ABSTRACT
MILLER, NATHAN FORREST. Characterization of Fungicide Sensitivity and Analysis of Microsatellites for Population Studies of Fusarium oxysporum f. sp. niveum Causing Fusarium Wilt of Watermelon. (Under the direction of Dr. Lina M. Quesada-Ocampo).
Fusarium oxysporum f. sp. niveum (FON) is the fungal causal agent of Fusarium wilt of watermelon, an economically important disease of watermelon in the United States and
worldwide. This vascular wilt disease has been difficult to manage because the host specific fungus forms chlamydospores that can survive for many years in the soil. It has been
traditionally managed with host resistance, crop rotation, and soil fumigation. However, the development of new FON races and the increased environmental regulation regarding fumigants have limited the efficacy of host resistance and soil fumigation for control of Fusarium wilt. Currently, there is only one systemic fungicide, the triazole fungicide prothioconazole, labeled for control Fusarium wilt in the field. This study examines additional fungicides that might be efficacious against Fusarium wilt of watermelon. A mycelium growth assay was used to assess the ability of ten fungicides to reduce vegetative growth of diverse FON isolates.
Prothioconazole and a new succinate dehydrogenase inhibitor fungicide, pydiflumetofen, were further examined against a panel of 98 Fusarium oxysporum isolates to scout for intrinsic activity and potential resistance. The resistance to pydiflumetofen was studied by sequencing fungal genes associated with insensitivity to this fungicide class.
2016, disease incidence per plot and disease severity were reduced with both fungicides using the drench treatments and the additional spray treatments. Yield data was taken in 2016, and fruit count was reduced when prothioconazole was applied as a drench treatment, whereas pydiflumetofenhad equal yield to the non-inoculated non-treated control treatments although there were no differences between the two products in terms of reducing disease.
Fusarium Wilt of Watermelon
by
Nathan Forrest Miller
A thesis submitted to the Graduate Faculty of North Carolina State University
in partial fulfillment of the requirements for the degree of
Master of Science
Plant Pathology
Raleigh, North Carolina 2017
APPROVED BY:
_______________________________ _______________________________ Dr. Lina M. Quesada-Ocampo Dr. James Kerns
Committee Chair
DEDICATION
BIOGRAPHY
Nathan Forrest Miller was born on June 4th, 1992, in Occidental, California, where he
lived for less than a year. The son of an oil company middle manager, he followed his parents from California to Springfield, Missouri, and then Ponca City, Oklahoma. He attended Ponca City High School, where he played tennis badly, ran cross country slowly, and marched out of step in the marching band.
In August 2010, Nathan attended the University of Tulsa, where he majored in Biology with minors in Chemistry and Spanish. In summer of 2011, he worked in western Nebraska with Dr. Charles Brown as a field assistant in a cliff swallow mark-recapture study. From 2012 to 2014, he worked as a research assistant in the aerobiology lab of Dr. Estelle Levetin, where he validated Juniperus ashei pollen forecasts. In the same lab, Nathan began an independent project isolating endophytic and epiphytic fungi from Cercis canadensis leaves.
ACKNOWLEDGMENTS
I would like to thank my committee members, Drs. Lina Quesada-Ocampo, Dr. Jim Kerns, and Dr. Tyler Harp for their continual feedback, understanding, and encouragement. I would like to especially thank Dr. Lina Quesada-Ocampo for pushing me to challenge myself in my work and encouraging me to interact with growers, extension agents, industry professionals, and fellow scientists. I would like to also thank Dr. Tyler Harp for encouraging me to visit the Vero Beach Research Center, where I got to learn how plant pathology research is done in an industry setting up close. I would like to acknowledge my funding from the Syngenta Crop Protection Graduate Fellowship, and I would like to thank them for the support.
I would like to thank the current and past members of the Quesada lab, including Emma Wallace, Andrew Scruggs, Camilo Parada, Nick Noel, Madison Stahr, Kim D’Arcangelo, Allie Druffel, Dr. Alamgir Rahman, Mike Adams, Hunter Collins, Saunia Withers, Dr. Elsa Góngora-Castillo, Dr. Liliana Cano, Emily Keller, Abel Walker, Zach Shea, Laura Williams, Kelsey Wynne, Lynde Ring, Christina Mara, Kayla Elswick, Aidan Shands, and Jesse Yamagata. Of this group, I would like to especially thank Mike Adams for teaching me about field experiments and Dr. Liliana Cano for mentoring me in bioinformatics. I would like to further thank Dr. Peter Ojiambo for his assistance with statistical analysis. Also, I would like to thank Dr. Estelle Levetin for inspiring me to love botany almost as much as she does.
TABLE OF CONTENTS
LIST OF TABLES ... viii
LIST OF FIGURES ... xi
CHAPTER I ... 1
Literature Review ... 1
Watermelon ... 1
Fusarium wilt of watermelon ... 2
Fusarium oxysporum f. sp. niveum biology ... 5
Disease cycle ... 5
Characterization of isolates ... 6
Genetics ... 8
Genomics ... 9
Microsatellites in Fusarium oxysporum ... 10
Succinate dehydrogenase inhibitor fungicides ... 11
Fungal succinate dehydrogenase inhibitor fungicide resistance ... 12
REFERENCES... 14
TABLES ... 18
CHAPTER II ... 19
Sensitivity of Fusarium oxysporum f. sp. niveum to fungicides in vitro and in field experiments for management of Fusarium wilt of watermelon ... 19
ABSTRACT ... 19
INTRODUCTION ... 20
METHODS ... 23
Isolate collection and long-term storage ... 23
Identification of fungicides with activity against FON isolates in vitro ... 24
Evaluation of Fusarium isolates for fungicide sensitivity in vitro ... 26
Confirmation of fungicide activity against FON in field experiments ... 26
Genetic diversity and succinate dehydrogenase gene sequence analysis ... 28
RESULTS ... 31
Identification of fungicides with activity against FON isolates in vitro ... 31
Evaluation of Fusarium isolates for fungicide sensitivity in vitro ... 32
Confirmation of fungicide activity against FON in field experiments ... 33
Genetic diversity and succinate dehydrogenase gene diversity ... 34
DISCUSSION ... 35
AKNOWLEDGEMENTS ... 40
REFERENCES... 41
TABLES ... 46
FIGURES... 56
CHAPTER III ... 66
Analysis of microsatellites in Fusarium oxysporum and their application for population analysis of Fusarium oxysporum f. sp. niveum causing Fusarium wilt of watermelon ... 66
ABSTRACT ... 66
INTRODUCTION ... 67
Identification and analysis of microsatellites in Fusarium oxysporum genomes ... 70
Isolate collection and DNA extraction ... 72
Microsatellite amplification and genotyping ... 73
Genetic diversity analysis of microsatellites and population study ... 75
RESULTS ... 77
Microsatellite analysis ... 77
Population structure analysis ... 79
DISCUSSION ... 80
AKNOWLEDGEMENTS ... 85
REFERENCES... 86
TABLES ... 93
LIST OF TABLES
Table 1: Watermelon genotypes used to differentiate races of Fusarium oxysporum f. sp. niveum
(FON). S refers to a genotype susceptible to that race whereas R refers to a genotype resistant to that FON race. ... 18 Table 2: Fusarium spp. isolates tested against both prothioiconazole and pydiflumetofen
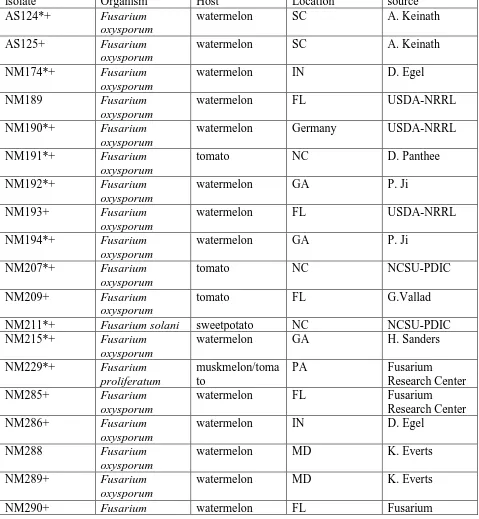
fungicides. The isolate name refers to the code in the culture collection, and the genus and species name refer to the nearest BLAST hit from the NCBI database. The country and state refer to where the fungus was first isolated, and the plant host refers to which plant host the fungus was first isolated. The isolate was kindly provided by the collaborator attributed in the final column. An asterisk (*) refers to isolates utilized in the expanded panel of fungicides. A plus sign (+) Refers to isolates that were included in the
phylogenetic study. ... 46 Table 3: Fungicides tested including active ingredient, brand name, fungicide class, and FRAC
code. ... 50 Table 4: Treatments for 2015 and 2016 field experiments. Drench treatments were applied at transplant and sprays were applied 14 days after transplant. ... 51 Table 5: Primers for the Internal transcribed spacer (ITS), Translation Elongation Factor 1α
(Tef-1α), and sdh genes. The sdhb and sdhc genes refer to the genes that code for the SdhB and SdhC subunits of the enzyme succinate dehydrogenase, associated with mutations that confer resistance to succinate dehydrogenase inhibitor fungicides. ... 52 Table 6: EC50 values in µg active ingredient/mL media for each fungicide tested with 10
isolates... 53 Table 7: Pairwise comparisons of EC50 values between fungicides. Values indicate differences
in EC50 between two fungicides and an asterisk (*) indicates significant differences after Bonferroni correction. ... 54 Table 8: Analysis of variance (ANOVA) table for total fruit count, marketable fruit count, and
weight by treatment in 2016 field experiments. There is no significant treatment effect for total fruit weight. SS stands for Sum of Squares and MS refers to Mean Sum of Squares. An asterisk (*) denotes a significant effect at the α=0.05 level. ... 55
Table 10: Microsatellite markers tested for amplification and polymorphism in the laboratory. GACGGCCAGT is the nucleotide sequence added to forward primers for fluorescent labeling and subsequent fragment analysis of PCR products. ... 94 Table 11: Isolates used for analysis including isolate code for the lab, isolate species, host from
where the isolate was collected from, the state where the isolate was collected, and the source. Isolates marked with an asterisk (*) were evaluated with gel electrophoresis in addition to fragment analysis. Isolates marked with a plus sign (+) were included in the population analysis... 97 Table 12: Total number and size of sequences examined in Fusarium oxysporum transcriptomes,
as well as the total number of microsatellites identified. The number of microsatellite containing sequences refers to the number of transcripts per transcriptome. ... 102 Table 13: Microsatellite frequency by repeat length and predicted transcriptome ... 103 Table 14: P-values from pairwise comparisons done with two sample T tests for microsatellite
relative density (DNA in microsatellites (Mb) / total DNA examined (Mb)) in dinucleotide repeats. Green highlighted values are significant at the alpha = 0.05 level, and red
highlighted values are significant at the alpha = 0.05 level with a Bonferroni correction.104 Table 15: P-values from pairwise comparisons done with two sample T tests for microsatellite
relative density (DNA in microsatellites (Mb) / total DNA examined (Mb)) in trinucleotide repeats. Green highlighted values are significant at the alpha = 0.05 level, and red
highlighted values are significant at the alpha = 0.05 level with a Bonferroni correction.105 Table 16: P-values from pairwise comparisons done with two sample T tests for microsatellite
relative density (DNA in microsatellites (Mb) / total DNA examined (Mb)) in
tetranucleotide repeats. Green highlighted values are significant at the alpha = 0.05 level, and red highlighted values are significant at the alpha = 0.05 level with a Bonferroni correction. ... 106 Table 17: P-values from pairwise comparisons done with two sample T tests for microsatellite
relative density (DNA in microsatellites (Mb) / total DNA examined (Mb)) in
pentanucleotide repeats. Green highlighted values are significant at the alpha = 0.05 level, and red highlighted values are significant at the alpha = 0.05 level with a Bonferroni correction. ... 107 Table 18: P-values from pairwise comparisons done with two sample T tests for microsatellite
relative density (DNA in microsatellites (Mb) / total DNA examined (Mb)) in
Table 19: P-values from pairwise comparisons done with two sample T tests for microsatellite relative density (DNA in microsatellites (Mb) / total DNA examined (Mb)) in total
microsatellites for each transcriptome. Green highlighted values are significant at the alpha = 0.05 level, and red highlighted values are significant at the alpha = 0.05 level with a Bonferroni correction. ... 109 Table 20: P-values from pairwise comparisons done with two sample T tests for relative
abundance (number of microsatellites divided by the number of transcripts examined) in the total microsatellites found in each transcriptome. The green symbolizes a significant difference at the alpha = 0.05 level, and red highlighted values are significant at the alpha = 0.05 level with a Bonferroni correction. ... 110 Table 21: Genetic diversity estimates for microsatellites tested for amplification and
polymorphism in the laboratory. N is the number of isolates with alleles at that particular locus. Na is the number of distinct alleles per locus. Ne is the number of effective alleles. I is Shannon’s Information index. h is gene diversity, and uh is unbiased gene diversity. PIC refers to polymorphism information content. ... 111 Table 22: Annotation and location of microsatellites tested for amplification and polymorphism
in the laboratory obtained from the FOXG genome (F. oxysporum f. sp. lycopersici 4275). ... 112 Table 23: Analysis of molecular variance (AMOVA) table with host as grouping factor. There is
no significant population differentiation between host groups either with or without clone-correction. Significance values attained by a randomization test with 999 permutations.113 Table 24: Analysis of molecular variance (AMOVA) table with location for isolate as the
LIST OF FIGURES
Figure 1: Percent disease incidence for each treatment was calculated per plot in 2015. The error bars represent the standard deviation for each treatment for each rating date. A in fungicide treatments on the horizontal axes refers to the drench application of the fungicide at
transplant, and AB refers to both a drench treatment at transplant and a foliar spray
application 14 days after transplant. Low and high refer to the rate of fungicide applied for each pydiflumetofenfungicide treatment (751.9 mL/ha and 1000.0 mL/ha, respectively).56 Figure 2: Percent disease incidence for each treatment was calculated per plot in 2016. The error
bars represent the standard deviation for each treatment for each rating date. A in fungicide treatments on the horizontal axes refers to the drench application of the fungicide at
transplant, and AB refers to both a drench treatment at transplant and a foliar spray
application 14 days after transplant. Low and high refer to the rate of fungicide applied for each pydiflumetofenfungicide treatment (751.9 mL/ha and 1000.0 mL/ha, respectively).57 Figure 3: Photos from the final rating date (July 24) in 2015. A is a Non-treated plot, B is a plot
with the pydiflumetofen treatment at a high rate applied as both a drench and a foliar spray, and C is a plot with the prothioconazole treatment applied as both a drench and a spray.58 Figure 4: Photos from the final rating date (July 14) in 2016. A is a Non-treated plot, B is a plot
with the pydiflumetofen treatment at a high rate applied as both a drench and a foliar spray, and C is a plot with the prothioconazole treatment applied as a drench, and D is a non-treated, non-inoculated plot. ... 59 Figure 5: relative Area Under the Disease Progress Curve (AUDPC) was calculated for the
incidence data, and was combined for the years 2015 and 2016. The relative AUDPC was plotted for each treatment and letters above bars indicate Tukey-Kramer groupings. A in fungicide treatments on the horizontal axes refers to the drench application of the fungicide at transplant, and AB refers to both a drench treatment at transplant and a foliar spray application 14 days after transplant. Low and high refer to the rate of fungicide applied for each pydiflumetofenfungicide treatment (751.9 mL/ha and 1000.0 mL/ha, respectively).60 Figure 6: relative Area Under the Disease Progress Curve (AUDPC) for severity data calculated
for each fungicide treatment in 2016. The relative AUDPC was plotted for each treatment and letters above bars indicate Tukey-Kramer groupings. A in fungicide treatments on the horizontal axes refers to the drench application of the fungicide at transplant, and AB refers to both a drench treatment at transplant and a foliar spray application 14 days after
Figure 7: Total count for 2016 yield data in melons. Letters above bars indicate Tukey-Kramer groupings; the same groupings were obtained for marketable count. A in fungicide treatments on the horizontal axes refers to the drench application of the fungicide at transplant, and AB refers to both a drench treatment at transplant and a foliar spray
application 14 days after transplant. Low and high refer to the rate of fungicide applied for each pydiflumetofenfungicide treatment (751.9 mL/ha and 1000.0 mL/ha, respectively). The non-treated control was inoculated, but the untreated non-inoculated non-treated control had no contact with FON and was used as a negative control. ... 62 Figure 8: A maximum likelihood phylogenetic tree paired with the EC50 values in ppm for
prothioconazole (black) and pydiflumetofen (gray). The numbers at the nodes refer to the bootstrap support or the percentage of replicate trees where isolates cluster together. .. 63 Figure 9: WebLogo diagram of amino acid distribution for SdhC. The letters reflect the amino
acid at that position in the polypeptide sequence, and the relative height of the letter reflects the percentage of isolates with that particular amino acid residue at that position. The Serine residue at position 222 and the Argenine residue at position 226 (starred) are not only conserved amongst Fusarium oxysporum isolates examined in this study but also amongst Ascomycota fungi... 64 Figure 10: WebLogo diagram of amino acid distribution for SdhB. The letters reflect the amino
acid at that position in the polypeptide sequence, and the relative height of the letter reflects the percentage of isolates with that particular amino acid residue at that position. The blue bar marks the conserved Iron-Sulfur subunit. ... 65 Figure 11: Minimum spanning network calculated with Bruvo’s genetic distance for all isolates
with host descriptors. The size and the number within each circle refer to the number of isolates of that particular multi-locus genotype (MLG). Thicker lines correspond to MLGs that are more closely related (smaller Bruvo’s genetic distance value between MLGs).115
Figure 12: Minimum spanning network calculated with Bruvo’s genetic distance for all isolates with location descriptors. The location refers to where the isolate was collected from. The size and the number within each circle refer to the number of isolates of that particular MLG. Thicker lines correspond to MLGs that are more closely related (smaller Bruvo’s genetic distance value between MLGs). ... 116 Figure 13: Minimum spanning network calculated with Bruvo’s genetic distance for isolates
collected from watermelon with location descriptors. The location refers to where the isolate was collected from. The size and the number within each circle refer to the number of isolates of that particular MLG. Thicker lines correspond to MLGs that are more closely related (smaller Bruvo’s genetic distance value between MLGs). ... 117
Figure 15: Delta K plot for STRUCTURE output when total dataset analyzed from K=1 to K=10 with burnin of 300,000 and MCMC of 500,000 with 15 iterations at each K. Two clusters were selected for analysis. ... 119 Figure 16: STRUCTURE plot with K=2 for the total dataset (N=73). The colors refer to the
genetic clusters predicted from STRUCTURE. Both clusters are represented in both the tomato and the watermelon isolates. Numbers refer to the host where the isolates were originally sampled: 1 is from ginger, 2 is muskmelon, 3 is sweetpotato, 4 is tomato, and 5 is watermelon. ... 120 Figure 17: Delta K plot for STRUCTURE output when total dataset analyzed from K=1 to K=10
with burnin of 300,000 and MCMC of 500,000 with 15 iterations at each K. Three clusters were selected for analysis. ... 121 Figure 18: STRUCTURE plot with K=3 for the FON dataset analyzed individually. Each color
CHAPTER I
Literature Review Watermelon
Watermelon (Citrullus lanatus) is a fruit crop in the Cucurbitaceae plant family.
Watermelon is an annual crop, and is planted in mid-April to June in North Carolina (Holmes et al. 2005). Transplants are grown for 4-6 weeks in the greenhouse before being transplanted in the field (Holmes et al. 2005). Watermelon growth is best in sandy and slightly acidic soils, and black plastic mulch with drip irrigation increases watermelon yield and decreases irrigation costs (Holmes et al. 2005). The most common cultivar grown from 2004 to 2006 in North Carolina was Liberty, a seedless triploid variety (USDA-NASS 2015).
Watermelon is widely cultivated in the United States (US) and around the world. China is the largest producer and accounts for 62% of the world’s watermelons (FAOSTAT 2017). In the US, North Carolina is the 7th largest producer of watermelons in terms of acreage harvested
in 2014, behind Florida, Georgia, California, Texas, South Carolina, and Indiana (USDA-NASS 2015). In that year, the value of watermelons produced in the state exceeded $34 million
(USDA-NASS 2015).
(Bruton et al. 2007). For example, diploid cultivars are directly seeded whereas more expensive triploid and hybrid cultivars are often transplanted from a greenhouse (Bruton et al. 2007). From an economic study done in 2006, directly seeding an open-pollinating diploid cultivar costs $0.01 to $0.02 per plant, whereas a triploid variety costs $0.28 (Taylor et al. 2006).
Fusarium wilt of watermelon
Fusarium wilt is among the most economically damaging watermelon disease in the world and has become more of a problem in recent years in North Carolina with the planting of susceptible triploid/seedless watermelon and the increase in prevalence of race 2 and 3 isolates.
Fusarium oxysporum f. sp. niveum (FON), the causal agent of this disease, infects the xylem and plugs the tissue with both mycelium and extracellular polysaccharide (Kleczewski and Egel 2011). This blockage will manifest itself as a wilt, causing a characteristic unilateral wilting in the plant (Kleczewski and Egel 2011). As the disease progresses, the leaves will often become chlorotic and necrotic. The wilting can be associated with heat, and it has been noted that this symptom can seemingly recover with cooler weather at night and with watering because the plant’s water requirement is lower under these conditions (Kleczewski and Egel 2011). In a
cross section of the stem or root, the xylem will appear darker (Kleczewski and Egel 2011). There are currently very few management strategies for Fusarium wilt. Traditionally, this disease has been managed by planting resistant cultivars. Some watermelon cultivars are
resistant to Fusarium oxysporum f. sp. niveum race 0, and some are resistant to F. oxysporum f. sp. niveum race 1. Other cultivars, including most hybrid and triploid watermelons, are
production of ammonia from the organic soil amendment of the killed vetch (Zhou and Everts 2004). There is also evidence that soil solarization without organic soil amendments can delay Fusarium wilt of watermelon onset and reduce disease incidence in the field (Martyn and Hartz 1986).
Another potential disease management tactic is to plant grafted plants with Fusarium wilt-resistant rootstocks. A study by Keinath and Hassell (2014) examined the efficacy of grafting resistant roots with susceptible scions, and found that there was reduced disease in the scion. The study concluded that not only was it efficacious, but that it was also cost effective if planted in a field with high disease pressure (Keinath and Hassell 2014). However, the cost of the grafted plants is very high, and there have been many different estimates. This study claimed that the cost of each grafted plant was $0.63 (Keinath and Hassell 2014). This is less than a 2006 economic model which estimated that the cost was approximately $0.75 per plant, and it has been estimated that the cost could be as high as $1.30 per grafted transplant (Taylor et al. 2006). While it is estimated that the costs will drop as this technology becomes increasingly available, it is still questionable whether grafted transplants are a financially viable option for growers.
Chemical management of this disease is very limited. Several in vitro and greenhouse studies have looked at different fungicides or chemicals that limit Fusarium oxysporum growth, but with only limited success. Amini and Sidovich (2010) demonstrated that carbendazim, prochloraz, benomyl, and bromuconazole all reduced mycelium growth of Fusarium oxysporum
labeled for tree trunk injection (EPA 2016). Other studies have found that difenoconazole is somewhat effective in controlling Fusarium avenaceum in that it slowed mycelia growth but did not succeed in killing the fungus (Kopacki and Wagner 2006).
Everts et al (2014)tested fungicides throughout the country to look at chemicals not labeled for this pathosystem at the time. This study found that the most efficacious chemicals were prothioconazole (trade name Proline; Bayer CropScience, Research Triangle Park, NC), thiophanate-methyl (Topsin M; United Phosphorous, Inc, King of Prussia, PA), and acibenzolar-S-methyl (Actigard; Syngenta Crop Protection, Greensboro, NC). Prothioconazole is an
ergosterol demethylation inhibitor (DMI). Thiophanate-methyl breaks down to form a beta-tubulin inhibitor, which effectively inhibits meiosis (Everts et al. 2014). Acibenzolar-S-methyl is a systemic acquired resistance-inducing chemical. After this study, Bayer CropScience included Fusarium wilt of watermelon management under a supplementary label for Proline (Everts et al. 2014). This study found prothioconazole to be effective with three drip
Fusarium oxysporum f. sp. niveum biology
Fusarium oxysporum f. sp. niveum is the causal agent of Fusarium wilt of watermelon. The pathogen is a filamentous ascomycete fungus that can produce three spore types. It can form straight and tapered septate macroconidia, as well as aseptate elliptical shaped microconidia (Leslie and Summerell 2006). These are produced on simple conidiophores singly or in
sporodochia. It is thought that F. oxysporum predominantly produces microconidia (Leslie and Summerell 2006). In adverse environments, such as a low carbon to nitrogen ratio in the
surrounding environment, the fungus can produce thick walled chlamydospores that can survive in the soil for up to 5 or 10 years (Carlile 1956). These can be formed on either simple
conidiophores or intercalarily within hyphae or macroconidia (Leslie and Summerell 2006). F. oxysporum f. sp. niveum mycelium is generally fluffy and aerial, and range in color from white to purple in color, but is typically pink. The metabolites produced by the fungus can stain the media red, pink, or purple (Leslie and Summerell 2006).
Disease cycle
The fungus infects the plant tissue as a germinating spore and growing hyphae penetrate the plant tissue through wounds or openings near the site of elongation for a root hair (Agrios 2005). The fungal hyphae eventually penetrate the vascular tissue and produce microconidia (Agrios 2005). The microconidia are then released into the xylem, which travel upward with the water and then these begin to colonize the watermelon’s vascular tissue further up in the plant
eventually colonizes all tissues of the plant, forming many spores when it reaches the plant surface that are spread by wind or splash transport (Agrios 2005). This disease is considered monocyclic, although there is debate with regards to the importance of conidia in the infection in other formae speciales (Kleczewski and Egel 2011). A report from Israeli tomato greenhouses demonstrated that the vascular wilt fungus F. oxysporum f. sp. lycopersici forms airborne macroconidia on the stem surface of living tomatoes, although F. oxysporum f. sp. lycopersici is traditionally a soil pathogen that does not form conidia on living tissues (Katan, Shlevin, and Katan 1997). This has not been otherwise been reported in F. oxysporum f. sp. lycopersici, and has not been reported in F. oxysporum f. sp. niveum, but this could have profound implications for the epidemiology of the disease. When the plant dies, the fungus forms conidia in
sporodochia on the dead leaves as well as chlamydospores in the soil directly on the mycelia (Agrios 2005). The chlamydospores are long lived, and can survive for many years in the soil (Agrios 2005). The fungus can also colonize the seed and cause disease in the infected seedlings the following year (Bruton et al. 2007; Kleczewski and Egel 2011).
Characterization of isolates
Isolates of Fusarium oxysporum have traditionally been classified into formae speciales
(f. sp.), defined by the host that an isolate can infect. For example, f. sp. niveum infects
watermelon whereas f. sp. lycopersici infects tomatoes. These distinctions are characterizations of convenience because they do not reflect evolutionary relationships (Gordon 1997).
niveum (0, 1, 2, and 3). All races can infect susceptible cultivars, but race 0 cannot infect melons with race 0 resistance. Only races 2 and 3 can infect all commercially produced watermelon cultivars (Table 1) (Zhou, Everts, and Bruton 2010). Race 2 and race 3 differ in their
aggressiveness towards the watermelon cultivar PI-296341-FR used for FON race testing, where race 3 more aggressive than race 2 (Zhou, Everts, and Bruton 2010). However, phylogenetic relatedness between races in a forme specialis is still unknown. What complicates matters is that races are often polyphyletic in origin, with only a few exceptions. For example, while race 3 in
F. oxysporum f. sp. niveum is monophyletic, isolates within the same clonal lineage can be either race 1 or race 2 (Zhou and Everts 2007). In Fusarium oxysporum, vegetative incompatibility can be used functionally to characterize strains in heterogeneous populations. If two isolates are vegetatively compatible and can form a heterokaryon, then they are presumed to be part of the same clonal lineage (Aanen, Glass, and Saupe 2013). When isolates can form a heterokaryon, they are then placed in the same vegetative compatibility group. Robust population and evolutionary analysis with genetic markers are needed to clarify relatedness of races within a
forma specialis of Fusarium oxysporum.
(2000), Indiana (2001), Georgia (2004), and South Carolina (Zhou, Everts, and Bruton 2010; Bruton, Fish, and Langston 2008; Egel, Harikrishnan, and Martyn 2005; Zhou and Everts 2001). Race 3 was discovered in Maryland and Delaware in 2000, although the report was not published until 2007 (Zhou, Everts, and Bruton 2010). Several resistance genes documented in tomato have been found that suggest a quantitative gene-for-gene relationship between host and pathogen (Michielse and Rep 2009). These host resistance genes and virulence genes in the pathogen define the race relationship between pathogen and host (Michielse and Rep 2009). Nonetheless, specific resistance genes recognizing pathogen virulence factors have not been described in F. oxysporum f. sp. niveum and watermelon.
Genetics
Fusarium oxysporum is a species complex of asexual fungi closely related to the
Gibberella fujikuroi complex (Fourie et al. 2009). O’Donnell et. al. (2009) created the Fusarium database that provides centralized information for research on the genus Fusarium. Within this website is the FUSARIUM-ID tool, which uses two gene loci, the Intergenic Spacer (IGS) and Elongation Factor region 1-α (EF-1α) to elucidate the phylogenetic relationships within the
10 het loci, and so there should be approximately 1,024 vegetative compatibility groups (VCGs) (Puhalla 1985). If two isolates belong to the same VCG, then they should have compatible het
loci and be able to form a heterokaryon and exchange genetic material (Fourie et al. 2009; Glass, Jacobson, and Shiu 2000).
A forma specialis infecting a host can have different VCGs (Fourie et al. 2009; Elias and Schneider 1990). For example, F. oxysporum f. sp. cubense (FOC), defined by the ability to infect banana, is comprised of 24 different VCGs, with many organisms that are not in FOC also in these VCGs (Fourie et al. 2009). This has also been found to hold true in both F. oxysporum f. sp. lycopersici (Elias and Schneider 1990) and F. oxysporum f. sp. niveum (Zhou and Everts 2007). As of 2009, there are 3 VCGs associated with F. oxysporum f. sp. niveum (O'Donnell et al. 2009). One example of a study where this VCG characterization was used was the
watermelon field F. oxysporum f. sp. niveum survey done by Zhou and Everts (Zhou and Everts 2007). As there were several VCGs in the same field, the study concluded that there was much more genetic diversity than previously expected. The study also concluded that the VCGs in the field did not correspond with the race distinctions (Zhou and Everts 2007).
Genomics
Several Fusarium genomes have been sequenced thus far, including F.graminearum, F. oxysporum f. sp. lycopersici, F. verticilloides, and F.‘solani’ f. sp. pisi (Ma et al. 2013).
Genomic studies have revealed that F. oxysporum genomes have more genes and noncoding DNA than other Fusarium genomes (Ma et al. 2013). It has also been found that there are “supernumerary” or “conditionally dispensable” chromosomes that can be transferred between
example, a chromosome transfer from a F. oxysporum f. sp. lycopersici isolate to a
nonpathogenic F. oxysporum isolate allowed the nonpathogenic isolate to infect tomato (Ma et al. 2010). This phenomena has also been found in F. ‘solani’ f. sp. pisi isolates that transfer chromosomes that allow formerly nonpathogenic isolates to infect pea (Ma et al. 2013). It is important to note that the Fusarium genome facilitates this horizontal chromosome transfer, as the “housekeeping” genes are centralized on separate chromosomes from those genes encoding
pathogenicity and secondary metabolites (Ma et al. 2010; Ma et al. 2013). In addition, it was found that the pathogenicity chromosomes are preferably transferred, while the “core
chromosomes” are not (Ma et al. 2010).
Microsatellites in Fusarium oxysporum
Population analysis can be done with the help of microsatellites, or simple sequence repeats (SSRs) that are repeated oligonucleotides of 2-6 base pairs found throughout the genome. A study from India looked at SSRs that could be used in differentiation of F. oxysporum
pathogen lineages (Mahfooz et al. 2012). This study looked at published F. oxysporum
expressed sequence tags (ESTs) to mine for SSRs in F. oxysporum f. sp. melonis (infects melon),
cucumerium (cucumber), and lycopersici (tomato). Primers were then developed to test for these different gene regions and tested across different formae speciales (melonis, cucumerium,
2013). Of these 30 markers, 21 produced amplicons in Fusarium udum, demonstrating the importance and application of SSR studies from ESTs and transcriptomes.
Another study used the full Fusarium oxysporum f. sp. lycopersici genome published by the Fusarium Comparative Database, operated by the Broad Institute of Harvard and MIT, to mine for microsatellites (Kumar et al. 2012). The study looked at the frequency and distribution of SSRs, and mapped them to chromosomes within the genome (Kumar et al. 2012). The findings concentrated on the length of the SSR motifs as well as the total length of the
microsatellite sequence. However, there was no molecular confirmation of the in silico data to determine the usefulness of the marker for population analysis (Kumar et al. 2012).
A third study looked at race diversity and evolution in Fusarium oxysporum f. sp. ciceris, which infects chickpea. The work was done with conserved genes used in fungal identification, genes involved in plant pathogenicity, as well as microsatellites (Demers, Garzón, and Jiménez-Gasco 2014). The microsatellite loci were found with molecular labeling of genomic DNA (Demers, Garzón, and Jiménez-Gasco 2014). Findings revealed that this particular forma specialis is monophyletic, using microsatellites to differentiate between races of the pathogen. However, analyses yielded very few polymorphic microsatellites for F. oxysporum f. sp. ciceris, which the authors attributed to the relatedness of the forma specialis (Demers, Garzón, and Jiménez-Gasco 2014).
Succinate dehydrogenase inhibitor fungicides
that are found on the mitochondrial membrane periphery, as well as two hydrophobic domains (SDHC and SDHD) found within the mitochondrial membrane (Walter 2012). The hydrophilic subunits are involved in the tricarboxylic acid cycle and provide the succinate dehydrogenase activity with a FAD cofactor in SDHA and iron-sulfur subunits in SDHB. The hydrophobic subunits comprise complex II of the respiratory chain and has heme groups that are involved with ubiquinone reduction (Walter 2012). The ubiquinone site is located between the B, C and D domains, near the heme group between the SDHC and SDHD domains and an iron-sulfur subunit in the SDHB domain (Walter 2012).
The first SDHI released was carboxin, introduced in the 1960s, which was effective against basidiomycete plant pathogens, but had limited effects on ascomycete fungi (1970). However, boscalid, a pyridine carboxamide, was introduced in 2003 and had efficacy against ascomycete fungi, like Botrytis spp., Sclerotinia spp., and Alternaria spp. (Avenot et al. 2014). The SDHI fungicide class (Fungicide Resistance Action Committee code 7) has expanded since the market release of Boscalid, and now includes active ingredients of various chemical
subclasses, including recent introductions of pyrazole carboxamides (Avenot et al. 2014).
Fungal succinate dehydrogenase inhibitor fungicide resistance
There is a medium to high risk for resistance development with SDHI fungicides. The Fungicide Resistance Action Committee (FRAC) has determined that SDHI fungicide
different binding, and, although they are in the same cross-resistance group, there is not complete cross-resistance between all chemicals in this fungicide class (Walter 2012). For example, within
Alternaria alternata populations, boscalid resistance was correlated with fluxapyroxad resistance, but was not positively correlated with either fluopyram or penthiopyrad (Walter 2012). This is because the chemicals bind to slightly different sites within the enzyme, and different mutations within the fungal succinate dehydrogenase (SDH) enzyme alter the binding site so that the enzyme is functional yet not inhibited by the fungicide.
REFERENCES
Aanen, D. K., N. L. Glass, and S. J. Saupe. 2013. 'Biology and genetics of vegetative
incompatibility in fungi.' in Alfons J. M. Debets (ed.), Cellular and Molecular Biology of Filamentous Fungi (ASM: Washington, D.C).
Agrios, G. N. 2005. Plant Pathology (Elsevier Academic Press: New York).
Amini, J., and D. F. Sidovich. 2010. 'The effects of fungicides on Fusarium oxysporum f. sp.
lycopersici associated with Fusarium wilt of tomato', Journal of Plant Protection Research, 50: 172-78.
Avenot, H. F., H. van den Biggelaar, D. P. Morgan, J. Moral, M. Joosten, and T. J. Michailides. 2014. 'Sensitivities of baseline isolates and boscalid-resistant mutants of Alternaria alternata from pistachio to fluopyram, penthiopyrad, and fluxapyroxad', Plant Disease, 98: 197-205.
Bruton, B. D., W. W. Fish, and D. B. Langston. 2008. 'First report of Fusarium wilt caused by
Fusarium oxysporum f. sp. niveum Race 2 in Georgia watermelons', Plant Disease, 92: 983-83.
Bruton, B. D., W. W. Fish, X. G. Zhou, K. L. Everts, and P. D. Roberts. 2007. "Fusarium wilt in seedless watermelons." In Southeast Regional Vegetable Conference, edited by W;. T .Kelly, 93-98. Savannah, Georgia.
Bruton, B. D., C. L. Patterson, and R. D. Martyn. 1988. 'Fusarium wilt (F. oxysporum f. sp.
niveum Race 2) of watermelon in Oklahoma', Plant Disease, 72: 734.
Carlile, M. J. 1956. 'A study of the factors influencing non-genetic variation in a strain of
Fusarium oxysporum', Microbiology, 14: 643-54.
Demers, J. E., C. D. Garzón, and M. D. M. Jiménez-Gasco. 2014. 'Striking genetic similarity between races of Fusarium oxysporum f. sp. ciceris confirms a monophyletic origin and clonal evolution of the chickpea vascular wilt pathogen', European Journal of Plant Pathology, 139: 309-24.
Di Pietro, A., M. P. Madrid, Z. Caracuel, J. Delgado-Jarana, and M. I. G. Roncero. 2003. 'Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus',
Molecular Plant Pathology, 4: 315-25.
Egel, D. S., R. Harikrishnan, and R. Martyn. 2005. 'First report of Fusarium oxysporum f. sp.
Elias, K. S., and R. W. Schneider. 1990. 'Vegetative compatability groups in Fusarium oxysporum f. sp. lycopersici', Phytopathology, 81: 159-62.
EPA, Environmental Protection Agency. 2016. "Pesticide Product Label Search." In.: Environmental Protection Agency.
Everts, K. L., D. S. Egel, D. Langston, and X-G. Zhou. 2014. 'Chemical management of Fusarium wilt of watermelon', Crop Protection, 66: 114-19.
Fourie, G., E. T. Steenkamp, T. R. Gordon, and A. Viljoen. 2009. 'Evolutionary relationships among the Fusarium oxysporum f. sp. cubense vegetative compatibility groups', Applied and environmental microbiology, 75: 4770-81.
Glass, N. Louise, David J. Jacobson, and Patrick K. T. Shiu. 2000. 'The genetics of hyphal fusion and vegetative incompatability in filamentous ascomycete fungi', Annual Review of Genetics, 34: 165-86.
Gordon, T. R. 1997. 'The evolutionary biology of Fusarium oxysporum', Annual review of phytopathology, 35: 111-28.
Holmes, Gerald J., David W. Monks, Jonathan R. Schultheis, Kenneth A. Sorenson, and Allan C. Thornton. 2005. "Crop profile for watermelons in North Carolina." In, edited by Jr. Stephen J. Toth.
Katan, Talma, E. Shlevin, and J. Katan. 1997. 'Sporulation of Fusarium oxysporum f. sp.
lycopersici on Stem Surfaces of Tomato Plants and Aerial Dissemination of Inoculum',
Phytopathology, 87: 712-19.
Keinath, A. P., and R. L. Hassell. 2014. 'Control of Fusarium Wilt of Watermelon by Grafting onto Bottlegourd or Interspecific Hybrid Squash Despite Colonization of Rootstocks by Fusarium', Plant Disease, 98: 255-66.
Kleczewski, N. M. , and D. S. Egel. 2011. 'A diagnostic guide for Fusarium wilt of watermelon',
Plant Health Progress.
Kopacki, M., and A. Wagner. 2006. 'Effect of some fungicides on mycelium growth of Fusarium avenaceum ( Fr .) Sacc . pathogenic to chrysanthemum (Dendranthema grandiflora
Tzvelev )', Agronomy Research, 4: 237-40.
Kumar, S., D. Maurya, S. Rai, L. Kashyap, and A. K. Srivastava. 2012. 'Computational mining and genome wide distribution of microsatellite in Fusarium oxysporum f . sp .
Kumar, S., S. Rai, D. K. Maurya, P. L. Kashyap, A. K. Srivastava, and M. Anandaraj. 2013. 'Cross-species transferability of microsatellite markers from Fusarium oxysporum for the assessment of genetic diversity in Fusarium udum', Phytoparasitica, 41: 615-22.
Leslie, J. F., and B. A. Summerell. 2006. The Fusarium laboratory manual (Blackwell Publishing: Ames, Iowa).
Ma, L-J., D. M. Geiser, R. H. Proctor, A. P. Rooney, K. O'Donnell, F. Trail, D. M. Gardiner, J. M. Manners, and K. Kazan. 2013. 'Fusarium pathogenomics', Annual review of
microbiology, 67: 399-416.
Ma, L-J., H. C. van der Does, K. A. Borkovich, J. J. Coleman, M-J. Daboussi, A. Di Pietro, M. Dufresne, M. Freitag, M. Grabherr, B. Henrissat, P. M. Houterman, S. Kang, W-B. Shim, C. Woloshuk, X. Xie, J-R. Xu, J. Antoniw, S. E. Baker, B. H. Bluhm, A. Breakspear, D. W. Brown, R. A. E. Butchko, S. Chapman, R. Coulson, P. M. Coutinho, E. G. J. Danchin, A. Diener, L. R. Gale, D. M. Gardiner, S. Goff, K. E. Hammond-Kosack, K. Hilburn, A. Hua-Van, W. Jonkers, K. Kazan, C. D. Kodira, M. Koehrsen, L. Kumar, Y-H. Lee, L. Li, J. M. Manners, D. Miranda-Saavedra, M. Mukherjee, G. Park, J. Park, S-Y. Park, R. H. Proctor, A. Regev, M. C. Ruiz-Roldan, D. Sain, S. Sakthikumar, S. Sykes, D. C.
Schwartz, B. G. Turgeon, I. Wapinski, O. Yoder, S. Young, Q. Zeng, S. Zhou, J.
Galagan, C. A. Cuomo, H. C. Kistler, and M. Rep. 2010. 'Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium', Nature, 464: 367-73.
Mahfooz, S., D. K. Maurya, A. K. Srivastava, S. Kumar, and D. K. Arora. 2012. 'A comparative in silico analysis on frequency and distribution of microsatellites in coding regions of three formae speciales of Fusarium oxysporum and development of EST-SSR markers for polymorphism studies', FEMS Microbiology Letters, 328: 54-60.
Martyn, R. D. 1987. 'Fusarium oxysporum f. sp. niveum Race 2: A highly aggressive race new to the United States', Plant Disease, 71: 233-36.
Martyn, R. D., and B. D. Bruton. 1989. 'An initial survey of the United States for races of
Fusarium oxysporum f. sp. niveum', HortScience, 24: 696-98.
Martyn, R. D., and T. K. Hartz. 1986. 'Use of soil solarization to control Fusarium wilt of watermelon', Plant Disease, 70: 762-66.
Michielse, C. B., and M. Rep. 2009. 'Pathogen profile update: Fusarium oxysporum', Molecular Plant Pathology, 10: 311-24.
O'Donnell, K., C. Gueidan, S. Sink, P. R. Johnston, P. W. Crous, A. Glenn, R. Riley, N. C. Zitomer, P. Colyer, C. Waalwijk, T. Van Der Lee, A. Moretti, S. Kang, H-S. Kim, D. M. Geiser, J. H. Juba, R. P. Baayen, M. G. Cromey, S. Bithell, D. A. Sutton, K. Skovgaard, R. Ploetz, H. C. Kistler, M. Elliott, M. Davis, and B. A. J. Sarver. 2009. 'A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex', Fungal Genetics and Biology, 46: 936-48.
Puhalla, J. E. 1985. 'Classification of strains of Fusarium oxysporum on the basis of vegetative compatibility', Canadian Journal of Botany, 63: 179-83.
Song, W., L. Zhou, C. Yang, X. Cao, L. Zhang, and X. Liu. 2004. 'Tomato Fusarium wilt and its chemical control strategies in a hydroponic system', Crop Protection, 23: 243-47.
Taylor, M. , B. Bruton, W. Fish, and W. Roberts. 2006. 'Cost benefit analyses of using grafted watermelons for disease control and the fresh-cut market', Cucurbitaceae: 277-85. USDA-NASS. 2015. "Vegetables 2014 summary." In, edited by USDA-NASS.
van den Bosch, F., R. Oliver, F. van den Berg, and N. Paveley. 2014. 'Governing principles can guide fungicide-resistance management tactics', Annu Rev Phytopathol, 52: 175-95. Walter, H. 2012. Bioactive heterocyclic compound classes (Wiley-VCH Verlag GmbH & Co.
KGaA: Weinheim, Germany).
Zhou, X. G., and K. L. Everts. 2001. 'First report of the occurrence of Fusarium oxysporum f. sp.
niveum Race 2 in commercial watermelon production areas of Maryland and Delaware',
Plant Disease, 85: 1291-91.
Zhou, X. G., and K. L. Everts. 2004. 'Suppression of Fusarium wilt of watermelon by soil amendment with hairy vetch', Plant Disease, 88: 1357-365.
Zhou, X. G., and K. L. Everts. 2007. 'Characterization of a regional population of Fusarium oxysporum f. sp. niveum by race, cross pathogenicity, and vegetative compatibility',
Phytopathology, 97: 461-9.
Zhou, X. G., K. L. Everts, and B. D. Bruton. 2010. 'Race 3, a new and highly virulent race of
TABLES
Table 1: Watermelon genotypes used to differentiate races of Fusarium oxysporum f. sp. niveum
(FON). S refers to a genotype susceptible to that race whereas R refers to a genotype resistant to that FON race.
Disease response to FON Cultivar or genotype Race 0 Race 1 Race 2 Race 3 Sugar Baby, Black Diamond S S S S
Charleston Gray R S S S
Calhoun Gray R R S S
CHAPTER II
Sensitivity of Fusarium oxysporum f. sp. niveum to fungicides in vitro and in field experiments for management of Fusarium wilt of watermelon
ABSTRACT
Fusarium wilt of watermelon, caused by Fusarium oxysporum f. sp. niveum (FON), is an economically important watermelon disease in the United States (US) and worldwide. This disease has traditionally been controlled in the US with resistant varieties, crop rotation, and soil fumigation. The development of new pathogen races and recent restrictions on fumigants have limited Fusarium wilt control. New fungicides that can be used in this system would benefit watermelon producers. This study examined the in vitro sensitivity of FON to fungicides and for control of Fusarium wilt of watermelon in the field. Fungicides prothioconazole,
pydiflumetophen, tebuconazole, difenoconazole, and propiconazole reduced F. oxysporum
INTRODUCTION
Fusarium wilt of watermelon is an economically important disease of watermelon in the United States (US) and worldwide. Watermelon is a major cucurbit crop in the southern US, where Florida, Texas, California, Georgia, Indiana, South Carolina, and North Carolina are the most productive states (USDA-NASS 2015). Fusarium wilt of watermelon presents itself in the field as a complete or unilateral wilt due to obstruction of the vascular system by the soilborne fungal pathogen Fusarium oxysporum f. sp. niveum (FON), which prevents the free passage of water in the xylem (Kleczewski and Egel 2011). The fungus produces thick walled survival propagules called chlamydospores that can survive for up to 20 years in the soil (Martyn 2014). The long-term survival of the pathogen makes the disease difficult to manage, and resistant watermelon cultivars and soil fumigants such as methyl bromide have been the main methods of disease control (Everts and Himmelstein 2015). However, methyl bromide was phased out in accordance with the Montreal Agreements due to damage to the ozone layer and fungicides for Fusarium wilt control are limited (Everts et al. 2014; King et al. 2008).
cultivars in production that are resistant to this race (Everts and Himmelstein 2015). In 2010, FON Race 3 was reported in Maryland and Delaware, and is virulent towards PI-296341-FR, which is resistant to FON Races 0, 1, and 2 (Zhou, Everts, and Bruton 2010). Thus, PI-296341-FR has been investigated as a source of resistance genes for resistance to FON Race 2, but is not produced commercially, as it produces small fruit without flavor (Martyn and Netzer 1991). Currently, no sources of resistance to FON Race 3 have been reported.
A field and greenhouse study conducted in 2014 found that only two out of eight fungicides tested reduced Fusarium wilt incidence. These fungicide active ingredients were prothioconazole and thiophanate-methyl (Everts et al. 2014). Of these fungicides,
prothioconazole, under the trade name Proline, is the only fungicide that received a label for controlling Fusarium wilt of watermelon. Prothioconazole (FRAC 2016) is a demethylase inhibitor fungicide that interferes with ergosterol synthesis in fungi, disrupting the normal growth and function of the fungal cell wall (Parker et al. 2011). Although the study examined applying the fungicides three times through a drip tape, the Proline label only allows for up to one soil application and up to two direct-banded sprays aimed at the soil line (Everts et al. 2014). These additional sprays may be less effective for disease management because the foliar spray would not maximize contact between the fungicide and the soilborne pathogen.
ergosterol is found across many different fungi, Mycosphaerella graminicola has been reported to have different effects under triazole treatment than yeasts (Parker et al. 2011). In addition, prothioconazole is demonstrated to bind in a competitive manner to CYP51 in M. graminicola, whereas other triazoles bind noncompetitively to the same enzyme (Parker et al. 2011).
Everts et. al. (2014) did not include carboxamide succinate dehydrogenase inhibitors (SDHIs) in their experiments, which act by inhibiting the ubiquinone-pocket of succinate dehydrogenase, also known as complex II, in the mitochondrial respiratory chain (Sierotzki and Scalliet 2013). Succinate dehydrogenase is an enzyme comprised of four nuclear encoded subunits, A, B, C, and D; mutations in subunits B, C, and D have been associated with resistance to SDHI fungicides in ascomycete fungi, including Alternaria alternata, Botrytis cinerea, and
Corynespora cassiicola (Avenot and Michailides 2010). Initially, SDHI fungicides were mostly active against basidiomycete fungi; nonetheless, in 2003, the SDHI boscalid showed activity against ascomycete fungi by inhibiting growth of Botrytis spp., Sclerotinia spp., and Alternaria
spp (Avenot and Michailides 2007). Many other SDHI fungicides have since been registered with activity against economically important ascomycete fungi. Interestingly, the SDHI fluopyram has been reported to also have activity against nematodes (Faske and Hurd 2015).
SDHI fungicides have all been so far classified into FRAC code 7. Chemically, these fungicides contain an amide bond, and are classified by the moiety on the carbonyl side of the bond, although the linker and hydrophobic rest are also variable (Sierotzki and Scalliet 2013). Although SDHIs are classified together by their mode of action, there are different cross-resistance patterns between different SDHI fungicides (Scalliet et al. 2012). For example,
resistant to fluopyram, fluxapyroxad, or penthiopyrad (Avenot et al. 2014). There are currently no SDHIs labeled for Fusarium oxysporum in the US (EPA 2016). One study examined the response of both Fusarium graminearum and Zymoseptoria tritici to isopyrazam and found several mutations that could explain differential fungicide responses in Zymoseptoria tritici
(Dubos et al. 2013). However, all F. graminearum isolates were insensitive to isopyrazam, and no amino acid substitutions among 40 isolates sequenced explained differential fungicide response (Dubos et al. 2013).
Disease control options for watermelon Fusarium wilt in the US are limited, only one fungicide is labeled, and there is little knowledge of fungicide efficacy and the occurrence of fungicide resistance in FON populations. To address this knowledge gap and provide insight regarding fungicide resistance in FON we aimed to: i) evaluate fungicides in vitro for their ability to inhibit mycelium growth of Fusarium oxysporum f. sp. niveum; ii) test the DMI prothioconazole and the SDHI pydiflumetofen against a diverse panel of F, oxysporum isolates from the US for inhibition of mycelium growth; iii) confirm the efficacy of prothioconazole and pydiflumetofen on watermelon Fusarium wilt in field experiments; and iv) determine the genetic diversity and occurrence of mutations conferring fungicide resistance in the succinate
dehydrogenase B and C genes of Fusarium oxysporum isolates.
METHODS
Isolate collection and long-term storage
surface-sterilized in a 1.85% NaClO solution for 5 minutes. The tissue was rinsed in sterile distilled water (SDW) and stem cross-sections were plated on Fusarium spp. selective Nash and Snyder media (Nash and Snyder 1962). Grown agar plugs were transferred to potato dextrose agar (PDA) (39 g PDA per 1 L of water, HiMedia, Mumbai, India) amended with 100 µg/mL ampicillin and 100 µg/mL rifampicin to remove bacterial contaminants. Isolates were single-spored by flooding plates with 1 mL of SDW, spreading 10 µL of the conidia suspension mixed with 90 µL of SDWon a water agar plate (10 g Select Agar per 1 L of water, BD Diagnostics, Sparks, MD), and transferring single germinating conidia after 12 hours onto PDA plates. Single-spored isolates were stored with two methods. In the first method, 2-4 agar plugs of monoconidial isolates were placed in 2 mL microcentrifuge tubes with 1mL of SDW and 2-3 sterilized hemp seeds, and these were stored in a refrigerator at 4˚C. These isolates were used as
temporary stocks, and were used in the experiments described here. In the other method, 2 agar plugs were placed in 2 mL cryotubes (Corning, NY) with 500 µL of potato dextrose broth (PDB, HiMedia). These were set at room temperature on an Advanced Digital Shaker (VWR
International, Radnor, PA) at 100 rpm for 2 days/48 hours, and then 500 µL sterile 1:1 glycerol: SDW was added to the tube, and these were stored at -80˚C for long term preservation of the isolates. Other isolates were kindly provided by collaborators (Table 2). These were single-spored and stored in the same conditions described.
Identification of fungicides with activity against FON isolates in vitro
ingredient (a. i.) pydiflumetophen, Syngenta Crop Protection, Greensboro, NC), Endura (boscalid; BASF Corporation, Research Triangle Park, NC), Folicur (a.i. tebuconazole; Bayer CropScience LP, Research Triangle Park, NC), Fontelis (a.i. penthiopyrad; Dupont Crop Protection, Wilmington, DE), Inspire (a.i. difenoconazole; Syngenta Crop Protection), Luna Privilege (a.i. fluopyram; Bayer CropScience), Mertect (a.i. thiabendazole; Syngenta Crop Protection), Quadris (a.i. azoxystrobin; Syngenta Crop Protection), Proline (a.i. prothioconazole; Bayer CropScience), and Tilt (a.i. propiconazole; Syngenta Crop Protection) (Table 3). Isolate plugs were taken from temporary storage and plated on PDA. After 7 days, 6 cm plugs were taken from the growing colony edge and plated on media amended with a single fungicide. Fungicide formulated products were diluted in water and added to autoclaved PDA media cooled to approximately 50˚C at 1 mL per 1 L volume to yield a final concentration of 10, 1, 0.1, and
0.01 µg active ingredient /mL media. SDW was added in the same volume to control plates. After 7 days of growing at 25˚C with a 12-hour light/dark cycle, mycelium growth of 10 FON isolates was measured twice at a perpendicular angle. Three technical replicates were used and the experiment was repeated once per isolate.
In vitro fungicide efficacy data for each concentration was divided by the average control treatment for that isolate and replication to yield relative growth at each fungicide concentration. Relative growth for each fungicide was analyzed as a four parameter log-logistic model with the R package “drc” (R Core Team 2016; Ritz and Streibig 2005). The model utilized was y = min
+ (max-min)/(1+10(Hillslope(log(x)-log(EC50)))), where “y” is the mycelium growth at “x” fungicide
parameter about the EC50 (Ritz and Streibig 2005). EC50s for each fungicide were analyzed by ANOVA and compared pairwise with a Bonferroni correction in Microsoft Excel (Excel for Mac 2011 Version 14.6.7).
Evaluation of Fusarium isolates for fungicide sensitivity in vitro
Since pydiflumetofen and prothioconazole showed mycelium growth inhibition in the first experiment, they were selected for evaluation in an expanded panel of 98 Fusarium spp. isolates, including 68 FON, 21 F. oxysporum f. sp. lycopersici (FOL), 4 F. oxysporum f. sp.
radicis-lycopersici (FORL), and 3 F. oxysporum isolates. There was one Fusarium proliferatum
isolate and one F. solani isolate also included in this panel. The fungicide concentrations in this study were 10, 1, 0.1, and 0.01 µg/mL active ingredient, and the experiment was conducted as described in the previous section. Calculations were done as previously described, but
fungicide*isolate combination factor was used as the grouping variable. EC50 values of prothioconazole and pydiflumetofen were plotted for each isolate, and a linear regression was calculated in Microsoft Excel.
Confirmation of fungicide activity against FON in field experiments
strength potato dextrose broth in 1L plastic flasks. Mycelium plugs were added to the broth, and they were grown in the dark on an Advanced Digital Shaker set at 200 rpm for 14 days. The solutions were filtered through four layers of sterile cheesecloth to remove mycelia, and spore concentration was calculated using a hemocytometer. Conidia suspensions were diluted in water to standard concentrations that differed for individual inoculation procedures. Plants were inoculated immediately before transplant by dipping the tray in a 107 conidia per mL solution for
20 minutes. Treatments and rates used are described in Table 4, and drench applications were performed by pouring 100 mL fungicide solution to the base of each plant. Non-treated control plots were inoculated with FON, and water was used in place of the fungicide. Additional FON inoculations were done 10 days after transplant by drenching the base of seedlings with a 100 mL suspension of 106 conidia/mL. Fungicide sprays were applied 14 days after transplant with a
CO2 pressurized backpack sprayer equipped with a single nozzle handheld boom with hollow
cone nozzles (TXVS-26) delivering 374.76 L/ha at 310 kPa with one pass per plot. Incidence and severity ratings were taken weekly for both trials, and yield ratings were taken on the final rating date in 2016 by counting and weighing the fruit per plot. Area under the disease progress curve (AUDPC) was calculated with trapezoidal integration for the 2015 and 2016 field disease incidence data and for severity data for 2016 using the R package “agricolae” (de Mendiburu
2016) and was normalized by dividing the AUDPC by the number of rating dates. These were analyzed as a generalized linear mixed model with PROC GLIMMIX in SAS with Tukey’s HSD
Genetic diversity and succinate dehydrogenase gene sequence analysis
Monoconidial Fusarium oxysporum cultures were grown from an agar plug in Potato Dextrose Broth on an Advanced Digital Shaker set at 150 rpm. The agar plug was removed, and mycelium was washed in SDW and vacuum filtrated to remove the excess media. The mycelium was placed in 2 mL microcentrifuge tubes with 2-3 2mm glass beads (Sigma Aldrich, St. Louis, MO) and lyophilized for 48 hours in a FreeZone 1 lyophilizer (LabConco, Kansas City, MO). The lyophilized mycelium was broken down with a Bead Ruptor 24 (Omni International,
Kennesaw, GA) for 5 seconds on speed 6 for two cycles with a rest period of 5 seconds between cycles. DNA was extracted with a modified published protocol where ethanol washes were added to purify the DNA (Ahmed et al. 2009). DNA samples were quantified with a NanoDrop Lite (Thermo Scientific, Waltham, MA, USA) and diluted to 50 ng/µL. DNA samples were stored at -20˚C.
Polymerase chain reactions (PCR) were performed in a T100 Thermal Cycler (Bio-Rad, Hercules, CA). Each reaction well contained 5 µL 2x GoTaq green master mix (Promega, Durham, NC), 3.5 µL filter-sterilized double distilled water, 0.5 µL DNA, and 0.5µL each forward and reverse primer for each locus diluted to 10µM. Primers ITS4 and ITS5 were used for the internal transcribed spacer (ITS) PCR reaction (White et al. 1990). The thermal cycler was set to 94˚C for 3 minutes, and there were 30 cycles of denaturation at 94˚C for 30 seconds, annealing at 50˚C for 30 seconds, and then elongation at 72˚C for 60 seconds. The final
and thermal cycling protocol, except the annealing temperature was set to 53˚C. For both PCR protocols, the samples were held at 4˚C until the samples were removed and stored at -20˚C.
Primer sequences from Fusarium graminearum for the sdhB and sdhC genes were obtained from previous literature (Dubos et al. 2013). The gene sequence and annotation for
sdhB and sdhC from F. graminearum and Zymoseptoria tritici were downloaded from GenBank. Additional sequences from Alternaria alternata, Botrytis cinerea, and Mycosphaerella
graminicola were utilized as reference sequences (Scalliet et al. 2012; Avenot, Sellam, and Michailides 2009; Staats and van Kan 2012; Yin, Kim, and Xiao 2011). These were compared with publicly available F. oxysporum genomic data from the Broad Institute with BLASTN alignments (E=1e-10) (Ma et al. 2010; Cuomo et al. 2007). Primers were designed from the F. oxysporum f. sp. lycopersici genome with Primer3 to flank the Iron-Sulfur binding units of sdhB
and the ubiquinone-binding domain of sdhC (Untergasser et al. 2012; Ma et al. 2010). The primers used in this study are in Table 5.
The PCR reagents were the same as in ITS for amplification of sdhB and sdhC, except for the respective primers for each reaction. For amplification of sdhC, the initial temperature of the PCR was set to 95˚C for 3 minutes. There were 35 cycles of denaturation at 95˚C for 30 seconds, annealing at 50˚C for 30 seconds, and extension at 72˚C for 60 seconds. The thermal cycler had a final extension at 72˚C for five minutes and then an infinite hold at 4˚C. The PCR
products were stored at -20˚C until further use. A touchdown PCR protocol was used for amplification of sdhB. The samples were heated to 95˚C for 3 minutes, followed by a cycle of
constant, until the annealing temperature reached 53˚C. The cycle with the annealing
temperature of 53˚C was repeated 25 times. There was a final extension of 5 minutes at 72˚C,
followed by an infinite hold at 4˚C. PCR products were run on a 1% agarose gel amended with Ethidium Bromide at 90 volts, and bands were analyzed with Quantity One 1-D Analysis software (Bio-Rad, Hercules, CA, USA).
Five µL of PCR product was combined with 2 µL of ExoSAP-IT (Affymetrix, Santa Clara, CA). Samples were placed in a thermal cycler, which was set to 37˚C for 15 minutes to degrade excess nucleotides and primers, and then set to 80˚C for 15 minutes to inactivate the
ExoSAP-IT reagent. 1 µL of the purified PCR product was combined with 1 µL 10µM forward primer and 10 mL filter sterilized double distilled water for sequencing at the Genomic Sciences Laboratory at North Carolina State University (Raleigh, NC). Sequencing reactions were done with a LifeTech 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA). Electropherograms were visualized using FinchTV software (Geospiza, Inc. Seattle, WA), and files were trimmed manually to remove low quality base calls. ITS and TEF PCR product sequences were compared with GenBank using BLAST for identity. ITS sequences for 71 isolates were aligned with a MUSCLE alignment in Geneious version 8.1.6, and the alignments were trimmed so that they were of equal length (Kearse et al. 2012). The same was done for the TEF sequence, and ITS and TEF sequences were concatenated for each isolate. A Maximum Likelihood consensus tree was inferred utilizing Tamura-Nei’s genetic distance in MEGA7 (Kumar, Stecher, and Tamura 2016; Tamura and Nei 1993). The tree was bootstrapped 1,000 times.
8.1.6 with the 6-frame translation function (Kearse et al. 2012). The translations of were aligned with MUSCLE v3.8.31, and the sequences that matched the reference amino acid sequences were selected for analysis (Edgar 2004; Dubos et al. 2013). FON amino acid sequences were aligned with reference strains used to develop primers, and amino acid substitutions were noted for sdhB and sdhC. Amino acid distributions were visualized with WebLogo (Doerks et al. 2002).
RESULTS
Identification of fungicides with activity against FON isolates in vitro
There were significant differences for the EC50 values after the Bonferroni correction between penthiopyrad and every other fungicide, and boscalid and every other fungicide (Table 6, Table 7). These two fungicides had higher EC50 values than other fungicides, and did not inhibit mycelium growth for the FONisolates. Fluopyram also had an EC50 value above 1 µg/mL. Thiabendazole exhibited an EC50 value above 1 µg/mL with no significant restriction of mycelium growth. All other fungicides yielded EC50 values between 0.1 and 1 µg/mL of a. i., and there were no significant differences between these values after the Bonferroni correction. The FRAC 3 fungicides examined in this study had low EC50 values, but only prothioconazole is labeled in this pathosystem and was further evaluated in the diversity panel and field trials. Of the FRAC group 7 fungicides, only pydiflumetofen was able to restrict growth and thus further examined alongside prothioconazole.
there were differences between fungicides in the lower limit term, which models the relative growth at higher concentrations of fungicides. There were significant differences after the Bonferroni correction between penthiopyrad and each of prothioconazole, tebuconazole, and difenoconazole. There were also significant differences between azoxystrobin and
prothioconazole and tebuconazole. The other fungicides were not statistically different after the Bonferroni correction in terms of how much they were able to restrict growth at the highest concentrations.
Evaluation of Fusarium isolates for fungicide sensitivity in vitro
Out of 98 isolates tested, there were no isolates that were insensitive to prothioconazole at 10 µg/mL. Sensitivity, intermediate sensitivity, and resistance are defined here as <30%, 30-90%, and >90% relative growth compared to the control at a given fungicide concentration, respectively (Petkar et al. 2017). However, there were 25 isolates that were intermediately sensitive to pydiflumetofen at 10 µg/mL, and the rest were sensitive to pydiflumetofen at that concentration.