Laser-assisted in-situ keratomileusis (LASIK) versus
photorefractive keratectomy (PRK) for myopia (Review)
Shortt AJ, Allan BDS, Evans JR
This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published inThe Cochrane Library 2013, Issue 1
T A B L E O F C O N T E N T S 1 HEADER . . . . 1 ABSTRACT . . . . 2
PLAIN LANGUAGE SUMMARY . . . .
2
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . . . .
5 BACKGROUND . . . . 6 OBJECTIVES . . . . 6 METHODS . . . . 8 RESULTS . . . . Figure 1. . . 9 Figure 2. . . 10 13 DISCUSSION . . . . 15 AUTHORS’ CONCLUSIONS . . . . 15 ACKNOWLEDGEMENTS . . . . 16 REFERENCES . . . . 20 CHARACTERISTICS OF STUDIES . . . . 42 DATA AND ANALYSES . . . .
Analysis 1.1. Comparison 1 LASIK versus PRK, Outcome 1 UCVA of 20/15 or better at two to four weeks
post-treatment. . . 43
Analysis 1.2. Comparison 1 LASIK versus PRK, Outcome 2 UCVA of 20/15 or better at six months post-treatment. 44 Analysis 1.3. Comparison 1 LASIK versus PRK, Outcome 3 UCVA of 20/15 or better at twelve months post-treatment. 45 Analysis 1.4. Comparison 1 LASIK versus PRK, Outcome 4 UCVA of 20/20 or better at two to four weeks
post-treatment. . . 46
Analysis 1.5. Comparison 1 LASIK versus PRK, Outcome 5 UCVA of 20/20 or better at six months post-treatment. 47 Analysis 1.6. Comparison 1 LASIK versus PRK, Outcome 6 UCVA of 20/20 or better at 12 months post-treatment. 48 Analysis 1.7. Comparison 1 LASIK versus PRK, Outcome 7 Within 0.50 D of target refraction at two to four weeks
post-treatment. . . 49
Analysis 1.8. Comparison 1 LASIK versus PRK, Outcome 8 Within 0.50 D of target refraction at six months
post-treatment. . . 50
Analysis 1.9. Comparison 1 LASIK versus PRK, Outcome 9 Within 0.50 D of target refraction at 12 months
post-treatment. . . 51
Analysis 1.10. Comparison 1 LASIK versus PRK, Outcome 10 Mean postoperative spherical equivalent at two to four weeks post-treatment. . . 52 Analysis 1.11. Comparison 1 LASIK versus PRK, Outcome 11 Mean postoperative spherical equivalent at six months
post-treatment. . . 53
Analysis 1.12. Comparison 1 LASIK versus PRK, Outcome 12 Mean postoperative spherical equivalent at 12 months
post-treatment. . . 54
Analysis 1.13. Comparison 1 LASIK versus PRK, Outcome 13 Lost one or more lines of BCVA at six months or more
post-treatment. . . 55
Analysis 1.14. Comparison 1 LASIK versus PRK, Outcome 14 Lost two or more lines of BCVA at six months or more
post-treatment. . . 56
Analysis 1.15. Comparison 1 LASIK versus PRK, Outcome 15 Final BCVA of 20/40 or less at six months or more
post-treatment. . . 57 57 ADDITIONAL TABLES . . . . 67 APPENDICES . . . . 78 WHAT’S NEW . . . . 78 HISTORY . . . . 78 CONTRIBUTIONS OF AUTHORS . . . . 79 DECLARATIONS OF INTEREST . . . . 79 SOURCES OF SUPPORT . . . . 79
DIFFERENCES BETWEEN PROTOCOL AND REVIEW . . . .
80
[Intervention Review]
Laser-assisted in-situ keratomileusis (LASIK) versus
photorefractive keratectomy (PRK) for myopia
Alex J Shortt1, Bruce DS Allan2, Jennifer R Evans3
1The Moorfields Eye Hospital/UCL Institute of Ophthalmology National Institute for Health Research Biomedical Research Centre, London, UK.2External Disease Service, Moorfields Eye Hospital NHS Foundation Trust, London, UK.3Cochrane Eyes and Vision Group, ICEH, London School of Hygiene & Tropical Medicine, London, UK
Contact address: Alex J Shortt, The Moorfields Eye Hospital/UCL Institute of Ophthalmology National Institute for Health Research Biomedical Research Centre, 162 City Road, London, EC1V 2PD, UK.a.shortt@ucl.ac.uk.
Editorial group:Cochrane Eyes and Vision Group.
Publication status and date:New search for studies and content updated (no change to conclusions), published in Issue 1, 2013. Review content assessed as up-to-date: 15 November 2012.
Citation: Shortt AJ, Allan BDS, Evans JR. Laser-assisted in-situ keratomileusis (LASIK) versus photorefractive keratectomy (PRK) for myopia.Cochrane Database of Systematic Reviews2013, Issue 1. Art. No.: CD005135. DOI: 10.1002/14651858.CD005135.pub3.
Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
A B S T R A C T Background
Myopia (also known as short-sightedness or near-sightedness) is an ocular condition in which the refractive power of the eye is greater than is required, resulting in light from distant objects being focused in front of the retina instead of directly on it. The two most commonly used surgical techniques to permanently correct myopia are photorefractive keratectomy (PRK) and laser-assisted in-situ keratomileusis (LASIK).
Objectives
To compare the effectiveness and safety of LASIK and PRK for correction of myopia by examining post-treatment uncorrected visual acuity, refractive outcome, loss of best spectacle-corrected visual acuity, pain scores, flap complications in LASIK, subepithelial haze, adverse events, quality of life indices and higher order aberrations.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library2012, Issue 11), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2012), EMBASE (January 1980 to November 2012), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to November 2012), themetaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/ search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 15 November 2012. We also searched the reference lists of the studies and the Science Citation Index.
Selection criteria
We included randomised controlled trials comparing LASIK and PRK for the correction of any degree of myopia. Data collection and analysis
Two authors independently assessed trial quality and extracted data. We summarised data using the odds ratio and mean difference. We combined odds ratios using a random-effects model after testing for heterogeneity.
Main results
We included 13 trials (1135 participants, 1923 eyes) in this review. Nine of these trials randomised eyes to treatment, two trials randomised people to treatment and treated both eyes, and two trials randomised people to treatment and treated one eye. None of the paired trials reported an appropriate paired analysis. We considered the overall quality of evidence to be low for most outcomes because of the risk of bias in the included trials. There was evidence that LASIK gives a faster visual recovery than PRK and is a less painful technique. Results at one year after surgery were comparable: most analyses favoured LASIK but they were not statistically significant. Authors’ conclusions
LASIK gives a faster visual recovery and is a less painful technique than PRK. The two techniques appear to give similar outcomes one year after surgery. Further trials using contemporary techniques are required to determine whether LASIK and PRK as currently practised are equally safe. Randomising eyes to treatment is an efficient design, but only if analysed properly. In future trials, more efforts could be made to mask the assessment of outcome.
P L A I N L A N G U A G E S U M M A R Y
Laser-assisted in-situ keratomileusis (LASIK) compared to photorefractive keratectomy (PRK) for correcting short-sightedness Myopia is the term used to describe short or near-sightedness, which means that you cannot see objects in the distance clearly. Most people with myopia wear spectacles or contact lenses. Glasses can be uncomfortable and are not practical for sport; contact lenses can be associated with corneal infections. For these reasons, some people choose to have surgery for myopia. Two commonly used surgical techniques are LASIK and PRK. Both these procedures use laser to remove corneal tissue and reshape the cornea. This review analyses the results from 13 clinical trials where 1923 eyes of 1135 participants were randomly treated with either LASIK or PRK. We considered the overall quality of evidence from these studies to be low. There was some evidence that LASIK gives a faster visual recovery than PRK, and is a less painful technique, although visual results one year after surgery were comparable. Surgical techniques are improving all the time and further trials are needed to see whether LASIK and PRK, as currently practised, are equally safe.
S U M M A R Y O F F I N D I N G S F O R T H E M A I N C O M P A R I S O N [ E xp la n a ti on ] L a se r-a ssi st e d in -si tu ke ra to m il e u si s (L A S IK ) ve rsu s p h o to re fr a ct iv e ke ra te ct o m y (P R K ) fo r m yo p ia P a ti e n t o r p o p u la ti o n : pa tien ts w ith m yo pi a S e tt in g s: In te rv e n ti o n : LA S IK C o m p a ri so n : P R K O u tc o m e s Il lu st ra ti ve co m p a ra ti ve ri sk s* (9 5 % C I) R e la ti ve e ff e ct (9 5 % C I) N o o f p a rt ic ip a n ts (st u d ie s) Q u a li ty o f th e e vi d e n ce (G R A D E ) C o m m e n ts A ssu m e d ri sk 1 C o rr e sp o n d in g ri sk P R K L A S IK U C V A o f 2 0 /2 0 o r b e tt e r Fo llo w -u p: 12 m on th s 6 0 0 p e r 1 0 0 0 7 1 1 p e r 1 0 0 0 (6 23 to 78 6) O R 1 .6 4 (1 .1 0 to 2. 45 ) 10 07 (7 st ud ies ) ⊕⊕ lo w 2 Ex cl ud in g 2 st ud ies at hi gh ris k of sel ec tio n bi as ga ve an O R of 1. 39 (0 .6 5 to 3. 00 ) W it h in 0 .5 0 D o f ta rg e t re fr a ct io n Fo llo w -u p: 12 m on th s 7 5 0 p e r 1 0 0 0 8 0 9 p e r 1 0 0 0 (7 48 to 86 3) O R 1 .4 5 (0 .9 9 to 2. 10 ) 10 07 (7 st ud ies ) ⊕⊕ lo w 2 Ex cl ud in g 2 st ud ies at hi gh ris k of sel ec tio n bi as ga ve an O R of 1. 33 (0 .9 0 to 1. 96 ) P o st o p e ra ti ve sp h e ri ca l e q u iv a le n t Fo llo w -u p: 12 m on th s Th e m ea n po st op er at iv e sp her ic al eq ui va len ti n th e in ter ven tio n gr ou ps w as 0 h ig h e r (0 .0 6 lo w er to 0. 04 hi gh er ) 58 9 (6 st ud ies ) ⊕⊕ lo w 2 L o st o n e o r m o re li n e s o f B C V A Fo llo w -u p: 6 m on th s or m or e 1 0 0 p e r 1 0 0 0 8 9 p e r 1 0 0 0 (5 4 to 14 3) O R 0 .8 8 (0 .5 1 to 1. 50 ) 74 6 (6 st ud ies ) ⊕⊕ lo w 2
F in a l B C V A o f 2 0 /4 0 o r le ss Fo llo w -u p: 6 m on th s or m or e 1 0 p e r 1 0 0 0 1 p e r 1 0 0 0 (0 to 19 ) O R 0 .1 2 (0 .0 1 to 1. 93 ) 44 2 (6 st ud ies ) ⊕ ve ry lo w 2 , 3 P a in sc o re s S ee co m m en t N ot es tim ab le 0 (3) S ee co m m en t 3 st ud ies rep or ted pa in sc or es ;s ig ni fic an tly m or e pa in ex per ien ced in th e P R K gr ou p *T he ba si s fo r th e a ssu m e d ri sk (e. g. th e m ed ia n co nt ro l gr ou p ris k ac ro ss st ud ies ) is pr ov id ed in fo ot no tes . Th e co rr e sp o n d in g ri sk (a nd its 95 % co nf id en ce in ter va l) is ba sed on th e as su m ed ris k in th e co m pa ris on gr ou p an d th e re la ti ve e ff e ct of th e in ter ven tio n (a nd its 95 % C I) . B C V A : bes ts pec ta cl e-co rr ec ted vi su al ac ui ty ; C I: co nf id en ce in ter va l; D : di op tr es ; R R : ris k ra tio ; O R : od ds ra tio ; U C V A : un co rr ec ted vi su al ac ui ty G R A D E W or ki ng G ro up gr ad es of ev id en ce H ig h q u a li ty : Fu rt her res ea rc h is ver y un lik el y to ch an ge ou r co nf id en ce in th e es tim at e of ef fec t. M o d e ra te q u a li ty : Fu rt her res ea rc h is lik el y to ha ve an im po rt an t im pa ct on ou r co nf id en ce in th e es tim at e of ef fec t an d m ay ch an ge th e es tim at e. L o w q u a li ty : Fu rt her res ea rc h is ver y lik el y to ha ve an im po rt an t im pa ct on ou r co nf id en ce in th e es tim at e of ef fec t an d is lik el y to ch an ge th e es tim at e. V e ry lo w q u a li ty : W e ar e ver y un cer ta in ab ou t th e es tim at e. 1M ed ia n ris k in P R K gr ou p ac ro ss st ud ies . 2N on e of th e tr ia ls w er e m as ked an d so w er e co ns id er ed to be at ris k of per fo rm an ce an d det ec tio n bi as ; in tw o tr ia ls al lo ca tio n w as no t pr op er ly co nc ea led an d th er ef or e th ey w er e at ris k of sel ec tio n bi as . 3O nl y tw o ev en ts , bo th ob ser ved in on e st ud y.
B A C K G R O U N D
Description of the condition
Myopia (also known as short-sightedness or near-sightedness) is an ocular condition in which the refractive power of the eye is greater than is required. The main determinants of refraction are the focusing power of the cornea and crystalline lens and the length of the eye. In myopia light from distant objects is focused in front of the retina instead of on it. This occurs because the corneal curvature is too strong or the eye is too long. As a result objects in the distance appear blurred. Near objects appear less blurred or may be seen clearly depending on the degree of myopia. People with myopia can be classified into two groups, those with low to moderate myopia (0 to < -6 dioptres) and those with moderate to high myopia (greater than -6 dioptres) (Sugar 2002).
The prevalence of myopia varies with age, country, ethnic group, level of education and occupation. The prevalence of myopia in Western populations is estimated to be approximately 25% (Kempen 2004; Sorsby 1960; Sperduto 1983). In some Asian populations myopia prevalence is as high as 70% to 90% (Chow 1990;Wong 2000). According to epidemiological evidence the prevalence of myopia is increasing, especially in Asian populations (Rajan 1995;Tay 1992). Most cases of myopia present in chil-dren of school age and young adults. The presenting complaint is difficulty reading objects at a distance and diagnosis is based on the results of refraction (spectacle testing). The exact cause of myopia is not yet clear, however there is substantial evidence that both genetic and environmental factors play a role in its aetiology (Fredrick 2002;Mutti 1996).
Description of the intervention
The most commonly used methods for correcting myopia are spec-tacle correction and contact lens wear. These conservative optical methods provide temporary correction. They each have functional limitations such as the problems encountered in wearing spectacles when showering or playing sports and the inconvenience of car-rying contact lens solutions and storage containers, or obtaining them in the event of unforeseen circumstances. Wearing contact lenses is not without risk as it has been shown to increase the risk of sight-threatening corneal infection (Dart 1998;Foulks 2006). Surgical procedures have been developed in an attempt to perma-nently correct myopia. These procedures involve an operation on either the cornea (corneal refractive procedures) or lens of the eye (lenticular refractive procedures) and work by reducing the focus-ing power of the cornea or lens respectively.
Corneal refractive procedures used to correct myopia include: • excimer laser refractive surgery: this is divided into two main procedure groups, ’surface treatments’ and ’flap treatments’;
◦ In surface treatments, the skin on the surface of the cornea is removed by physical scraping or peeling and the laser is
applied to the surface of the main body of the cornea, known as the stroma. The laser corrects the shape of the corneal stroma and therefore abolishes myopia. The surface skin can be left to heal naturally with the aid of a contact lens (as in PRK) or the removed dead skin can be replaced and may act like a bandage whilst new skin regenerates below it, as in laser epithelial keratomileusis (LASEK) or epipolis (Greek for surface) LASIK which is also known as EpiLASIK. All of these are categorised as surface treatments.
◦ Flap treatments use a blade or a femtosecond laser to cut a thin flap on the surface of the cornea. This flap is peeled back and the excimer laser is applied within the body of the corneal stroma. The flap is replaced at the end of the procedure. This flap treatment is called laser-assisted in-situ keratomileusis (LASIK). A recent variant of LASIK is sub-Bowmans
keratomileusis (SBK) which differs from LASIK only in that the thickness of the flap is substantially less. Hence SBK is also referred to as ’thin-flap LASIK’.
• incisional procedures: radial keratotomy or astigmatic keratotomy (a blade is used to make cuts in the cornea to alter its shape);
• tissue and synthetic implants: epikeratophakia,
keratophakia and intracorneal rings (in these techniques human corneal tissue or synthetic devices are inserted into the cornea to change its shape).
Lenticular refractive procedures used to correct myopia include: • clear lens extraction with or without intraocular lens insertion (this operation is identical to cataract surgery and is also called refractive lens exchange);
• phakic intraocular lens insertion.
Why it is important to do this review
The two most commonly used surgical techniques to correct my-opia are PRK and LASIK. Both these techniques use the oph-thalmic excimer laser to remove corneal tissue and reshape the cornea thus reducing its refractive power. The number of PRK and LASIK procedures being performed has rapidly increased over the last 20 years (Leaming 2004). These interventions are performed on healthy eyes and the vast majority of patients are under 60 years of age. It is important that patients are informed about and un-derstand the effectiveness, limitations, safety, complications and relative merits of these procedures.
PRK was the first technique to employ the ophthalmic excimer laser for correction of myopia (Epstein 1994; Goodman 1989; Munnerlyn 1988). Later Pallikaris et al described laser in-situ keratomileusis, which is now widely known as LASIK (Pallikaris 1990;Pallikaris 1991). PRK gained FDA approval before LASIK and was initially more widely performed; but LASIK uptake grew rapidly in the late 1990s and LASIK quickly became the dominant method of laser refractive correction for myopia. This shift was
not based on any clear evidence of a superior visual outcome for LASIK but rather upon other factors such as those summarised in reviews bySugar 2002andSutton 2010:
• earlier post-treatment stabilisation of visual acuity; • less post-treatment patient discomfort;
• faster improvement in visual acuity;
• possibly improved predictability and stability; • less stromal haze formation;
• easier enhancement procedure.
The most feared outcome of either procedure is loss of vision, specifically loss of best-corrected vision. In PRK this is most likely to occur due to corneal haze (an inflammatory reaction of the cornea to treatment). Mitomycin C (MMC) is a chemotherapy agent which when applied to the cornea following PRK may re-duce the risk of this complication in high-risk patients (Lee 2005). Corneal haze is rare following LASIK (SUMMIT 1998) but com-plications arising from the creation of a flap can result in loss of best-corrected vision (Gimbel 1998;Lin 1999;Stulting 1999; Sugar 2002). When managed appropriately the refractive and vi-sual outcomes following flap complications are comparable to those in uncomplicated cases (Ito 2004;Sharma 2005). Corneal ectasia, a distortion of the shape of the cornea, is another rare but potentially serious complication of LASIK (Chuck 2008;Pallikaris 2001; Randleman 2003;Sugar 2002). Careful screening of pa-tients preoperatively for risk factors such as high myopia, forme fruste keratoconus and low residual stromal thickness post-treat-ment is important to minimise the risk (Randleman 2003). There have been concerns about the long-term stability of laser refrac-tive surgery. While individual cases of regression (the eye becom-ing long- or short-sighted again followbecom-ing an initially successful treatment) can occur, data from studies demonstrates that overall the outcome of both LASIK and PRK remains stable in the long term (Dirani 2010;O’Doherty 2006;Rajan 2004;Sekundo 2003; Stephenson 1998).
The initial version of this Cochrane review (Shortt 2006a) was the first systematic review comparing LASIK and PRK. The data available at that time confirmed that:
• visual recovery is faster following LASIK than PRK; • final uncorrected visual acuity may be superior following LASIK but the result was sensitive to exclusion of a large study which was at high risk of bias;
• there is no difference in post-treatment refraction (accuracy) between LASIK and PRK;
• LASIK is safer in that it resulted in fewer eyes losing 2 or more lines of visual acuity than PRK.
Since this review was first published there have been two major changes in the way LASIK and PRK are performed. Firstly, con-temporary PRK and LASIK treatments are invariably wavefront-guided whereas the studies included in the original review were not. This means that the laser uses a more detailed set of data about an eye to create a customised pattern of laser treatment specifically
tailored to that eye. In theory this should result in more accurate treatments and superior outcomes although the results of studies are inconsistent. A recent meta-analysis showed no clear evidence of a benefit of wavefront-guided over non-wavefront-guided abla-tions (Fares 2011).
The second major shift in practice is in the method of flap creation in LASIK which can be achieved using a mechanical microker-atome or the more recently developed femtosecond laser. The rate of intraoperative flap complications using a mechanical microker-atome is approximately 4% (Gimbel 1998;Lin 1999; Stulting 1999;Sugar 2002) and for the femtosecond laser is approximately 3% (Moshirfar 2010). The thickness of a microkeratome LASIK flap is in the order of 150 to 180 microns whereas a femtosecond laser flap is approximately 90 to 110 microns and is more pre-dictable (Binder 2004;Slade 2008;Sutton 2010). It is postulated that as a result the biomechanical properties of the cornea following SBK are equivalent to those following PRK (Dawson 2008). Fem-tosecond lasers also cause less epithelial injury (Moshirfar 2010). There is some evidence that femtosecond laser flaps result in better uncorrected distance visual acuity (UDVA) postoperatively than mechanical microkeratomes (Durrie 2005;Tran 2005), however a recent randomised controlled trial (RCT) comparing microker-atome and femtosecond laser flap creation for LASIK did not show any difference in efficacy, accuracy and safety measures in the early and mid-term follow-up, although it was found that femtosecond flaps may induce fewer aberrations (Zhang 2011).
This updated version of the review includes data from trials that use these newer technologies and re-evaluates the evidence for the relative efficacy and safety of these procedures.
O B J E C T I V E S
To compare laser-assisted in-situ keratomileusis (LASIK) versus photorefractive keratectomy (PRK) for the correction of myopia by examining post-treatment uncorrected visual acuity, refractive outcome, loss of best spectacle-corrected visual acuity, pain scores, flap complications in LASIK, subepithelial haze, adverse events, quality of life indices and higher order aberrations.
M E T H O D S
Criteria for considering studies for this review
Types of studies
Types of participants
We only considered trials in which the participants were men and women over 18 years of age and under 60 years of age undergo-ing laser-assisted in-situ keratomileusis (LASIK) or photorefrac-tive keratectomy (PRK) for any degree of myopia. Sub-Bowmans keratomileusis (SBK) is considered as sufficiently similar to LASIK that data from trials comparing SBK and PRK are included. Par-ticipants under 18 years of age were excluded due to the frequent change in refractive error still occurring in this age group. Partic-ipants over 60 years of age were excluded on the basis that some degree of cataract is observed in many of these people and corneal refractive procedures will not correct aberrations or reduced visual acuity caused by cataract.
As most people with myopia have some degree of astigmatism this review included individuals with up to 3 dioptres (D) of myopic astigmatism. People undergoing treatment for correction of refrac-tive errors other than primary myopia, for example post corneal graft, were excluded, as were people with any other co-existing ocular disease or any systemic disease that is associated with ab-normal or impaired wound healing.
Types of interventions
We included studies in which LASIK (including SBK) was com-pared with PRK for correction of myopia.
Types of outcome measures
See the ’Differences between protocol and review’ section for sum-mary of, and justification for, changes to outcome measures for this update.
Effectiveness measures
At two to four weeks, six months and 12 months:
• Proportion of eyes with uncorrected visual acuity (UCVA) ◦ 20/15 or better
◦ 20/20 or better
• Proportion of eyes within ±0.50 D of target refraction • Mean postoperative spherical equivalent
Safety measures
At six months or more after treatment, proportion of eyes: • lost 1 or more lines of best spectacle-corrected visual acuity (BCVA)
• lost 2 or more lines of BCVA • with final BCVA of 20/40 or worse
Adverse effects • Refractive stability • Pain scores • Subepithelial haze
• Flap-related complications in LASIK eyes • Optical side effects
• Higher order aberrations
Quality of life measures
Any standardised quality of life measured such as the Refractive Status and Vision Profile (RSVP) or National Eye Institute Re-fractive Quality of Life (NEI-RQL)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Tri-als (CENTRAL) 2012, Issue 11, part ofThe Cochrane Library. www.thecochranelibrary.com(accessed 15 November 2012), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-In-dexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2012), EMBASE (January 1980 to November 2012), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to November 2012), themetaRegister of Controlled Trials (mRCT) ( www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) ( www.who.int/ictrp/search/en). We did not use any date or lan-guage restrictions in the electronic searches for trials. We last searched the electronic databases on 15 November 2012. See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4),mRCT (Appendix 5), ClinicalTrials.gov (Appendix 6), and the ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of the studies included in the review for information about further trials. We also searched the Science Citation Index to find studies that have cited the identified trials. We did not handsearch journals or conference proceedings for this review as considering the resources required this was not felt to be sufficiently likely to identify includable data.
Data collection and analysis
Two authors working independently assessed the titles and ab-stracts resulting from the searches. We obtained full-text copies of all potentially or definitely relevant studies. The two review au-thors assessed these full-text copies to determine whether they met the criteria for inclusion in the study.
Data extraction and management
The two authors independently extracted data using a form devel-oped by the Cochrane Eyes and Vision Group. The results were compared and we resolved any discrepancies by discussion. One author entered the data into RevMan (RevMan 2011) and the second author checked the data.
Where the method of randomisation was unclear or where data for outcomes were not available in the published study report we contacted the authors for clarification and additional data. The au-thors ofForseto 2000,Hjortdal 2005andSchallhorn 2009kindly provided such information and additional unpublished data.
Assessment of risk of bias in included studies
Two authors independently assessed risk of bias using the Cochrane Collaboration’s tool for assessing risk of bias according to Chapter 8 of theCochrane Handbook for Systematic Reviews of Interventions(Higgins 2011). Disagreements were resolved by dis-cussion. We contacted trial authors for clarification on any param-eter graded as ’unclear’.
Measures of treatment effect
We used the odds ratio as the measure of effect for dichotomous variables and the mean difference for continuous variables.
Unit of analysis issues
Ideally studies that randomly allocated eyes to treatment should report a paired analysis. In the event, none of the paired studies included in this review did the analysis appropriately. This meant that it was not possible to calculate the intra-class correlation coef-ficient and adjust the analyses accordingly (Elbourne 2002). The analyses therefore assume that the response of any eye to one treat-ment is not related to the response of the fellow eye. This is a con-servative assumption as, in the presence of correlation, adjusting for the pairing would have reduced the width of the confidence intervals.
Assessment of heterogeneity
We assessed heterogeneity by examining the graphs (forest plots) to see whether the direction of effect was similar in all studies and whether the confidence intervals for the individual study estimates overlapped. We also considered the I2statistic (Higgins 2003).
We took an I2statistic value of 50% or more to indicate substantial
inconsistency in study results such that a pooled result may not be informative.
Data synthesis
We pooled data using a random-effects model, unless fewer than three trials were available for analysis, or the number of events was low, in which case we used a fixed-effect model.
Subgroup analysis and investigation of heterogeneity We did two subgroup analyses. Firstly, we compared effects in studies that recruited people with low to moderate myopia com-pared to those that recruited people with moderate to high my-opia and secondly, we compared effects in studies conducted from 2008 onwards with those done prior to this as surgical techniques had improved (Table 1).
We did not explore heterogeneity further.
Sensitivity analysis
Two trials (Schallhorn 2009andWang 1997) had significant prob-lems with allocation concealment and we repeated relevant analy-ses excluding these trials.
R E S U L T S
Description of studies
See:Characteristics of included studies;Characteristics of excluded studies.
Results of the search
The original electronic searches performed in the first version of this review identified 949 reports up to 2005. There were six RCTs from this period that met the inclusion criteria (el Danasoury 1999;el Maghraby 1999;Forseto 2000;Hjortdal 2005;SUMMIT 1998;Wang 1997). An update search was run in November 2012 which yielded a total of 578 records. The Trials Search Co-ordi-nator scanned the search results and removed 336 records which were not relevant to the scope of the review. We assessed a total of 242 records against the inclusion criteria for the review. We obtained full-text copies of seven reports and all were suitable for inclusion in the review (Barreto 2010;Durrie 2008;Hatch 2011; Moshirfar 2010;Schallhorn 2009;Wallau 2008;Manche 2011). No ongoing studies or studies awaiting classification were identi-fied.
Included studies
Below is a summary of the included studies. Further details can be found in the ’Characteristics of included studies’ table.
Types of participants
Participants in all trials were men and women aged 18 or over with stable refraction for one year or more. Exclusion criteria included previous refractive or other ocular surgery, central corneal thick-ness of less than 490 microns by ultrasound pachymetry, kerato-conus or suspected keratokerato-conus on corneal topography, active oc-ular disease, dry eyes and systemic diseases likely to affect corneal wound healing (for example, connective tissue disease). The range of myopia treated varied between studies (Table 2).
Types of interventions
All eyes underwent LASIK or PRK as a day case procedure under topical anaesthesia.
The surgical procedure used to perform PRK was standard be-tween studies. Mitomycin C (MMC) was used as an adjunct to prevent post-PRK corneal haze in some contemporary studies. The LASIK technique varied widely. Flap creation was performed using a microkeratome in early studies whereas in contemporary studies this was performed using the femtosecond laser. The ex-cimer laser manufacturer varied between studies but not within studies. Treatment nomograms varied between studies as did the target refraction although in the majority of trials the target refrac-tion was emmetropia. From 2008 onwards, both PRK and LASIK were performed using wavefront-guided technology.Table 1 sum-marises the techniques used in each study.
Types of outcomes measures
Table 3summarises which trials reported which outcome mea-sures.
Unit of analysis
In two studies, participants were randomly assigned to LASIK or PRK and then received the same treatment in both eyes (Schallhorn 2009;Wang 1997). Two studies included only one eye of each patient in the study (Hjortdal 2005;SUMMIT 1998). InSUMMIT 1998the eye to be included was decided ad hoc by the principal investigator. InHjortdal 2005it is not clear how this decision was made. The remaining studies allocated one eye to LASIK and the fellow eye to PRK.Durrie 2008used a ran-domisation system which accounted for ocular dominance and en-sured an equal number of dominant eyes in each treatment group. Hatch 2011andManche 2011 also randomised the dominant eye to the first treatment.Wallau 2008randomised right eyes to the first treatment.el Danasoury 1999andel Maghraby 1999 both randomised the eye to be treated first and the procedure to be performed. In the remaining studies (Barreto 2010;Forseto 2000;Moshirfar 2010) it is unclear how the eye that was to be randomised was chosen.
Excluded studies
We excluded 19 studies and reasons for exclusion are provided in the ’Characteristics of excluded studies’ table.
Risk of bias in included studies
’Risk of bias’ assessment is summarised inFigure 1andFigure 2
Figure 1. ’Risk of bias’ graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 2. ’Risk of bias’ summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Sequence generation was adequate in almost all studies. InBarreto 2010andWang 1997it was not clear how eyes were randomised to treatments and inSchallhorn 2009participants were ranked by refractive error then assigned sequential numbers; odd numbers were assigned to one treatment and even numbers to the other.
Allocation concealment
Allocation concealment was clearly described in seven studies and not reported in three studies (Figure 2). Allocation was not con-cealed inSchallhorn 2009(as discussed above) andWang 1997. In Wang 1997a substantial number of people with eyes randomised to undergo LASIK refused this treatment and insisted on hav-ing PRK. The reason cited was that the patients could not afford LASIK.
Blinding
Blinding (masking) of participants and personnel (performance bias)
None of the studies attempted to mask, or reported efforts to mask, participants and personnel and so we considered all studies at high risk of performance bias.
Blinding (masking) of outcome assessment (detection bias) Masking of outcome assessment was reported in three studies (Hatch 2011;SUMMIT 1998;Wallau 2008). InHatch 2011the method of masking was not reported in detail but the study was described as masked and the following statement made:“After the study was completed, the results were compiled and the data unmasked for statistical analysis”which implies that the data were masked un-til the point of the statistical analysis. InSUMMIT 1998 “Preop-erative and follow-up visits included a detailed ophthalmologic exam-ination with manifest refraction by two independent observers at each visit.” and “All [corneal topography] maps were graded by two masked observers”.Wallau 2008made the most convincing efforts to mask visual acuity assessment“During follow-up examinations, a single examiner was unaware of which procedure was done in each eye and slit-lamp microscopy was always the last examination to be performed at each appointment”, however the report did not indicate whether or not this attempt to mask was successful.
We considered the masking of visual acuity separately to other out-comes but in general the assessment was the same. The exception wasSUMMIT 1998where it was specifically stated that some non visual acuity outcomes were masked but it was not clear whether or not visual acuity assessment was masked or not.
Incomplete outcome data
In general follow-up in the individual trials was good. Eight of the 12 included trials randomly allocated eyes to treatment. This meant that differential loss to follow-up was not possible therefore we graded most trials as being at low risk of attrition bias.
Selective reporting
See outcome reporting matrix (Table 3). In general the reasons why data were not reported were unclear, although not all studies reported all outcomes.
Effects of interventions
See:Summary of findings for the main comparison Laser-assisted in-situ keratomileusis (LASIK) versus photorefractive keratectomy (PRK) for myopia
Effectiveness measures
Uncorrected visual acuity (UCVA) 20/15 or better
Four trials reported UCVA 20/15 or better two to four weeks after treatment (n = 566) (Analysis 1.1). All four trials reported that more participants treated with laser-assisted in-situ keratomileusis (LASIK) achieved this very good vision compared to people treated with photorefractive keratectomy (PRK) (pooled odds ratio 5.89 95% confidence interval (CI) 3.34, 10.39). This pooled odds ratio corresponds to a risk ratio of 3.30 (95% CI 2.43 to 4.15) assuming a risk of 0.16 in the PRK group (based on median risk in the included studies).
Five trials reported UCVA 20/15 or better six months after treat-ment (n = 682) (Analysis 1.2). All five trials provided effect esti-mates consistent with LASIK or PRK being more effective (con-fidence intervals included 1) and the pooled odds ratio was 1.13 (95% CI 0.75 to 1.69). This pooled odds ratio corresponds to a risk ratio of 1.08 (95% CI 0.82 to 1.35) assuming a risk of 0.36 in the PRK group (based on median risk).
Two trials reported UCVA 20/15 or better at 12 months after treatment (n = 372) (Analysis 1.3). The pooled odds ratio was 1.08 (95% CI 0.58 to 2.00) The risk ratio was 1.01 (95% CI 0.91 to 1.07) assuming a control group risk of 0.87.
UCVA 20/20 or better
Eight trials reported UCVA 20/20 or better two to four weeks after treatment (n = 1079) (Analysis 1.4). All eight trials reported that more participants treated with LASIK achieved this very good vision compared to people treated with PRK (pooled odds ratio 3.69, 95% CI 2.55 to 5.36).This pooled odds ratio corresponds
to a risk ratio of 1.85 (95% CI 1.62 to 2.05) assuming a risk of 0.37 in the PRK group (based on median risk).
Ten trials reported UCVA 20/20 or better six months after treat-ment (n = 1113) (Analysis 1.5). Five trials found more people re-ceiving LASIK achieved 20/20 or better, four trials found more people receiving PRK achieved this good vision, and one trial found no difference. However, for nine out of 10 of these trials the confidence intervals included 1 and therefore the results were con-sistent with greater beneficial effect of either LASIK or PRK. The pooled odds ratio was 1.41 (95% CI 1.00 to 2.00). This pooled odds ratio corresponds to a risk ratio of 1.06 (95% CI 1 to 1.10) assuming a risk of 0.82 in the PRK group (based on median risk). Seven trials reported UCVA 20/20 or better at 12 months after treatment (n = 1007) (Analysis 1.6). Five out of these seven trials found in favour of LASIK and the pooled odds ratio was 1.64 (95% CI 1.10 to 2.45). This pooled odds ratio corresponds to a risk ratio of 1.17 (95% CI 1.04 to 1.29) assuming a risk of 0.63 in the PRK group (based on median risk).
±0.50 D of target refraction
Six trials reported whether or not participants were within 0.50 D of their target refraction two to four weeks after treatment (n = 455) (Analysis 1.7). There was substantial heterogeneity in the results (I2= 58%). Results ranged from an odds ratio of 0.26 in favour of PRK (Hjortdal 2005) to an odds ratio of 7.07 in favour of LASIK (Manche 2011).
Eight trials reported this outcome at six months after treatment (n = 567) (Analysis 1.8). Again individual study results were variable but there was no statistical evidence of inconsistency (I2= 0%) and the pooled odds ratio was 1.11 (95% CI 0.74 to 1.67). The corresponding risk ratio was 1.03 (95% CI 0.90 to 1.14) based on a median risk of 0.69 in the PRK group.
Seven trials reported achievement of target refraction 12 months after treatment (n = 1007 participants) (Analysis 1.9). Trial results varied from an odds ratio of 0.44 in favour of PRK (Hjortdal 2005) to an odds ratio of 3.76 in favour of LASIK (Schallhorn 2009). Five out of these seven trials found in favour of LASIK and the pooled odds ratio was 1.45 (95% CI 0.99 to 2.10). The corresponding risk ratio was 1.06 (95% CI 1 to 1.10) based on a median risk of 0.0.83 in the PRK group.
Mean spherical equivalent
Nine trials reported mean spherical equivalent two to four weeks after treatment (n = 1041) (Analysis 1.10). The results were in-consistent (I2= 83%) and a pooled value is not appropriate here. Differences ranged from -0.60 D to 0.14 D.
Nine trials reported mean spherical equivalent six months after treatment (n = 1024) (Analysis 1.11). Again the results were in-consistent (I2= 59%). Differences ranged from -0.26 D to 0.60 D.
Six trials reported mean spherical equivalent 12 months after treat-ment (n = 599) (Analysis 1.12). Results were consistent (I2= 0%) with a pooled mean difference of -0.01 (95% CI -0.06 to 0.04).
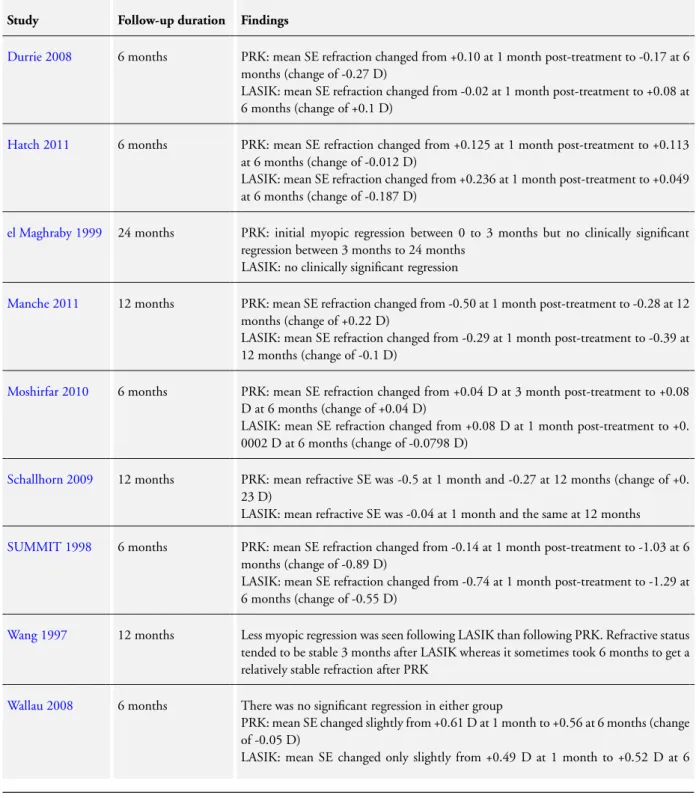
Stability of refraction
Nine studies examined refractive stability (Table 4). Only two of these studies reported a change in mean spherical equivalent refraction of more than 0.25 D over the study period.Durrie 2008 reported a change of -0.27 D in the PRK group between month one and six but no significant shift in the LASIK group.SUMMIT 1998found a change of -0.89 D in the PRK group and -0.55 D in the LASIK group between month one and six.
Safety measures
Lost 1 or more lines of best corrected visual acuity (BCVA) Six trials reported whether or not participants lost 1 or more lines of BCVA six or more months after treatment (n = 746) (Analysis 1.13). Two trials reported no events (Forseto 2000;Hatch 2011). For the other four trials there was no consistent pattern; the pooled Peto odds ratio was 0.88 (95% CI 0.51 to 1.50).
Lost 2 or more lines of BCVA
Eleven trials reported whether or not participants lost 2 or more lines of BCVA six or more months after treatment (n = 1446 ) (Analysis 1.14). Overall, 34 of these 1446 people (2.35%) treated with LASIK or PRK lost 2 or more lines of BCVA. Five trials reported no events (Durrie 2008; el Danasoury 1999; Forseto 2000;Hatch 2011;Manche 2011). For the other six trials, three trials found odds ratios close to 1 and three trials found odds ratios favouring LASIK. No individual trial found a statistically significant effect but the pooled Peto odds ratio favoured LASIK: 0.47 (95% CI 0.23 to 0.98).
Final BCVA of 20/40 or worse
Six trials reported final BCVA of 20/40 or worse six or more months after treatment (n = 442) (Analysis 1.15). There were only two events, both in the LASIK arm ofSUMMIT 1998.
Subgroup analyses
Two sets of subgroup analyses are presented (Appendix 8; Appendix 9).
InAppendix 8 the studies are divided up into those recruiting patients with different degrees of myopia. We divided studies into those treating low to moderate myopia (0 to -6 D) or moderate to
high myopia (- 6 D to -15 D). In general there was no strong sta-tistical evidence of any major differences in effect between LASIK and PRK at different levels of myopia. The exception was mean spherical equivalent at two to four weeks after treatment. There appeared to be little difference between LASIK and PRK in people with low myopia (0 to -6 D) but a pooled mean difference of -0.56 D in people with high myopia (-6 D to -15 D). However, only two trials contributed to the high myopia group and this difference in effect between subgroups was not evident at six or 12 months. InAppendix 9the studies are divided up into those conducted before 2008 and those conducted from 2008 onwards. There were some subgroup differences but no particular pattern to these. In some analyses, the two techniques were more similar - that is likely to show an pooled effect of around 1 for dichotomous outcomes, and 0 for continuous outcomes - in more recent studies (Analysis 1.5;Analysis 1.6;Analysis 1.10;Analysis 1.11;Analysis 1.12) and in other analyses LASIK/PRK appeared more different in more recent studies (Analysis 1.4;Analysis 1.7;Analysis 1.8;Analysis 1.9;Analysis 1.13;Analysis 1.14).
Sensitivity analyses
Due to concerns as to the potential high risk of bias inSchallhorn 2009andWang 1997we excluded these studies and repeated the relevant analyses (Appendix 10).
In general excluding these studies did not change the effect esti-mates although inAnalysis 1.6the effect became non-significant, probably as a result of lower numbers included in the analysis.
Adverse effects Pain scores
One study used questionnaires to assess intraoperative pain (el Danasoury 1999) and another two studies used questionnaires to assess postoperative pain (Durrie 2008;el Maghraby 1999). These found that intraoperative pain was less with PRK and that post-operative pain was less after LASIK. The findings are summarised inTable 5.
Subepithelial haze at six to 12 months post-PRK
Nine studies reported data for subepithelial haze post-PRK. These are summarised inTable 6.
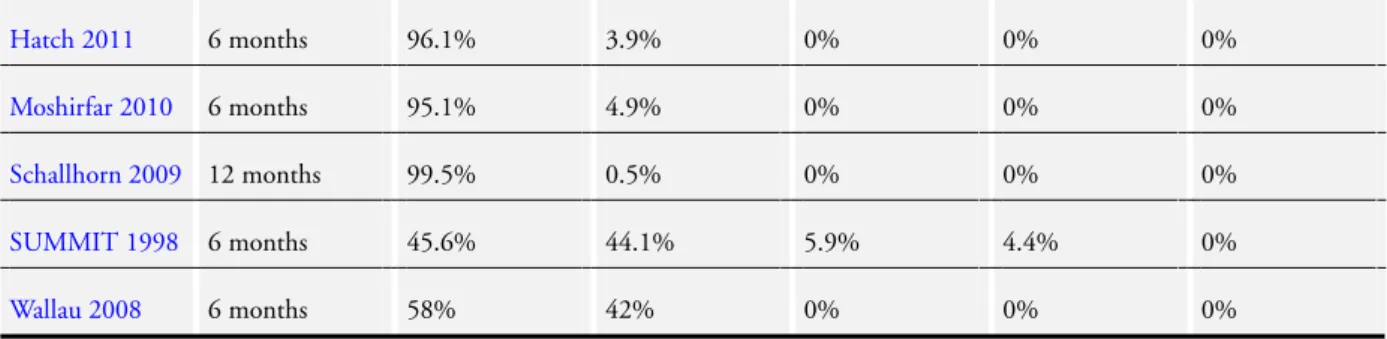
Flap-related complications in LASIK eyes
Six studies reported flap-related complications. The rate of inci-dence ranged from 0.7% to 15% but only one participant lost 2 or more lines of BCVA as a result of a flap complication. The overall rate of flap complications was 3.8%. These results are summarised inTable 7.
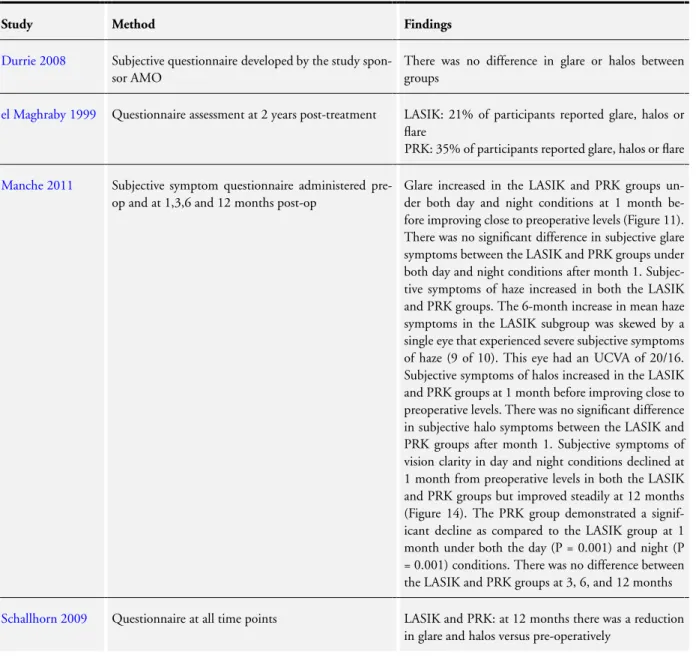
Optical side effect
Six studies examined optical side effects such as glare, halo and monocular diplopia. Some participants in both groups experi-enced optical side effects but onlyel Maghraby 1999found any dif-ference between interventions with symptoms arising more com-monly following PRK. Their findings are summarised inTable 8.
Higher order aberrations (HOAs)
Seven studies reported data for this outcome which is summarised inTable 9. All but one of these studies show that both LASIK and PRK result in a statistically significant increase in HOAs.The exception isDurrie 2008which found that HOAs were reduced in both PRK and LASIK compared with pre-operatively. When postoperative HOAs were compared between LASIK and PRK, only one study found a statistically significant difference, there being fewer HOAs in the LASIK group (Schallhorn 2009).
Quality of life measures
No studies reported data on quality of life.
D I S C U S S I O N Summary of main results
The principal findings of this updated review are as follows. • Visual recovery is faster following laser-assisted in-situ keratomileusis (LASIK) than photorefractive keratectomy (PRK).
• There is weak evidence that visual acuity at six months and 12 months may be superior with LASIK.
• There is little evidence to suggest any difference in accuracy between LASIK and PRK at 12 months post-treatment.
• There is little evidence to suggest any difference in safety between LASIK and PRK at six months or more post-treatment.
• Sub-analysis of data from studies from 2008 onwards that used modern techniques revealed no significant difference between the contemporary forms of these techniques for any of the efficacy or safety outcomes beyond the two to four-week time point.
In the previous version of this review we found that visual recovery is faster following LASIK than PRK, which is confirmed again in this update. We previously found that there was no clear differ-ence in the efficacy of the two procedures. The addition of data from seven new trials (Barreto 2010;Durrie 2008;Hatch 2011; Moshirfar 2010;Schallhorn 2009;Wallau 2008;Manche 2011)
has not altered these findings. The most significant finding of the inaugural review was that LASIK was safer than PRK. The addi-tional data has altered this finding and no strong evidence of a difference could be found.
Overall completeness and applicability of evidence
It is widely accepted that visual recovery following LASIK is more rapid than following PRK. This is again supported by the data presented in this updated review. Our subgroup analyses show that this benefit is present across all degrees of refractive errors and regardless of whether newer technologies such as femtosecond laser flap creation and wavefront-guided ablation are used or not. From the patients’ perspective, the more rapid visual recovery and less postoperative pain following LASIK are the main advantages of LASIK over PRK. The pain scores in the studies we found support the generally accepted view that LASIK is associated with less postoperative pain but more intraoperative discomfort than PRK. This is important when counselling patients about their operation.
When individual studies are examined the only trials which in-dividually found a difference between the treatments wereWang 1997,Hjortdal 2005andSchallhorn 2009. As discussed in the results section,Wang 1997is at significant risk of bias. It is a large trial and carries significant weight in the analyses, therefore per-forming a sensitivity analysis on the relevant outcomes to which it contributes was essential.
At first glance, LASIK appeared to have superior efficacy with the odds of achieving a visual acuity of 20/20 or better at six months and 12 months significantly more likely than with PRK. The superior visual outcome at 12 months could be accounted for by the more accurate refractive outcomes seen with LASIK at 12 months. However, looking at the data more clearly, these findings were limited to the subgroup of patients with refractive errors up to -6 D. More importantly the results were sensitive to the exclusion ofWang 1997. When analyses were performed without this potentially biased study, no differences were found in any of these outcomes.
The duration covered by this study encompasses huge advances in the technology and techniques used. To evaluate whether there was any difference in the contemporary forms of treatment, using femtosecond laser flap creation and wavefront-guided treatments, we stratified outcomes into studies from 2008 onwards in which these were used and those pre 2008 which employed older tech-nology. This analysis demonstrated that contemporary studies do not show any difference in efficacy or accuracy between PRK and LASIK whereas older studies show a possible difference in favour of LASIK. This suggests that modern PRK with mitomycin C (MMC) and wavefront-guided treatments is equally as effective and accurate as LASIK.
We previously found that LASIK was potentially safer than PRK, based upon the fact that fewer LASIK eyes lost 2 or more lines of best spectacle-corrected visual acuity (BCVA) and this finding was not sensitive to the exclusion ofWang 1997. In this updated review, whilst there is still a significant difference in the loss of 2 or more lines of vision, which is less common in LASIK (odds ratio (OR) 0.47, 95% confidence interval (CI) 0.23 to 0.98) this disappears onceWang 1997is excluded (OR 0.52, 95% CI 0.22 to 1.26). Hence the updated data in this review does not support this finding. This difference is likely explained in the overall analysis by the inclusion of data from additional studies that has reduced the significant weight that Wang et al carried previously. When we performed a sub-analysis according to the surgical technology used it showed that prior to 2008 LASIK was less likely to result in loss of 2 or more lines of BCVA whereas in contemporary studies there was no difference between treatments. The implication for patients is that they can be reasonably confident that the risks involved are equal for both treatments.
In several outcome analysesHjortdal 2005appears to have a dif-ferent outcome from other studies and in some instances this re-sulted in the detection of statistical heterogeneity. This may in part be explained by the mean postoperative spherical equivalent outcomes for this trial. The forest plots of mean postoperative spherical equivalent refraction data are of limited value on their own but must be interpreted in conjunction with the raw spherical equivalent data. These data demonstrate that for prescriptions up to -6 D at two to four weeks post-treatment, the mean difference in refractive error between the two treatments was only 0.10 D (95% CI 0.04 to 0.16). There was no significant difference be-tween treatments at six or 12 months. The data on refractive sta-bility indicate that at one year post-treatment, neither procedure has a significant degree of change in refraction (Table 4). The follow-up duration reported in these studies was variable. Only eight of the 12 studies reported 12-month follow-up data and of those studies using contemporary techniques and published post 2008, only two of six reported 12-month follow-up data. The remainder reported six-month data. It is possible that this duration of follow-up is inadequate to determine the final visual outcomes for either or both of these procedures. There is evidence that vision may continue to improve after one year with continued resolution of haze (Rajan 2004). Recovery of corneal innervation and restoration of a normal tear film and ocular surface may also take longer than 12 months (Calvillo 2004;Murphy 1999). The incidence of visually significant corneal haze following PRK was found to range from 0% to 13% with an overall average of 3.3%. Only one instance of visually significant haze due to diffuse lamellar keratitis following LASIK was reported. The risk of sig-nificant haze after PRK is an important difference between these procedures, which in three of the recent trials was addressed using MMC as an adjunct (Table 1). Of the six contemporary studies three reported data on optical side effects such as glare and halos. None of these found any difference between PRK and LASIK.
The rate of flap-related complications in LASIK participants ranged from 0.7% to 15% and only one participant lost 2 or more lines of BCVA. This is in keeping with the published rate of approximately 4% (Gimbel 1998;Lin 1999;Moshirfar 2010; Stulting 1999;Sugar 2002). Of the six post 2008 trials which used femtosecond lasers for flap creation only two reported flap complications. It is not possible to comment on the relative safety of mechanical versus femtosecond laser flap creation based on the current data set.
We incorporated an additional outcome measure of higher order aberrations into this study. All eight studies added to this update reported such data. The outcomes were remarkably similar. All but one study showed that in both PRK and LASIK there was a significant increase in higher order aberrations following treat-ment versus pre-operatively. There was no difference between the two treatments in the amount or type of aberrations induced by treatment.
Quality of the evidence
Overall we graded the quality of the evidence as low or very low (Summary of findings for the main comparison). This was largely because of the potential for risk of bias in the included studies. The major difficulty in combining the results of randomised con-trolled trials included in this review was the heterogeneity of out-come measures and follow-up intervals reported. Improved com-pliance with suggested methods for reporting visual and refractive results of trials involving refractive procedures has made this up-date significantly easier than the initial review. Our framework of clearly defined outcome measures at fixed time points has worked well in both versions of this review. The downside of using such rigid outcome measures is that it is difficult to extract complete data sets from each trial. Hence not all trials could be included in each of the outcome analyses. In this updated version we have modified the outcome measures to try to detect more subtle dif-ferences in visual outcome (BCVA less than or equal to 20/15) and safety (loss of 1 or more lines of BCVA). This approach was limited by the fact that a minority of studies reported such data. The methodological quality of the trials that we have included is in some cases satisfactory but not without flaws. The quality is summarised inFigure 1and inFigure 2. Masking of participants and personnel when performing the procedure was not possible because of the nature of the procedures so studies were not ex-cluded on the basis of this. Masking of outcome assessment was not performed in all studies and is a potential source of significant bias. We had concerns about the quality of two trials (Schallhorn 2009;Wang 1997) and we examined the effect of including these data using a sensitivity analysis. The mixture of study designs (uni-lateral versus bi(uni-lateral treatment) posed a problem with data syn-thesis. In order to include data from all study types we assumed that the response of any eye to one treatment is in no way related to or predictable from the response of the fellow eye and we therefore
treated data as unpaired for all studies. By doing this we may have lost the power to detect changes that a paired analysis may have found. However, if we were to analyse the paired and unpaired data separately we would have had only three trials with paired data and two trials with unpaired data. As not all trials reported data for each outcome and time point the outcomes that could be analysed in a meaningful way would be minimal. Accepting these limitations we were able to combine the data from these different trials and perform statistical analysis on the results.
A U T H O R S ’ C O N C L U S I O N S Implications for practice
This review demonstrates that LASIK results in a more rapid re-covery of visual acuity post-treatment than PRK. The visual and refractive outcomes of these two procedures are comparable, espe-cially when modern techniques and technology are used.
Implications for research
Further research should focus on using contemporary techniques and equipment and on using more sensitive outcome measures, later time points and a questionnaire instrument designed to mea-sure vision-related quality of life. Further trials using contempo-rary techniques are required to determine whether LASIK and PRK as currently practised are equally safe. Randomising eyes to treatment is an efficient design, but only if analysed properly. In future trials, more efforts could be made to mask the assessment of outcome.
A C K N O W L E D G E M E N T S
The Cochrane Eyes and Vision Group Trials Search Co-ordinator prepared and executed the electronic searches for this review. We thank Marie Diener-West for her comments on the update of the review, Catey Bunce, Jennifer Burr, Swaroop Vedula and Richard Wormald for their comments on the final draft of the review and Suzanne Brodney-Folse, Duguld Bell and Marco Anelli for their comments on the protocol for this review. We thank Anupa Shah for her comments and assistance throughout the review process. Bruce Allan (co-author) and Richard Wormald (Co-ordinating Editor for CEVG) acknowledge financial support for their CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Insti-tute of Ophthalmology for a Specialist Biomedical Research Cen-tre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
R E F E R E N C E S
References to studies included in this review
Barreto 2010 {published data only}
Barreto J Jr, Barboni MT, Feitosa-Santana C, Sato JR, Bechara SJ, Ventura DF, et al.Intraocular straylight and contrast sensitivity after contralateral wavefront-guided
LASIK and wavefront-guided PRK for myopia.Journal of
Refractive Surgery2010;26(8):589–93.
Durrie 2008 {published data only}
Durrie DS, Slade SG, Marshall J. Wavefront-guided excimer laser ablation using photorefractive keratectomy and
sub-Bowman’s keratomileusis: a contralateral eye study.Journal
of Refractive Surgery2008;24(1):S77–4.
Slade SG, Durrie DS, Binder PS. A prospective contralateral eye study comparing thin-flap LASIK (sub-Bowman keratomileusis) with photorefractive keratectomy.
Ophthalmology2009;116(6):1075–82.
el Danasoury 1999 {published data only}
el Danasoury MA, el Maghraby A, Klyce SD, Mehrez K. Comparison of photorefractive keratectomy with excimer laser in situ keratomileusis in correcting low myopia (from -2.00 to -5.50 diopters). A randomized study.
Ophthalmology1999;106(2):411-20; discussion 420-1.
el Maghraby 1999 {published data only}
El Maghraby A, Salah T, Waring GO 3rd, Klyce S, Ibrahim O. Randomized bilateral comparison of excimer laser in situ keratomileusis and photorefractive keratectomy for 2.50
to 8.00 diopters of myopia.Ophthalmology1999;106(3):
447–57.
Forseto 2000 {published and unpublished data}
Forseto ADS, Nosé RAM, Nosé W. PRK versus LASIK for
correction of low and moderate myopia.Arquivos Brasileiros
De Oftalmologia2000;63(4):257–62.
Hatch 2011 {published data only}
Hatch BB, Moshirfar M, Ollerton AJ, Sikder S, Mifflin MD. A prospective, contralateral comparison of photorefractive keratectomy (PRK) versus thin-flap LASIK: assessment of
visual function.Clinical Ophthalmology2011;5(1):451–7.
Hjortdal 2005 {published and unpublished data} Hjortdal JO, Moller-Pedersen T, Ivarsen A, Ehlers N. Corneal power, thickness, and stiffness: results of a prospective randomized controlled trial of PRK and LASIK
for myopia.Journal of Cataract and Refractive Surgery2005;
31(1):21–9.
Ivarsen A and Hjortdal J. Seven-year changes in corneal
power and aberrations after PRK or LASIK.Investigative
Ophthalmology and Visual Science2012;53(10):6011–6016.
Manche 2011 {published data only}
Manche EE, Haw WW. Wavefront-guided laser in situ keratomileusis (Lasik) versus wavefront-guided
photorefractive keratectomy (Prk): a prospective randomized eye-to-eye comparison. (An American
Ophthalmological Society Thesis). Transactions of the
American Ophthalmological Society2011;109:201–20.
Moshirfar 2010 {published data only}
Moshirfar M, Gardiner JP, Schliesser JA, Espandar L, Feiz V, Mifflin MD, et al.Laser in situ keratomileusis flap complications using mechanical microkeratome versus
femtosecond laser: retrospective comparison. Journal of
Cataract and Refractive Surgery2010;36(11):1925–33.
Schallhorn 2009 {published and unpublished data} Schallhorn SC. Personal communication May 2009. Schallhorn SC. Results of a randomized perspective comparison of advanced surface ablation and sub-bowman keratomileusis. American Academy of Ophthalmology Annual Meeting, New Orleans 2007 - Refractive Surgery Subspecialty Day. 2007.
SUMMIT 1998 {published data only}
Hersh PS, Abbassi R. Surgically induced astigmatism after photorefractive keratectomy and laser in situ keratomileusis.
Summit PRK-LASIK Study Group.Journal of Cataract and
Refractive Surgery1999;25(3):389–98.
∗
Hersh PS, Brint SF, Maloney RK, Durrie DS, Gordon M, Michelson MA, et al.Photorefractive keratectomy versus laser in situ keratomileusis for moderate to high myopia. A
randomized prospective study.Ophthalmology1998;105(8):
1512-22, discussion 1522-3.
Hersh P S, Scher K S, Irani R. Corneal topography of photorefractive keratectomy versus laser in situ keratomileusis. Summit PRK-LASIK Study Group.
Ophthalmology1998 Apr;105(4):612–9.
Hersh PS, Steinert RF, Brint SF. Photorefractive keratectomy versus laser in situ keratomileusis: comparison of optical side
effects. Summit PRK-LASIK Study Group.Ophthalmology
2000;107(5):925–33.
Wallau 2008{published data only}
Wallau AD, Campos M. Photorefractive keratectomy with mitomycin C versus LASIK in custom surgeries for myopia:
a bilateral prospective randomised clinical trial.Journal of
Refractive Surgery2008;24(4):326–36.
Wang 1997 {published data only}
Wang Z, Chen J, Yang B. Comparison of laser in situ keratomileusis and photorefractive keratectomy to correct
myopia from -1.25 to -6.00 diopters.Journal of Refractive
Surgery1997;13(6):528–34.