adults. Since the effects of glucose did not extend to a demanding working memory task in my study, this provides some support that effects of glucose are more specific and do not just relate to task difficulty. Compared to older adults with higher body weight, I found that older adults with lower body weight appeared to have lower associative memory scores in the glucose relative to the saccharin condition. This finding provided tentative evidence that lack of glucose attenuation of the associative deficit in my study may have been due to a less than optimal dose of glucose. The effectiveness of a range of glucose dosages upon memory has only been examined in young adult females (Messier et al., 1998). Based upon the dosage recommendation from that study, 24 g glucose may have enhanced the memory of older adult participants in my study. Considering that older adults regulate glucose differently than young adults, a study examining the effectiveness of a range of glucose dosages in older adults is needed to determine the optimal mg/kg dosage for associative memory enhancement in this age group.
blood-brain barrier may further slow the delivery of glucose resulting in transient hypoglycemia in the hippocampus during cognitively demanding activities (McNay 2005; Syková, 2001; Syková et al., 2002). These effects may ultimately impair associative memory in older adults. If orally administered glucose improves the associative deficit in older adults and has little effect upon associative memory in young adults, this may tentatively suggest that age-related physiologic changes relating to the transport and diffusion of glucose to the hippocampus contribute to the development of the associative deficit in older adulthood, and that the older adult hippocampus may have greater functional capacity for the encoding of associations when it is supplied with adequate glucose. Investigating whether glucose attenuation of the associative deficit is found in older adults with IFG or IGT would also be important. This discovery might provide insight regarding particular mechanisms (e.g., insulin resistance) that may be appropriate to target in an intervention to improve associative memory.
memory may be at least partly mediated by insulin increase. Future research should seek to determine the specific physiologic mechanisms that account for glucose facilitation of episodic and associative memory. Isolating these mechanisms is necessary for the
REFERENCES
Allen, J.B., Gross, A.M., Aloia, M.S., & Billingsley, C. (1996). The effects of glucose on non-memory cognitive functioning in the elderly. Neuropsychologia, 34, 459-465. American Diabetes Association. (2003). Follow-up report on the diagnosis of diabetes
mellitus. Diabetes Care, 26, 3160-3167.
Armoni, M., Rafaeloff, R., Barzilai, A., Eitan, A., & Karnieli, E. (1987). Sex differences in insulin action and glucose transport on transporters in human omental adipocytes. Journal of Clinical Endocrinology & Metabolism, 65, 1141-1146.
Baltes, P.B. (1987). Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Developmental Psychology, 23, 611-626.
Baltes, P.B., Staudinger, U.M., & Lindenberger, U. (1999). Lifespan psychology: Theory and application to intellectual functioning. Annual Review of Psychology, 50, 471-507.
Banks, W.A. (2004). The source of cerebral insulin. European Journal of Pharmacology, 490, 5-12.
Bialystok, E., & Craik, F.I.M. (2006). Lifespan Cognition. New York: Oxford University Press.
Bruehl, H., Sweat, V., Hassenstab, J., Polyakov, V., & Convit, A. (2009). Cognitive impairment in nondiabetic middle-aged and older adults is associated with insulin resistance. Journal of Clinical and Experimental Neuropsychology, 32, 487-493.
Chalfonte, B.L., & Johnson, M.K. (1996). Feature memory and binding in young and older adults. Memory & Cognition, 24, 403-416.
Chobanian, A., Bakris, G., Black, H., Cushman, W., Green, L., Izzo, J., ….Rocella, E.J. (2003). The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. Journal of the American Medical Association, 289, 2560-2571.
Coltheart, M. (1981). The MRC Psycholinguistic Database. Quarterly Journal of Experimental Psychology, 33A, 497-505.
Convit, A. (2005). Links between cognitive impairment in insulin resistance: An explanatory model. Neurobiology of Aging, 26, S31-S35.
associated with poor memory performance and hippocampal atrophy among normal elderly. Proceedings of the National Academy of Sciences, 100, 2019-2022.
Cowie, C.C., Rust, K.F., Ford, E.S., Eberhardt, M.S., Byrd-Holt, D.D., Li, C., ….. Geiss, L.S. (2009). Full accounting of diabetes and pre-diabetes in the U.S. population in 1988 – 1994 and 2005 – 2006. Diabetes Care, 32, 287-294.
Craft, S., Murphy, C., & Wemstrom, J. (1994). Glucose effects on complex memory and nonmemory tasks: The influence of age, sex, and glucoregulatory response.
Psychobiology, 22, 95-105.
Craft, S., & Watson, G.S. (2004). Insulin and neurodegenerative disease: Shared and specific mechanisms, Lancet Neurology, 3, 169-178.
Craik, F.I.M. (1986). A functional account of age differences in memory. In F. Klix & H. Hagendorf (Eds.), Human memory and cognitive capabilities, mechanisms, and performance (pp. 409-422). New York: Elsevier.
Dahle, C.L., Jacobs, B.S., & Raz, N. (2009). Aging, vascular risk, and cognition: Blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychology and Aging. 24, 154 – 162.
Duelli, R. & Kuschinsky, W. (2001). Brain glucose transporters: Relationship to local energy demand. News in Physiological Science, 16, 71-76.
Engle, R.W., Tuholski, S.W., Laughlin, J.E., & Conway, A.R. (1999). Working memory, short-term memory and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General, 128, 309-331.
Gage, F.H., Kelly, P., & Björklund, A. (1984). Regional changes in brain glucose
metabolism reflect cognitive impairments in aged rats. The Journal of Neuroscience, 4, 2856-2865.
Gagnon, C., Greenwood, C. E., & Bherer, L. (2010). The acute effects of glucose ingestion on attentional control in fasting healthy older adults. Psychopharmacology, 211, 337- 346.
Gschanes, A., Boado, R., Sametz, W., & Windisch, M. (2000). The drug cerbrolysin and its peptide fraction E021 increase the abundance of the blood-brain barrier GLUT1 glucose transporter in brains of young and old rats. Histochemistry Journal, 32, 71-77.
Greenwood, C., & Winocur, G. (2005). High-fat diets, insulin resistance and declining cognitive function. Neurobiology of Aging, 26S, S42-S45.
Hale, P.J., Wright, J.V., & Nattrass, M. (1985). Differences in insulin sensitivity between normal men and women. Metabolism, 34, 1133-1138.
Hall, J.L., Gonder-Frederick, L.A., Chewning, W.W., Silvera, J., & Gold, P.E. (1989). Glucose enhancement of performance on memory tests in young and aged humans. Neuropsychologia, 27, 1129-1138.
Kaplan, R.J., Greenwood, C., Winocur, G., & Wolever, T. (2000). Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enriched with glucose and dietary carbohydrates. American Journal of Clinical Nutrition, 72, 825-836. Katzman, R., Brown, T., Fuld, P., Peck, A., Schecter, R., & Shimmel, H. (1983). Validation
of a short orientation-memory-concentration test of cognitive impairment. American Journal of Psychiatry, 140, 734-739.
Kondo, H., Morishita, M., Osaka, N., Osaka, M., Fukuyama, H., & Shibasaki, H. (2004). Functional roles of the cingulo-frontal network in performance on working memory. NeuroImage, 21, 2-14.
Korol, D.L., & Gold, P.E. (1998). Glucose, memory, and aging. American Journal of Clinical Nutrition, 67, 764S-771S.
Kucera and Francis, W.N. (1967). Computational analysis of present-day American English. Providence: Brown University Press.
Lamberts, S. (2008). Endocrinology and aging. In H.M Kronenberg, S. Melmed, K.
Polonsky, & P.R. Larsen (Eds.), Williams Textbook of Endocrinology (11th edition) (pp. 1185-1199). Philadelphia: Saunders.
Lamport, D.J., Lawton, C.L., Mansfield, M.W., & Dye, L. (2009). Impairments in glucose tolerance can have a negative impact on cognitive function: A systematic research review. Neuroscience and Biobehavioral Reviews, 33, 394-413.
Laight, D.W., Carrier, M.J., & Änggård, E.E. (2000). Antioxidants, diabetes and endothelial dysfunction. Cardiovascular Research, 47, 457-464.
Lyon, M.E., Baskin, L.B., Braakman, S., Presti, S., Dubois, J., & Shirey, T. (2009). Interference studies with two hospital-grade and two home-grade glucose meters. Diabetes, Technology, & Therapeutics, 11, 641-647.
Manning, C.A., Hall, J.L., & Gold, P.E. (1990). Glucose effects on memory and other neuropsychological tests in elderly humans. Psychological Science, 1, 307-311. Manning, C.A., Parsons, M.W., Cotter, E.M., & Gold, P.E. (1997). Glucose effects on
declarative and non-declarative memory in healthy elderly and young adults, Psychobiology, 25, 103-108.
McNay, E. (2005). The impact of recurrent hypoglycemia on cognitive function in aging. Neurobiology of Aging, 26S, S76-S79.
McNay, E.C., Fries, T.M., & Gold, P.E. (2000). Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task.
Proceedings of the National Academy of Sciences, 97, 2881-2885.
McNay, E.C., & Gold, P.E. (2001). Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. Journals of Gerontology Series a – Biological Sciences and Medical Sciences, 56, B66-B71.
McNay, E.C., & Gold, P.E. (2002). Food for thought: Fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation, Behavioral and Cognitive Neuroscience Reviews, 1, 264-280.
Meyer, C., Pimenta, W., Woerle, H.J., Van Haeften, T., Szoke, E., Mitrakou, A. & Gerich, J. (2006). Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care, 29, 1909–2006.
Messier, C. (2004). Glucose improvement of memory: A review. European Journal of Pharmacology, 490, 33-57.
Messier, C., Gagnon, M., & Knott, V. (1997). Effect of glucose and peripheral glucose regulation on the elderly. Neurobiology of Aging, 18, 297-304.
Messier, C., Pierre, J., Desrochers, A., & Gravel, M. (1998). Dose-dependent action of glucose on memory processes in women: Effect on serial position and recall priority. Cognitive Brain Research, 7, 221-233.