Lab 24: Determining Kby Half-Titration of a Weak Acid
Full text
Figure
Related documents
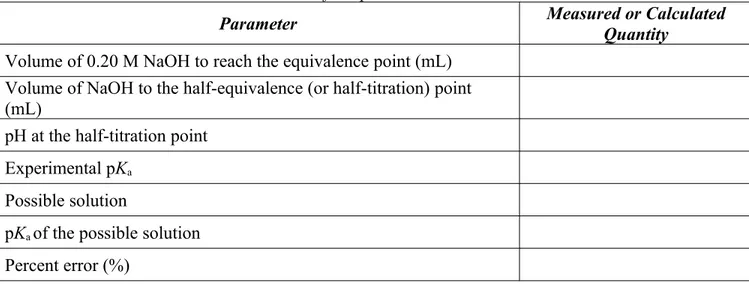
In this lab, the identity of an unknown weak acid will be determined by constructing a titration curve, estimating the value of pK a , and determining the molar mass of the acid
Have students perform the acid-base titration lab between a strong acid and a strong base and the titration between a weak acid and a strong base. Have students design a lab to
Volume of NaOH(aq) added (mL) Titration of a Diprotic Acid with Sodium Hydroxide Solution.. essentially two titration curves
When our page is error free, then we have just successfully made our first Resume webpage with Cascading Style Sheet code webpage using HTML programming. * World Class CAD
Under continuous gas lift, high pressure gas enters the tubing through gas lift valves continuously, maintaining a constant flowing bottomhole pressure. This action reduces the
COBIT was developed to align IT resources and processes with business objectives, quality standards, monetary controls, and security needs. COBIT is composed of four domains:
titration, (3) evaluate a pH titration curve and determine the pH of a weak acid / strong base titration at the equivalence point, (4) use the molar solubility, as determined from
The amount of OH =- formed from the equilibrium reaction shown by equation (8) is negligible. You will then plot all the pH measurements made in this experiment against the