EXPERIENCE AND REASON—Briefly Recorded
‘‘In Medicine one must pay attention not to plausible theorizing but to experience and reason together. . . . I agree that theorizing is to be approved, provided that it is based on facts, and systematically makes its deductions from what is observed. . . . But conclusions drawn from unaided reason can hardly be serviceable; only those drawn from observed fact.’’ Hippocrates: Precepts. (Short communications of factual material are published here. Comments and criticisms appear as letters to the Editor.)
A Family Cluster of Streptococcal
Toxic Shock Syndrome in Children:
Clinical Implication and
Epidemiological Investigation
ABSTRACT. Background. Most invasive group A streptococcal (GAS) disease occurs sporadically. Reports of family clusters of these infections are scanty, and most invasive disease occurs in adults. We describe a family cluster of streptococcal toxic shock syndrome (STSS) in-volving 3 children and present the results of an epidemi-ologic investigation.
Patients and Methods. During a 16-day period, 3 chil-dren in a family developed STSS with an interval of 7 and 9 days, respectively, between the onset of disease. Cases 2 and 3 had GAS isolated from blood culture. Case 2 was fatal. Pharyngeal culture survey of the family mem-bers and schoolchildren was conducted. Antibiogram, serotyping, detection of exotoxin genes, and random am-plified polymorphic DNA patterns of the disease strains and survey strains were examined.
Results. One of 15 family members sampled—the sis-ter of the index case—and 7 (5.6%) of 125 schoolchildren sampled had GAS isolated from pharyngeal cultures. Of the 10 strains examined, 2 isolates from the patients, 1 from the sister of index case, and 2 from the classmates of case 2 (the fatal case) had an identical pattern of both genotype and phenotype.
Conclusion. We describe a family cluster of STSS in-volving 3 children caused by a single clone and provide additional data regarding invasive GAS infection subse-quent to household contact. Additional studies should be conducted in conjunction with surveillance to define bet-ter the magnitude of risk in household contacts and to identify settings in which subsequent infections may occur.Pediatrics2001;107:1181–1184;group A Streptococ-cus, streptococcal toxic shock syndrome, family cluster, children.
ABBREVIATIONS. GAS, group A streptococcus/streptococcal; STSS, streptococcal toxic shock syndrome; CRP, C-reactive pro-tein; BUN, blood urea nitrogen; PCR, polymerase chain reaction; RAPD, random amplified polymorphic DNA; APPCR, arbitrarily primed polymerase chain reaction; MIC, minimal inhibitory con-centration.
U
ntil the late 1980s, the incidence of severe
group A streptococcal (GAS) infections had
declined markedly.
1,2Since 1987, a
resur-gence of invasive GAS infections has been reported
in the form of the recently described streptococcal
toxic shock syndrome (STSS).
3– 8For the most part
the infections have occurred sporadically and have
rarely been associated with clusters of cases.
9 –14Re-ports of family clusters of invasive GAS infection are
scant, and, where reported, most invasive diseases
have occurred in adults.
10,13–16In this report, we describe a family cluster of STSS
involving 3 children and the results of an
epidemio-logic investigation.
PATIENTS
During a 16-day period, 3 children in an extended
family developed STSS. Cases 2 and 3 are siblings
and are also cousins of the index case. The interval
between the onset of diseases was 7 and 9 days,
respectively. All 3 patients were previously healthy
males. There was no recent history of varicella and
upper respiratory infection in them. Except for the
lack of bacterial evidence in case 1, all 3 cases met the
consensus definition of STSS proposed in 1993
8—
that is, hypotension in combination with at least 2 of
the following: renal impairment, coagulation or liver
abnormalities, adult respiratory distress syndrome,
rash, and necrotizing fasciitis.
Case 1
A 4-year-old boy was admitted to Chang Gung Children’s Hospital on February 25, 1999, with a 1-day history of fever, abdominal pain, vomiting, diarrhea, and general malaise. On ad-mission, the patient appeared lethargic with sunken eyes and dry lips. His temperature was 38.8°C; blood pressure, 75/32 mm Hg; pulse rate, 128 beats per minute; and respiratory rate, 32 breaths per minute. A diffuse erythematous rash on the trunk and neck was noted. Initial laboratory studies showed a leukocyte count of 16 100/mm3(21% band forms, 75% segmented neutrophils, 4% lymphocytes), a platelet count of 156 000/mm3, and a C-reactive protein (CRP) value of 357 mg/L (normal, 0 –10). Sixteen hours later, his blood pressure dropped to 63/25 mm Hg, the thrombo-cyte count decreased to 68 000/mm3, and blood urea nitrogen (BUN) increased to 38 mg/dL (normal,⬍20). Intravenous saline and dopamine were administered. Therapy with penicillin G and ceftizoxime was initiated. The patient’s condition stabilized 2 days later. Skin exfoliation over the trunk and extremities was noted on February 27 and persisted until March 20. Therapy with penicillin was continued for 14 days total, and recovery was uneventful. No GAS was isolated from either blood or throat cultures.
Case 2
An 8-year-old boy developed fever, chills, vomiting, diarrhea, and general malaise 7 days after his cousin (case 1) became ill. Two days later, he was brought to a local hospital because of coldness
Received for publication Apr 27, 2000; accepted Aug 30, 2000.
and cyanosis of the limbs and dyspnea. At the emergency depart-ment, he appeared cyanotic and tachypneic. His temperature was 35.9°C; blood pressure, 64/38 mm Hg; pulse rate, 157 beats per minute; respiratory rate, 40 breaths per minute. Moist rales were found over the right lung field, and tenderness over the epigastric area was detected. Initial artery blood gas showed severe meta-bolic acidosis (pH 7.003, HCO37.9 mmol/L) and hypoxemia (Pao2 28.4 mmol/L). Laboratory studies resulted in the following: leu-kocyte count, 4600/mm3(87% segmented neutrophils, 8% lym-phocytes, 5% monocytes); platelet count, 49 000/mm3; CRP value, 315 mg/L; BUN, 57 mg/dL; serum creatinine, 3.8 mg/dL; aspar-tate transaminase, 166 mg/dL; alanine transaminase, 100 mg/dL; and creatine phosphokinase, 3501 U/L (normal,⬍170) with 5.5% MB fraction. Chest roentgenography showed segmental pneumo-nia over the right lower lung field.
Because of cyanosis and severe respiratory distress, the patient was intubated. Intravenous saline was infused and dopamine was administered. Therapy with ampicillin was started. The patient’s condition deteriorated despite this management for 2 hours. An attempt was made to transfer the patient to a medical center; unfortunately, he progressed to a state of shock and unrespon-siveness on the way and died. The blood culture taken at the local hospital subsequently yieldedStreptococcus pyogenes.
Case 3
A 6-year-old boy, a younger brother of case 2, was admitted to Chang Gung Children’s Hospital on March 13, 1999 (17 days after the index case became ill) because of fever, vomiting, diarrhea, and abdominal pain for 1 day. On admission, his temperature was 38.9°C; blood pressure, 77/44 mm Hg; pulse rate, 140 beats per minute; and respiratory rate, 26 breaths per minute. A diffuse erythematous rash on the trunk was noted. Initial laboratory studies showed a leukocyte count of 7000/mm3(41% band forms, 55% segmented neutrophils, 4% lymphocytes), platelet count of 271 000/mm3, and CRP value of 22 mg/L. The patient was trans-ferred to the pediatric intensive care unit immediately because of impending shock. Intravenous saline was infused, and inotropic agents, including dopamine, dobutamine, and epinephrine, were administered. Therapy with ceftriaxone, amikacin, and vancomy-cin was initiated, and 1 dose of intravenous immunoglobulin (2 g/kg) was infused. Two days later, the platelet count decreased to 43 000/mm3, petechia were noted, and thus a platelet transfusion was administered. The patient’s condition was stabilized on the fourth hospital day, and the inotropic agents were discontinued gradually. A blood culture taken on admission yieldedS pyogenes.
Antibiotics were shifted to penicillin alone, and this was contin-ued for 14 days total, with the addition of rifampin for the last 4 days. Recovery was uneventful.
METHODS
When we became aware that these cases represented a family cluster of STSS, we immediately notified the health bureau of the local government and conducted a pharyngeal culture survey of the family members and certain exposed schoolmates.
Survey of Family Members and Schoolchildren
The 3 cases lived in a large, 3-generation family, consisting of 7 small households. These families lived next to one another in a large 3-floor condominium building with 7 units. Except during festivals, the family meals were prepared and eaten separately. The children usually played together and sometimes shared food and water with each other. There were 34 persons in this extended family, but 13 members had been residing out of town. Excluding the 3 cases of STSS, pharyngeal culture for GAS was obtained from 15 of the 18 members at home, including 7 adults and 8 children. Another family, relatives of the cases, lived in the neighbor-hood (several hundred meters away) and consisted of 3 adults and 5 children. Three children—aged 5, 8 and 10 years, respectively— had high fever, sore throat, and mild cough on March 17 (the survey day). Pharyngeal culture was obtained from all of the 8 relatives.
At that time, case 3 and the elder sister of the index case (aged 5 years) attended a kindergarten class in a separate building, several hundred meters away from the elementary school. The children stayed in the kindergarten during the daytime of each weekday. Case 2 (class A, second grade) and his elder sister (class
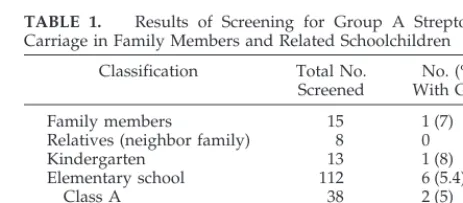
B, third grade) and elder brother (class C, fifth grade) attended the elementary school. Except for the whole day on Tuesday, the pupils in class A spent the morning time in the school every day; whereas the pupils in classes B and C stayed in the school during the daytime of each weekday except Wednesday. On March 17, 1999, pharyngeal cultures were systematically obtained from the children attending the kindergarten (13 children), class A (38 children), class B (38 children), and class C (36 children). The subject numbers and results of the pharyngeal culture survey are shown in Table 1. All pharyngeal specimens were collected using the Venturi Transystem (Copan, Italia Brescia, Italy), then trans-ported to and processed in our microbiologic laboratory within 4 hours.
Laboratory Evaluation Methods for GAS Isolates Isolates were confirmed asS pyogeneswith the use of standard techniques. Antimicrobial susceptibility of the isolates was deter-mined by the E-test (PDM Epsilometer, AB Biodisk, Solna, Swe-den). The T-protein patterns of the isolates were determined by the slide agglutination method, as previously described.17M se-rotyping is not feasible in Taiwan. The presence ofspeA,speB,and
speCgenes as well as serotypes M1, M6, and M12 was assessed with the polymerase chain reaction (PCR).17Random amplified polymorphic DNA (RAPD) patterns by means of arbitrarily primed polymerase chain reaction (APPCR) of the isolates were performed with 2 arbitrary oligonucleotide primers OPA01: 5⬘ -CAGGCCCTTC-3⬘and OPC-05: 5⬘-GATGACCGCC-3⬘. The reac-tion mixture for PCR and PCR condireac-tions were followed as de-scribed previously.18
RESULTS
Of the 15 family members screened, only 1 child,
who was the elder sister of the index case, had GAS
isolated from pharyngeal culture. None of the 8
neighborhood family members was a carrier of GAS.
One of 13 children in the kindergarten sampled had
a throat culture positive for
S pyogenes. Of the
ele-mentary schoolchildren sampled, positive results
were noted in 2 children (8%) from class A, 4
chil-dren (11%) from class C, and none from class B,
respectively. Overall, 6% of the children tested were
positive for
S pyogenes. The results of the pharyngeal
culture survey are shown in Table 1. Including 2
isolates from the blood cultures of cases 2 and 3, a
total of 10 isolates were obtained for analysis.
Of the 10 isolates examined, 2 isolates from the
patients’ blood cultures (strains 1 and 2), 1 isolate
from the sister of the index case (strain 5), and 2
isolates (strains 3 and 4) from class A had an identical
antimicrobial susceptibility profile, T-type (serotype
T4), and RAPD pattern (type A). Four isolates
(strains 6 –9) from class C and 1 isolate (strain 10)
from the kindergarten had an identical antimicrobial
susceptibility profile and T-type (serotype T12), but
for RAPD pattern, strains 9 and 10 were similar (type
B), strains 7 and 8 were similar (type C), and strain 6
TABLE 1. Results of Screening for Group A Streptococcal Carriage in Family Members and Related Schoolchildren
Classification Total No. Screened
No. (%) With GAS
Family members 15 1 (7)
Relatives (neighbor family) 8 0
Kindergarten 13 1 (8)
Elementary school 112 6 (5.4)
Class A 38 2 (5)
Class B 38 0
Class C 36 4 (11)
was unique (type D). The detailed antimicrobial
sus-ceptibility profiles, serotypes, and RAPD patterns
(Fig 1) of these 10 isolates are shown in Table 2.
These 10 isolates were not type M-1, M-6, or M-12.
SpeB
gene could be detected in all 10 isolates while
speA
and
speC
genes could not.
DISCUSSION
This outbreak of STSS, caused by a single clone of
S pyogenes, involved 3 children in a family.
Person-to-person transmission of
S pyogenes
among these
cases was documented by both genotyping and
phe-notyping methods of the related isolates. Although
the index case lacked bacterial evidence of GAS
in-fection, the diagnosis of STSS was presumed by the
epidemiologic evidence of subsequent cases and the
recovery of the same clone of
S pyogenes
from the
pharyngeal culture of his sister, accompanied by a
similar clinical manifestation. Previous reports
10,13–16regarding family clusters of GAS infection indicate
that children experience the vast majority of mild
GAS infections, and play a major role in the spread of
infection within families; most invasive GAS
infec-tions occur in adults, with the rate increasing with
age. However, the risk of secondary cases of invasive
infections in children cannot be ignored,
10a fact
highlighted by the 2 subsequent cases of STSS in
children, 1 fatal, reported in this article. To our
knowledge, this is the first report of familial
trans-mission of STSS in children in Taiwan.
Pharyngeal GAS infections can be acquired from
either symptomatically or asymptomatically infected
persons. GAS transmission occurs easily among close
contacts in family, child care, school, and military
training settings. After the introduction of the
organ-ism, the GAS carrier rate is typically higher, ranging
from 20% to 80%,
19 –21among family members,
schoolchildren, and military personnel. Cockerill et
al
21demonstrated that the frequency of the outbreak
clone among pharyngeal carriers was significantly
higher in schoolchildren in the outbreak area. In the
present study, the positive rate of pharyngeal culture
for GAS did not increase and was still
⬍
10% among
the schoolchildren as well as family members. In the
investigation of an outbreak of scarlet fever at a child
care center reported from Taiwan,
18the incidence of
throat carriage of GAS among 131 children and 24
staff members without scarlet fever at the center was
also
⬍
10%. It is possible that GAS colonization can
occur in sites other than the throat and that GAS may
be transmitted through routes other than exposure to
large respiratory droplets.
The strains of GAS prevailing in Taiwan appeared
to differ in different times and regions. During 1992
and 1993, the isolates of serotype T12 accounted for
42.3% of 78 clinical isolates and 87.5% of the 24
isolates recovered from throat swab samples from
southern Taiwan.
22Serotype T4 was the
predomi-nant type of 83 clinical isolates from northern Taiwan
during 1996 to 1998.
23However, minimal inhibitory
concentrations (MICs) of erythromycin were high
Fig 1. Gel electrophoresis of APPCR products obtained withprimers OPC5 (upper panel) and OPA1 (lower panel). Lane M represents molecular weight marker (1 kb ladder); lanes 1 and 2, isolates from Cases 2 and 3; lanes 3 and 4, isolates from class A; lane 5, isolates from the sister of the index case; lanes 6 to 9, isolates from class C; lane 10, isolate from the kindergarten; and lanes 11 to 15, unrelated reference isolates.
TABLE 2. Antimicrobial Susceptibility Profiles, T-Types, and Genotype Characteristics of 10 Isolates ofS pyogenesInvestigated in a Family Cluster of Streptococcal Toxic Shock Syndrome
Strain No.
Source of Isolates
MIC (mg/L)* T-Type RAPD Pattern†
CT CH PG EM CM VA OPA1‡ OPC‡5
1 Case 2 0.023 3 0.016 4 0.094 0.38 4 A A
2 Case 3 0.023 3 0.023 6 0.094 0.5 4 A A
3 Class A 0.032 3 0.016 12 0.125 0.5 4 A A
4 Class A 0.023 4 0.012 8 0.094 0.5 4 A A
5 Sister of index case 0.023 3 0.016 8 0.094 0.5 4 A A
6 Class C 0.047 0.064 0.032 0.094 0.125 0.5 12 C D
7 Class C 0.064 0.047 0.032 0.094 0.125 0.5 12 B C
8 Class C 0.047 0.064 0.032 0.094 0.125 0.5 12 B C
9 Class C 0.047 0.047 0.032 0.094 0.047 0.5 12 B B
10 Kindergarten 0.047 0.047 0.032 0.094 0.125 0.5 12 B B
among these isolates, and more than half of all
iso-lates from both periods were resistant to
erythromy-cin (MIC
⬎
0.5
g/mL). In the present study,
eryth-romycin MICs were high in the outbreak strains
(serotype T4).
It has been documented that the presence of
speA
and its encoding toxin SPEA is strongly correlated
with invasive GAS disease and STSS. In a previous
study from Taiwan,
17speA
was present in only 13%
of 8 STSS-associated strains, while
speB
was present
in all of 72 invasive disease, including STSS, isolates.
A similar picture (negative
speA
but positive
speB)
was also noted in all 25 strains isolated from an
investigation of an outbreak of scarlet fever at a child
care center in Taiwan.
18The same is true in both the
outbreak and colonizing strains in this study.
The issue of whether antimicrobial prophylaxis is
warranted in prevention of invasive GAS disease
among household contact was recently discussed by
a working group on prevention of invasive GAS
infection, and a consensus statement was
pub-lished.
16No definite recommendation could be made
at that time because of insufficient data. The present
report, although still inconclusive, provides
addi-tional data of subsequent invasive GAS infection
after household contact to support the strategy of
antimicrobial prophylaxis. However, additional
studies should be conducted in conjunction with
sur-veillance to define better the magnitude of risk in
household contacts and to identify settings in which
subsequent infections occur.
Yhu-Chering Huang, MD, PhD* Po-Ren Hsueh, MD‡
Tzou-Yien Lin, MD* Dah-Chin Yan, MD§ Shao-Hsuan Hsia, MD储
*Division of Pediatric Infectious Diseases ‡Department of Laboratory Medicine, National
Taiwan University Hospital §Pediatric Allergy/Immunology 储Pediatric Critical Care Medicine Chang Gung Children’s Hospital Chang Gung University
Kweishan, Taoyuan, Taiwan
REFERENCES
1. Massel BF, Chute CG, Walker AM, Kurland GS. Penicillin and the marked decrease in morbidity and mortality from rheumatic fever in the United States.N Engl J Med. 1988;318:280 –286
2. Stevens DL. Invasive group A streptococcal infections: the past, present and future.Pediatric Infect Dis J. 1994;13:561–566
3. Cone LA, Woodard DR, Schilevert PM, Tomory GS. Clinical and bac-teriologic observations of a toxic shock-like syndrome due to Strepto-coccus pyogenes.N Engl J Med. 1987;317:146 –149
4. Hribalova V.Streptococcus pyogenesand the toxic shock syndrome.Ann Intern Med. 1988;108:772
5. Stevens DL, Tanner MH, Einship J, et al. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A.N Engl J Med. 1989;321:1– 8
6. Begovac J, Marton E, Lisic M, Beus I, Bozinovic D, Kuzmanovic N. Group A beta-hemolytic streptococcal toxic shock-like syndrome. Pedi-atr Infect Dis J. 1990;9:369 –370
7. Hoge CW, Schwartz B, Talkington DF, Breiman RF, MacNeill EM, Englender SJ. The changing epidemiology of invasive group A strepto-coccal infections and the emergence of streptostrepto-coccal toxic shock-like syndrome: a retrospective population-based study. JAMA. 1993;269: 384 –389
8. Working Group on Severe Streptococcal Infections. Defining the group
A streptococcal toxic shock syndrome: rationale and consensus defini-tion.JAMA. 1993;269:390 –391
9. Centers for Disease Control. Nursing home outbreaks of invasive group A streptococcal infections—Illinois, Kansas, North Carolina, and Texas.
MMWR Morb Mortal Wkly Rep. 1990;39:577–579
10. Schwartz B, Elliott JA, Butler JC, et al. Clusters of invasive group A streptococcal infections in family, hospital, and nursing home settings.
Clin Infect Dis. 1992;15:277–284
11. Centers for Disease Control and Prevention. Outbreak of invasive group AStreptococcusassociated with varicella in a childcare center—Boston, Mass. MMWR Morb Mortal Wkly Rep. 1997;46:944 –949
12. Cannaday P, McNitt T, Horn K, Goodpasture H, Gentry LO. A family outbreak of serious streptococcal infection.JAMA. 1976;236:585–587 13. DiPresio JR, File TM, Stevens DL, Gardner WG, Petropoulos G, Dinsa K.
Spread of serious disease-producing M3 clones of group A Streptococ-cus among family members and health care workers.Clin Infect Dis. 1996;22:490 – 495
14. Gamba M-A, Martinelli M, Schaad HJ, et al. Familial transmission of a serious disease-producing group A Streptococcus clone: case report and review.Clin Infect Dis. 1997;24:1118 –1121
15. Davies HD, McGeer A, Schwartz B, et al. Invasive group A streptococcal infections in Ontario, Canada.N Engl J Med. 1996;335:547–554 16. Working Group on Prevention of Invasive Group A Streptococcal
In-fections. Prevention of invasive group A streptococcal disease among household contacts of case-patients: Is prophylaxis warranted?JAMA. 1998;279:1206 –1210
17. Hsueh PR, Wu JJ, Tsai PJ, Liu JW, Chuang YC, Luh KT. Invasive group A streptococcal disease in Taiwan is not associated with the presence of streptococcal pyrogenic exotoxin genes.Clin Infect Dis. 1998;26:584 –589 18. Hsueh PR, Teng LJ, Lee PI, et al. Outbreak of scarlet fever at a hospital day care center: analysis of strain relatedness with phenotypic and genotypic characteristics.J Hosp Infect. 1997;36:191–200
19. Dingle JH, Badger GF, Jordan WS. Streptococcal infections. In: Dingle JH, Badger FG, Jordan WS, eds.Illness in the Home.Cleveland, OH: Case Western Reserve University; 1964:97–117
20. Holmstrom L, Nyman B, Rosengren M, Wallander S, Ripa T. Outbreaks of infections with erythromycin-resistant group A streptococci in child day care centers.Scand J Infect Dis. 1990;22:179 –185
21. Cockerill FR III, MacDonald KL, Thompson RL, et al. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children.JAMA. 1997; 277:38 – 43
22. Hsueh PR, Chen HM, Huang AH, Wu JJ. Decreased activity of eryth-romycin againstStreptococcus pyogenes in Taiwan. Antimicrob Agents Chemother. 1995;39:2239 –2242
23. Tseng HY, Hsueh PR, Lee PI, Huang LM, Lee CY, Luh KT. Extremely high proportion of M-phenotype in clinical isolates of erythromycin-resistantStreptococcus pyogenesin Taiwan. The 158th Scientific Meeting of the Chinese Taipei Pediatric Association; April 1999
Glutaric Acidemia, Type I, Missed
by Newborn Screening in an Infant
With Dystonia Following
Promethazine Administration
ABSTRACT. We report a child initially diagnosed with promethazine-induced dystonia despite a lack of re-sponse to diphenhydramine therapy. On further evalua-tion, the child was diagnosed with glutaric acidemia, type I (GA-I), an autosomal recessive inborn error of metabolism caused by the deficiency of glutaryl-CoA dehydrogenase. The characteristic clinical feature of
Received for publication Jul 21, 2000; accepted Oct 5, 2000.
Reprint requests to (D.D.K.) Division of Medical Genetics, Duke University Medical Center, DUMC 3528, Durham, NC 27710. E-mail: koeb001@mc. duke.edu
GA-I is an acute encephalopathic and neurologic crisis typically occurring during a catabolic state. Despite slow improvement, many patients do not fully recover from a neurologic crisis, and residual neurologic morbidity can be significant. Although newborn screening using tan-dem mass spectrometry is expected to enable presymp-tomatic diagnosis of GA-I, this patient was not detected by newborn screening with tandem mass spectrometry. Therefore, a high suspicion of GA-I must be maintained in the evaluation of childhood dystonia, even when new-born screening results are reportedly normal.Pediatrics
2001;107:1184 –1187;glutaric acidemia type I, inborn errors of metabolism, promethazine, dystonia, newborn screen-ing.
ABBREVIATIONS. GA-I, glutaric acidemia, type I; MS/MS, tan-dem mass spectrometry.
G
lutaric acidemia, type I (GA-I) is an
autoso-mal recessive inborn error of metabolism
with an incidence of about 1 in 30 000.
1It is
caused by the deficiency of glutaryl-CoA
dehydro-genase, an essential enzyme in the catabolism of the
amino acids tryptophan, lysine, and hydroxylysine.
As a result of this metabolic block, excessive
glutaryl-CoA is shunted into alternate metabolic pathways
leading to accumulation of the metabolites glutaric
acid, 3-hydroxyglutaric acid, and glutarylcarnitine.
Like many metabolic disorders, GA-I is often
diffi-cult to diagnose because of its nonspecific and
inter-mittent presentation. Typically, patients with GA-I
present late in infancy with dystonia, and
occasion-ally acidosis. Postnatal macrocephaly is seen in the
majority of affected individuals from late infancy
onward. Accurate diagnosis requires metabolic
test-ing, including urine organic acid analysis and, more
recently, plasma acylcarnitine analysis. Urine
or-ganic acid analysis characteristically reveals elevated
glutaric and 3-hydroxyglutaric acids (the latter
me-tabolite is pathognomonic), although the urine
or-ganic acid analysis result may be normal in
asymp-tomatic individuals.
1The plasma or blood spot
acylcarnitine profile reveals elevated
glutarylcarni-tine, which also may be intermittent.
2In GA-I and
other disorders of metabolism, samples for
biochem-ical testing obtained when the patient is symptomatic
are most likely to be informative. Ultimately,
defi-ciency of glutaryl-CoA dehydrogenase may be
dem-onstrated in cultured fibroblasts (skin biopsy) and
leukocytes.
1,3Confusion with other, common disorders may
de-lay the diagnosis GA-I, and the differential diagnosis
includes trauma (given the presence of subdural
he-matomas), benign macrocephaly, drug toxicity, and
cerebral palsy.
4,5We report here a patient with GA-I
presenting at 11 months of age with acute-onset
dys-tonia after gastroenteritis and administration of
promethazine.
CASE REPORT
The patient was born at term via spontaneous vaginal delivery weighing 3409 g. The immediate neonatal period was unremark-able, and she was discharged from the hospital at 24 hours of age. At that time, a pilot study of newborn screening by tandem mass spectrometry (MS/MS) was being conducted in North Carolina,
and this patient had an abnormal initial acylcarnitine newborn screen with mildly elevated glutarylcarnitine. A repeat sample appeared to be normal, and according to the state laboratory protocol for evaluation of abnormal newborn screens at that time no additional diagnostic testing was recommended. The patient was breastfed for the first 6 months of life and was weaned to low iron cow’s milk-based formula and solid foods without difficulty. She received regular well-child care including scheduled immu-nizations. Psychomotor development progressed normally: at 11 months of age she had several recognizable words, used crawling for locomotion, and was taking rare steps.
At 11 months of age, she developed symptoms consistent with a viral gastroenteritis including vomiting and diarrhea. She re-portedly had adequate fluid intake and urinary output. Prometh-azine suppositories were prescribed to control vomiting, and 2 doses were given (6.25 mg, 6 hours apart; 0.6 mg/kg/dose). She was found in her crib that evening with dystonic posturing con-sisting of leftward eye deviation and intermittent stiffness of the left side of her body alternating with extreme hypotonia. No tonic-clonic movements were seen. At the local emergency room, midazolam was administered before loading doses of phenobar-bital and phenytoin for presumed new-onset seizures. Evaluation for acute infection was unrevealing; however, ceftriaxone was administered empirically. Serum electrolytes were normal (calci-um, phosphorus and magnesium were not measured). A com-puted tomography scan of the head was reported to show no intracranial lesions or structural abnormalities. Alternating dys-tonic posturing and hypotonia continued despite anticonvulsant therapy. The patient was transferred to a tertiary care center for hospital admission and additional evaluation.
Physical examination at the time of admission revealed a fussy infant with intermittent left-sided body stiffening and head posi-tion preference, and the remainder of the physical examinaposi-tion was unremarkable. Given the apparent lack of clinical response to adequate anticonvulsant therapy, the diagnostic possibility of promethazine-associated dystonia was suspected and treatment with approximately 1 mg/kg intravenous diphenhydramine ev-ery 6 hours was started. The patient’s dystonia improved slowly during the subsequent 48 hours, although hypotonia with inter-mittent, sporadic movements, primarily involving the tongue, continued. The patient was discharged from the hospital with a prescription for oral diphenhydramine to treat intermittent epi-sodes of abnormal posturing. Intermittent dystonic movements continued over the next week.
Neurology evaluation 1 week after the initial presentation re-vealed clinical improvement in muscle tone and voluntary motor control, with residual continuous choreoathetoid movements of the hands, facial dyskinesia, and intermittent stiffening of the legs (Fig 1). Baclofen (Watson Laboratories, Corona, CA) , 5 mg twice a day, was initiated for treatment of spasticity. Additional
ation for persistent dystonia included urine organic acids that showed modest elevations of glutaric acid and 3-hydroxyglutaric acid, and a plasma acylcarnitine profile (Fig 2) that revealed elevated glutarylcarnitine (ratio to internal standard 0.38, normal
⬍0.11). These abnormalities were consistent with the diagnosis of GA-I. Therapy consisting of restricted protein intake and supple-mental riboflavin (100 mg/d) and carnitine (100 mg/kg/d) was initiated when these results became available.
The family history was significant for consanguinity with the patient’s parents being second cousins. There was no family his-tory of seizures, movement disorders, birth defects, genetic dis-eases, or metabolic disorders.
At 1- and 3-month follow-up visits after diagnosis the patient had slow, but steady, improvement in motor skills. The patient’s parents reported that she continued to have intermittent abnormal movements of her tongue, right foot, and both hands, especially when fatigued. She laughed, smiled, and vocalized, but had no recognizable words. She had not resumed crawling but did pull to a stand and walk in a walker. Growth parameters, including head circumference, were normal. Physical examination revealed some posturing of the extremities, with no facial grimacing. Reflexes were brisk, without clonus, and there was no spasticity. Follow-up urine organic acids showed a mild elevation of 3-hydroxyglutaric acid. A concurrent plasma acylcarnitine profile revealed elevated glutarylcarnitine with a signal to internal standard ratio of 0.33. Nutritional therapy consisting of restricted tryptophan (10 mg/ kg/d) and lysine (60 mg/kg/d) and protein (1.2 g/kg/d) was initiated. Supplemental riboflavin (100 mg/d) and carnitine (100 mg/kg/d) were continued.
A magnetic resonance imaging examination of the brain at 18 months of age revealed subtle linear bands of signal abnormality involving the lateral aspects of the putamen bilaterally, compati-ble with known GA-I. There was no frontotemporal atrophy or widening of the Sylvian fissures, findings commonly seen in pa-tients with symptomatic GA-I.
DISCUSSION
The presenting symptoms of this infant, a
neuro-logic crisis late in infancy with dystonia,
choreoath-etoid movements and hypotonia, is typical for GA-I.
Unfortunately, the diagnosis was confounded by the
administration of promethazine, another
well-docu-mented cause of acute onset dystonia especially in
infants and young children. Although it is a
phe-nothiazine, promethazine has significant antiemetic
and antihistamine properties and is reported to have
very few psychotropic effects. In general, the
phe-nothiazines have well-documented neurologic
ad-verse effects including neuroleptic malignant
syn-drome, seizures, and extrapyramidal syndromes
consisting of acute dystonic reactions, akathisia,
aki-nesia, and tardive dyskinesia.
6,7These adverse
ef-fects are more common in younger children (
⬍
2
years of age) and when given in relatively high
doses. Anticholinergic therapy with 1 or 2 parenteral
doses (1 mg/kg given intravenously or
intramuscu-larly) of diphenhydramine reportedly ameliorates
promethazine-induced dystonia within 15 to 30
min-utes.
8Additional childhood medications commonly
associated with acute dystonic reactions include the
antiemetic and promotility agents cisapride and
met-oclopramide (Reglan, A. H. Robins Company,
Rich-mond, VA),
9and dextromethorphan.
10The potential
toxicity of ingested medications must always be
con-sidered with the onset of new symptoms in an ill
child.
Fortunately for this patient, alternative diagnoses
were considered and appropriate biochemical testing
ultimately led to the correct diagnosis, appropriate
treatment, and family education regarding GA-I. The
additional historical information that her parents
were second cousins raised the likelihood of a
ge-netic disorder, as consanguinity is not uncommon in
rare, autosomal recessive conditions. A high index of
suspicion for disorders of metabolism in children
with neurologic crises will ensure the prompt
acqui-sition of metabolic testing and arrival at the
appro-priate diagnosis.
Individuals with GA-I have signs and symptoms
resulting from excess glutaric acid and related
me-tabolites, primarily within the central nervous
sys-tem. The characteristic clinical feature of GA-I is the
acute neurologic crisis typically after a catabolic state
such as an acute illness with fever, decreased oral
intake, or vomiting. The neurologic crisis is best
de-scribed as encephalopathic with irritability and
leth-argy that can progress to coma. Additional
logic findings may include repetitive movements,
seizures, or abnormal posturing. Despite slow
im-provement, many patients do not fully recover from a
neurologic crisis. Residual morbidity can be significant;
hypotonia, ataxia, choreoathetoid movements,
spastic-ity, dystonia, and dyskinesia have all been reported.
The most significant physical sign in GA-I is
mac-rocephaly; in fact, macrocephaly may be the only
physical sign in otherwise asymptomatic infants.
Most commonly, infants develop progressive
macro-cephaly with markedly accelerated rates of head
cir-cumference growth in the first few months of life.
11Not all children with GA-I have macrocephaly; this
patient did not.
In patients with GA-I, the main therapeutic goal is
to prevent neurologic crises, associated neurologic
symptoms, and neurodevelopmental decline. The
mainstay of treatment is to reduce the dietary intake
of tryptophan and lysine thus reducing stress across
the metabolic block caused by the deficiency of
glu-taryl-CoA dehydrogenase. A dietary prescription
typically involves a generalized protein restriction,
using age-appropriate foods or formula/breast milk,
and use of a supplemental medical formula lacking
lysine and tryptophan to provide the recommended
daily allowances for the essential amino acids.
Ad-ditional recommended therapy includes
pharmaco-logic doses of riboflavin (100 mg/d, by mouth),
which serves as a cofactor for glutaryl-CoA
dehydro-genase and facilitates any residual enzyme activity.
In GA-I, dietary therapy and riboflavin
supplemen-tation has been shown to significantly reduce the risk
of neurologic complications.
4Carnitine
supplemen-tation (50 –100 mg/kg/d divided twice a day, by
mouth) has been shown to increase the urinary
ex-cretion of glutaric acid and replenish reduced body
carnitine stores.
4During an acute neurologic crisis,
additional protein restriction and carbohydrate
sup-plementation are introduced to prevent or reverse
endogenous protein catabolism.
The major impetus for early diagnosis of metabolic
disorders is the availability of effective therapy.
Newborn screening programs that include
acylcarni-tine analysis by MS/MS hold significant promise for
early and presymptomatic detection of a variety of
these inborn errors of metabolism.
2,12,13Although the
patient described here had an abnormal blood
acyl-carnitine profile at birth, the repeat specimen was
reported to be normal, thus, newborn screening
ul-timately failed to indicate the diagnosis of GA-I.
Newborn screening is a powerful tool to
poten-tially diagnose presymptomatic infants; however, it
should not be considered a diagnostic test. The
ex-perience of this case and others has prompted the
North Carolina State Laboratory to adjust the signal
ratio cutoff for glutarylcarnitine to increase the
sen-sitivity of the newborn screening test for GA-I, and
this is now suggested as a general recommendation
for laboratories screening for GA-I by MS/MS. To
allow presymptomatic diagnosis and treatment of
GA-I, we recommend a complete evaluation,
includ-ing both a plasma acylcarnitine profile and a urine
organic acid analysis, of any patient with elevated
glutarylcarnitine in a blood spot acylcarnitine
pro-file. A recently developed stable-isotope dilution
as-say for 3-hydroxyglutaric acid
14in urine by gas
chro-matography/mass spectrometry shows promise for
detection of patients with GA-I, because
3-hydroxy-glutaric acid was consistently present in urine of the
patients studied. This assay should be considered for
additional evaluation of infants with elevated
glu-tarylcarnitine in the newborn screen, when urine
organic acids and the plasma acylcarnitine profile are
normal. If thorough follow-up evaluations are
pur-sued for infants with abnormalities in newborn
screening suggestive of GA-I, additional patients
could treated before the onset of symptoms.
Wendy E. Smith, MD David S. Millington, PhD Dwight D. Koeberl, MD, PhD
Division of Medical Genetics Department of Pediatrics Duke University Medical Center Durham, NC 27710
Philip S. Lesser, MD
Carolina Neurological Clinic Charlotte, NC 28203
REFERENCES
1. Goodman SI, Frerman FE. Organic acidemias due to defects in lysine oxidation: 2-ketoadipic acidemia and glutaric acidemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds.The Metabolic and Molecular Bases of Inherited Disease. 7th ed. New York, NY: McGraw-Hill, Inc; 1995: 1451–1460
2. Millington DS, Kodo N, D. L. Norwood, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism.J Inherit Metab Dis. 1990;13:321–324
3. Christensen E. Improved assay of glutaryl-CoA dehydrogenase in cul-tured cells and liver: application to glutaric aciduria type I.Clin Chim Acta.1983;129:91–97
4. Hoffmann GF, Zschocke J. Glutaric aciduria type 1: from clinical, bio-chemical and molecular diversity to successful therapy.J Inherit Metab Dis. 1999;22:381–391
5. Morris AM, Hoffmann GF, Naughten ER, Monavari A, Collins JE, Leonard JV. Glutaric aciduria and suspected child abuse.Arch Dis Child. 1999;80:404 – 405
6. Casey DE. Neuroleptic-induced acute dystonia. In: Lang AE, Weiner WJ, eds.Drug-Induced Movement Disorders. Mt Kisco, New York: Futura Publishing Co, Inc; 1992:21–39
7. DeGrandi T, Simon JE. Promethazine-induced dystonic reaction.Pediatr Emerg Care. 1987;3:91–92
8. Burns M. The Antipsychotic Drugs. In: Haddad LM, Shanon MW, Winchester JF, eds.Clinical Management of Poisoning and Drug Overdose. 3rd ed. WB Saunders Company; 1998:628 – 641
9. Batts KF, Munter DW. Metoclopramide toxicity in an infant.Pediatr Emerg Care. 1998;14:39 – 41
10. Warden CR, Diekema DS, Robertson WO. Dystonic reaction associated with dextromethorphan ingestion in a toddler.Pediatr Emerg Care. 1997; 13:214 –215
11. Nyhan WL, Ozand PT. Atlas of Metabolic Diseases. London, United Kingdom: Chapman and Hall Medical; 1998:46 –52
12. Rashed MS, Ozand PT, P. Bucknall M, Little D. Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acid profiling using automated electrospray tandem mass spectrometry. Pe-diatr Res. 1995;38:324 –331
13. Millington DS, Chace DH, Hillman SI, Kodo N, Terada N. Diagnosis of metabolic disease. In: Matsuo T, Caprioli RM, Gross ML, Seyama Y, eds.
Biological Mass Spectrometry: Present and Future. New York, NY: John Wiley and Sons, Ltd; 1994;559 –579