Management of Group A
Streptococcal Sore Throat for
the Prevention of Acute
Rheumatic Fever
© Ministry of Health 2011
Published by: New Zealand Guidelines Group (NZGG) PO Box 10 665, The Terrace, Wellington 6145, New Zealand
ISBN (Electronic): 978-1-877509-60-5
Copyright
The copyright owner of this publication is the Ministry of Health, which is part of the New Zealand Crown. Content may be reproduced in any number of copies and in any format or medium
provided that a copyright acknowledgement to the New Zealand Ministry of Health is included and the content is neither changed, sold, nor used to promote or endorse any product or service, or used in any inappropriate or misleading context. For a full copyright statement, go to
www.health.govt.nz/about-site/copyright.
Funding and independence
This work was funded by the Ministry of Health. The work was researched and written by NZGG employees or contractors. Appraisal of the evidence, formulation of recommendations and reporting are independent of the Ministry of Health.
Statement of intent
NZGG produces evidence-based best practice guidelines to help health care practitioners, policy-makers and consumers make decisions about health care in specific clinical circumstances. The evidence is developed from systematic reviews of international literature and placed within the New Zealand context.
While NZGG guidelines represent a statement of best practice based on the latest available evidence (at the time of publishing), they are not intended to replace the health practitioner’s judgment in each individual case.
Citation: New Zealand Guidelines Group. Management of Group A Streptococcal Sore Throat. Wellington: New Zealand Guidelines Group; 2011.
Contents
Acknowledgments ... v
About the evidence review ... v
Purpose... v
The need for a guidance ... v
Scope of the evidence review... v
Target audience ... v
Treaty of Waitangi ... vi
Key point development process... vi
Definitions... vi
Summary ... 1
Key messages ...1
1 Introduction and context ... 2
GAS throat infection ...2
Acute rheumatic fever...2
GAS throat infection in New Zealand ...3
Acute rheumatic fever in New Zealand...3
Ethnic disparities ...9
Signs and symptoms of GAS throat infection ... 13
2 Rapid Antigen Diagnostic Tests ... 15
Rapid Antigen Diagnostic Test in people with a current sore throat ... 15
Rapid Antigen Diagnostic Test in people with a res olved sore throat ... 40
Timing of testing... 41
3 Antibiotic treatment ... 42
Antibiotic type ... 42
Antibiotic dose ... 51
Antibiotic duration... 60
4 Asymptomatic GAS infection... 70
4.1 Prevalence of GAS sore throat ... 70
Relationship between prevalence of asymptomatic GAS throat infection and rheumatic fever... 72
5 Community swabbing ... 75
Rheumatic fever outbreaks ... 75
Swabbing asymptomatic community members and households in areas of outbreak ... 77
Appendix 1: Methods... 84
Cont ribut ors ... 84
Research process ... 85
Research questions ... 85
Reviewing the literat ure ... 87
E vidence appraisal ... 89
Appendix 2: Abbreviations and glossary... 92
Abbreviations ... 92
Glossary ... 94
Acknowledgments
NZGG would like to thank Dr Richard Milne and his co-authors for granting us permission to use their analysed data on incidence of acute rheumatic fever in New Zealand, and Dr Rajesh Khanna, DHB (Paed), MPH; Co-ordinator, National Child Health Research Centre, National Institute for Health and Family Welfare, Delhi, for reviewing the analysis of Rapid Antigen Diagnostic Tests.
About the evidence review
Purpose
The purpose of this evidence review is to provide an evidence-based summary of current New Zealand and overseas evidence to inform best practice in the
management of people with Streptococcal A infection of the throat (pharyngitis) especially with the aim of preventing one of the more serious sequalae: Acute rheumatic fever (ARF).
The need for a guidance
Acute rheumatic fever rates in New Zealand have failed to decrease since the 1980s and remain some of the highest reported in a developed country. 1, 2 In response to this ongoing problem, the Ministry of Health wished to understand whether there were specific strategies for managing Group A beta-hemolytic streptococcal throat infection (GAS) throat infections that could help to lower the rate of ARF and prevent chronic rheumatic heart disease.
Scope of the evidence review
The evidence review specifically addresses the diagnosis of people with suspected GAS throat infection using Rapid Antigen Diagnostic tests, and the management of people with confirmed GAS throat infection using antibiotics. The review also provides information on asymptomatic GAS throat infection and community swabbing. It should be noted that the management of GAS throat infection in people with confirmed ARF, acute or chronic rheumatic heart disease or in people with recurrent GAS throat infection is beyond the scope of this work and has been excluded.
Target audience
The evidence review and guidance is intended primarily for the providers of care for New Zealanders with GAS throat infection.
Treaty of Waitangi
The New Zealand Guidelines Group acknowledges the importance of the Treaty of Waitangi to New Zealand, and considers the Treaty principles of partnership, participation and protection as central to improving Māori health.
NZGG’s commitment to improving Māori health outcomes means we work as an organisation to identify and address Māori health issues relevant to each piece of guidance. In addition, NZGG works to ensure Māori participation is a key part of the development process. It is important to differentiate between involving Māori in the guidance development process (the aim of which is to encourage participation and partnership), and specifically considering Māori health issues pertinent to the topic at all stages of the development process. While Māori participation in guidance
development aims to ensure the consideration of Māori health issues by the expert advisory group, this is no guarantee of such an output; the entrenched barriers Māori may encounter when involved in the health care system (in this case guidance development) need to be addressed. NZGG attempts to challenge such barriers by specifically identifying points in the development process where Māori health must be considered and addressed. In addition, it is expected that Māori health is considered at all points in the guidance in a less explicit manner.
Key point development process
NZGG convened a multidisciplinary expert advisory group (EAG) comprising members nominated by a diverse range of stakeholder groups. The research questions
developed by the Ministry of Health and NZGG were discussed with the EAG and were used to inform the search of the published evidence, from which systematic evidenced-based statements for best practice were derived. A one-day, face-to-face meeting of the full EAG was held, plus additional teleconferences, at which evidence was reviewed and key practice points were developed.
Full methodological details are provided in Appendix 1.
Definitions
Several common terms are currently in use for Group A beta-haemolytic streptococcal pharyngitis. NZGG has elected to use the term ‘GAS throat infection’ throughout this document in an attempt to keep the document clear and easy to read.
Summary
Key messages
Antibiotics should be initiated as soon as possible as there is no evidence to support current practice of delaying treatment by up to nine days and there is no evidence to support any other recommendation about the timing of treatment.
Children at high risk of developing rheumatic fever should continue to receive empiric (immediate) antibiotic treatment and the presence of GAS should continue to be confirmed by laboratory culture.
To establish asymptomatic carriage rate in the school population, where an
intervention is planned, all consented children should be swabbed before and after the intervention, regardless of symptoms to allow evaluation of programme
effectiveness.
There is reliable evidence about the efficacy of rapid antigen diagnostic tests, which give a result much faster than swabbing and testing.
Once daily amoxicillin is the first choice for antibiotic treatment for a GAS throat infection. Studies comparing amoxicillin with penicillin V report comparable outcomes. Amoxicillin is likely to achieve better compliance because of its daily dosing and ability to be taken with food compared with penicillin V’s more frequent dosing and the requirement to take it on an empty stomach.
1
Introduction and context
GAS throat infection
Streptococcal pharyngitis is caused by a Group A beta-haemolytic streptococcal infection and can trigger an inflammatory response in pharyngeal cells that causes many of the signs and symptoms of streptococcal pharyngitis.3 Group A streptococcus (GAS) is a bacterium often found in the throat and on the skin and can be carried by people who have no symptoms of illness.4 It affects the pharynx including the tonsils and possibly the larynx. After an incubation period of 2 to 5 days5, 6 there is an abrupt onset of illness with sore throat and fever.7 The tonsils and pharynx are inflamed and tonsillar exudate may be present.3 Throat pain is typically described as severe and is associated with difficulty in swallowing.3 Symptom severity varies and the presence of classically associated symptoms such as headache, malaise or gastrointestinal symptoms may be present in only 35% to 50% of patients.3
GAS sore throat is a communicable disease, spread through close contact with an infected individual. A definitive diagnosis is made based on the results of a throat culture. One of the more serious complications is acute rheumatic fever (ARF). Evidence indicates that antibiotic treatment for GAS throat infection in communities where the complication is common can reduce progression to ARF by more than two-thirds.8
Acute rheumatic fever
Acute rheumatic fever is an autoimmune response to infection with GAS bacteria. In New Zealand this response is primarily thought to be due to GAS throat infections. Though there has been discussion of the role of GAS skin infections in ARF (skin sepsis), convincing evidence has yet to be found to support this theory.9
The ensuing generalised inflammatory response to the GAS infection occurs in certain organs; the heart, joints, central nervous system (ie, brain) and skin. Inflammation of the heart (carditis) can cause long-term damage to the heart valves requiring heart valve replacement surgery. The consequence of recurrent exposure to ARF is the development of rheumatic heart disease (RHD) which may include valvular disease and cardiac myopathy and sequlae such as heart failure, atrial fibrillation, systemic embolism, stroke, endocarditis and the requirement for cardiac surgery.10 In the 1990s RHD was responsible for 120 deaths per year in New Zealand.1
GAS throat infection in New Zealand
While most sore throats are thought to be viral in origin, estimates of the numbers of sore throats due to GAS vary widely.3 Evidence on rates is slim. A review completed by the World Health Organization11 investigated the current evidence in relation to the burden of GAS infections on a worldwide scale and estimated that in children in developing countries (New Zealand was included in this group given the high rates of rheumatic fever in specific communities within New Zealand) the number of sore throats due to GAS could be as high as 40%.11
This estimate was based on the findings from three studies from populations where ARF is common: New Zealand (primarily in Māori and Pacific communities), Kuwait and Northern India. As the authors state, a positive GAS finding was not confirmed with serology and hence the true rate may be lower. New Zealand data is currently being collected in a school-based sore throat swabbing programme in Opotiki.12 Interim data shows that between October 2009 and December 2010, 8% of children reporting sore throats who were swabbed had a GAS infection (211 positive swabs of 2489 taken). Data collection is ongoing and analysis of trends would currently be premature.12 This data supports those accepted estimates that between 3% and 36% of sore throats are due to a GAS infection.3
There is currently no national data collected by ESR (Environmental Science and Research) for GAS infections in New Zealand independent of the notification of rheumatic fever.
Acute rheumatic fever in New Zealand
Acute rheumatic fever is reported two ways in New Zealand. The most current data, available publically in rate form, is that reported by the ESR as part of its annual surveillance of notifiable diseases. ESR collects this data from the regional public health units. Local District Heath Boards (DHBs) and treating hospital clinicians are required to use a specific ARF reporting process to notify regional public health
services of the ARF cases hospitalised within their region; this data is then reported to ESR by each region (who each have their own database to hold this data). This data may be reported from the DHBs to the regional public health units late and in bundles or not at all, given it requires a separate reporting process.
The second source of ARF data in NZ comes from the National Minimum Dataset (NMDS). This is a centralised dataset, in which all hospital encounters are coded within the hospitals themselves and entered straight into the database, the direct report nature does mean the NMDS data is viewed as more reliable and valid. However, given the large numbers of data involved in the NMDS, rates for ARF are not calculated on an annual basis.
Case Numbers of acute rheumatic fever
Acute rheumatic fever appears to have been virtually eradicated from most ‘developed’ countries yet rates in New Zealand have failed to decrease since the 1980s and remain some of the highest reported in a developed country.1, 2
The Ministry of Health’s ESR Annual Surveillance Report of notifiable disease has reported annually between 100 and 150 cases over the last decade (all ages).13 In 2010, 155 initial cases and 13 recurrent cases of rheumatic fever were notified (for all ages),14 while analysis of the hospital admissions and ICD discharge data provided in the NMDS indicated that from 1987 to 2008 there were between 150 and 230 cases per year (all ages).13
Hospitalisation data indicates that the primary episode of ARF usually occurs in
children aged between 5 to 14 years (Figure 1.1)1, 2 and a recent analysis of the NMDS hospitalisation data (using data up to 2009) reported 115 index cases of ARF in
children aged 5 to14 years in 2009 (Table 1.1).15 In 2010, approximately 75% (117 cases) of initial attack ARF cases notified were in those aged less than 15 years, with the highest age-specific rate in the 10 to 14 years age group (25.4 per 100 000 population, 75 cases).14
Figure 1.1 Number of hospitalisations between 2004 and 2010 for acute rheumatic fever by age
Table 1.1 Annual index cases by year and ethnicity for children 5 to 14 years of age 1993 2009 %change Ratio of 2009 to 1993 Cases Māori 32 62 +98% 2.0 Pacific Islands 17 48 +185% 2.9 European/Other 17 5 -168% 0.3 Total 64 115 +79% 1.8
Source: Milne, R., D. Lennon, et al. (2010). Burden and cost of rheumatic fever and rheumatic heart disease in New Zealand: focus on school age children. A report to the Ministry of Health. Auckland, New Zealand, Health Outcomes Associates Limited.
Rates of acute rheumatic fever
It is reported that rates of ARF in New Zealand since 1980 have remained at about 15 cases per 100,000 children aged 5 to 15 years of age.13
An analysis of hospitalisation data between 2000 and 200915 found a mean incidence rate for New Zealand children (all ethnicities) of 17.2 per 100,000, and distinct
inequalities in the rates between different ethnic groups (Table 1.2).
Table 1.2 ARF incidence rates for New Zealand children 5 to 14 years of age (2000–2009)
Māori Pacific Non-Māori/Pacific Total Rate ratio* Māori Pacific Mean 40.2 81.2 2.1 17.2 19.5 39.3 -95%CI 36.8 73.4 1.6 16.1 15.5 31.3 +95%CI 43.8 89.6 2.5 18.2 24.5 49.8 CI = confidence interval
* Compared to non-Māori/Pacific
Source: Milne, R., D. Lennon, et al. (2010). Burden and cost of rheumatic fever and rheumatic heart disease in New Zealand: focus on school age children. A report to the Ministry of Health. Auckland, New Zealand, Health Outcomes Associates Limited.
Of concern is that the inequality between ethnic groups has been widening over time. In the period studied (1993–2009) incidence rates increased by 79% and 73% for Māori and Pacific children respectively and declined by 71% for non-Māori/Pacific categories, with an overall increase of 59%15 (Figure 1.2). Māori and Pacific children 5 to 14 years of age accounted for 92% of new cases of ARF in the period 2000 to 2009 and comprised 30% of children in the 2006 census.15
Figure 1.2 Annual index cases and incidence rates for acute rheumatic fever in 1993–2009 for children 5 to 14 years of age
Source: Milne, R., D. Lennon, et al. (2010). Burden and cost of rheumatic fever and rheumatic heart disease in New Zealand: focus on school age children. A report to the Ministry of Health. Auckland, New Zealand, Health Outcomes Associates Limited.
The notification rates from ESR since 2000 for all ages and ethnicities are displayed in Figure 1.3 for both initial and recurrent attacks.14
Figure 1.3 Rates of notified rheumatic fever per 100,000 from 2000 to 2010
Source: ESR, 2011
Acute rheumatic fever in New Zealand by region
ESR reports rates for initial ARF attack by DHB, ethnic group, age and sex for the 2010 year. The highest rate of notified cases in 2010 was in Tairawhiti DHB (15.1 per
100,000 population, 7 cases), followed by Counties Manukau (10.6 per 100,000, 52 cases) and Northland (10.2 per 100,000, 16 cases) DHBs.14
However, given the small numbers, rates by DHB are more meaningful if examined over time. Analysis of the 2000 to 2009 hospitalisation data found that Counties Manukau DHB had the highest mean annual incidence rate for children (93.9 per 100,000) and contributed 298/700 cases (43%).15 Ninety-nine percent of index cases in Counties Manukau were in children of Māori or Pacific ethnicity. Table 1.3 displays incidence for the 2000 to 2009 years by DHB, ethnicity and decile.
Table 1.3 Index ARF cases and incidence rates for deciles 9 and 10 children aged 5 to 14 years, by District Health Board
Index ARF cases in 2000-2009 Mean annual incidence per 100,000
DHBa Māori Pacific
Non- Māori/
Pacific Total Māori Pacific
Non- Māori/
Pacific Total Counties Manukau 111 183 4 298 115.8 121.6 5.6 93.9
Northland 62 1 4 67 99.7 48.3 13.6 71.5
Capital and Coast 9 23 3 35 50.9 102.2 16.1 59.5
Aucklandb 13 49 5 67 58.3 86.8 12.1 55.7 Bay of Plenty 39 3 5 47 63.7 147.1 17.8 51.5 Tairawhiti 19 1 1 21 60.5 85.5 11.7 51.0 Hawke's Bay 27 7 3 37 60.9 107.5 12.2 49.0 Lakes 19 5 1 25 50.5 196.1 6.6 45.2 Waikato 43 3 4 50 60.4 36.6 7.3 37.2 Midcentral 10 2 0 12 43.2 51.7 0.0 22.7 Remaining 11c 20 14 7 41 18.8 29.6 3.9 12.4 Total 372 291 37 700 64.9 96.0 7.5 51.0 Top 10 DHBs 332 278 28 638 75.1 104.1 8.7 61.9 % total casesd 95% 95% 81% 94% Na Na Na Na % populatione 81% 84% 64% 76% Na Na Na Na
CCDHB=Capital and Coast DHB; CMDHB=Counties Manukau DHB; DHB=District Health Board; Na=not applicable
a
Sorted by total incidence rate
b
Waitemata patients were also hospitalised at Auckland hospital (ADHB)
c
Includes five North Island and all six South Island DHBs
d
Percentage of all index cases occurring in the top10 DHBs
e
Percentage of NZ population 5–14 years of age
Source: Milne, R., D. Lennon, et al. (2010). Burden and cost of rheumatic fever and rheumatic heart disease in New Zealand: focus on school age children. A report to the Ministry of Health. Auckland, New Zealand, Health Outcomes Associates Limited .
International rates of acute rheumatic fever
International comparisons for rates of ARF are problematic (due to global data quality issues) and estimates of the annual number of ARF cases must be considered a very crude estimate.11, 16 The World Health Organization estimates median incidence of 10 per 100,000 in established market economies; the data was not stratified by initial and recurrent attack.11 Recent data derived from Aboriginal communities in Australia indicates an incidence of 374 cases per 100,000,11 which is extremely high. Data on rates of ARF in Aboriginal communities is probably most usefully compared with data on the incidence in Māori and Pacific communities, rather than overall New Zealand incidence.
A systematic review which focused only on prospective population-based studies of first incidence of ARF (all ages) computed a mean yearly incidence rate of ≤10 cases per 100,000 in the USA and Western Europe and less than 10 cases per 100,000 in Eastern Europe, Australia and the Middle East.18 The only study that met the inclusion
criteria for the Australasian area was a New Zealand study from 1984 authored by Talbot.17 This was assessed by the authors as being of high quality. In that study, overall incidence in New Zealand was reported as being 22 per 100,000 in a population of people aged less than 30 years. A subgroup analysis from the Talbot study showed an incidence of greater than 80 per 100,000 for Māori. Again the authors highlighted the paucity of high quality population-based prospective studies of ARF around the world.
Mortality data related to ARF is also problematic.11 Reliable cause-specific mortality data relating to ARF and RHD are only available from indigenous populations living in relative poverty in wealthy countries (such as New Zealand). However, the New Zealand data cited is relatively old (1985–1987); age standardised mortality for RHD (with or without rheumatic fever) for non-Māori were reported at 2.0 per 100,000 per year, and 9.6 per 100,000 per year for Māori.11
Ethnic disparities
As has been highlighted in earlier sections, Māori and Pacific children experience a disproportionally high rate of ARF in New Zealand and rates of disparity are
widening1,15 (Figure 1.2). In the 10 years to 2005, the 5 to 14 year-olds rate for non-Māori and Other children was reported to be 3.0 per 100,000 (lower than the age standardised rate for all people of 3.4 per 100,000), while for Māori and Pacific children rates were 34.1 and 67.1 per 100,000 respectively.1 More recent analysis has found this disparity to have increased: for the period from 2000 to 2009, Māori children experienced an initial ARF rate of 40.2 per 100,000 (CI 36.8 to 43.8, p=.05), Pacific children 81.2 per 100,000 (CI 73.4 to 89.6, p=.05) and non-Māori children 2.1 per 100,000 (CI 1.6 to 2.5, p=.05) (Table 1.2).
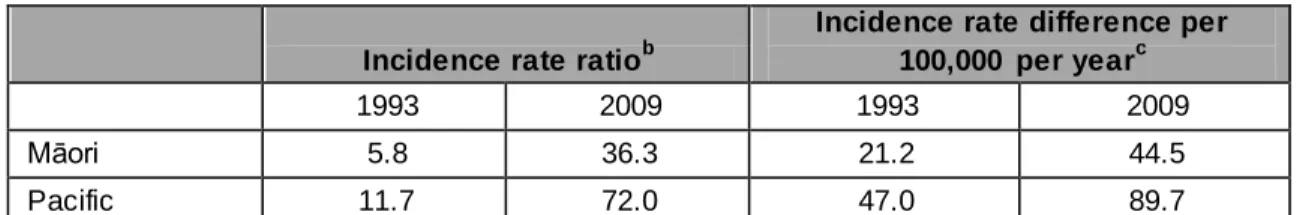
From 1996 to 2005, the New Zealand European and Others ARF rate decreased significantly while Māori and Pacific peoples’ rates increased. Compared with New Zealand European and Others, rate ratios were 10.0 for Māori and 20.7 for Pacific peoples.1 These disparities continued to increase after 2005. Incidence rates between 2000 and 2009 for children 5 to 14 years were about 20-fold higher for Māori children and 40-fold higher for Pacific children in this age group compared with non-Māori/Pacific categories.15 Rate ratios for Māori children were 19.5 and for Pacific children were 39.3, when compared with non-Māori children (Table 1.2). During 1993 and 2009 the ethnic disparity for Māori and Pacific children compared with non-Māori/Pacific children widened both in relative terms (the ratio of incidence rates) and in absolute terms (the difference in incidence rates) (Table 1.4).
Table 1.4 Changes in ethnic disparity over time for children 5 to 14 years of age during the period 1993–2009a
Incidence rate ratiob
Incidence rate differenceper 100,000 per yearc
1993 2009 1993 2009
Māori 5.8 36.3 21.2 44.5
Pacific 11.7 72.0 47.0 89.7
a
Based on linear regression of incidence rates on year
b Incidence rate of Māori or Pacific children divided by that for non-Māori/Pacific children c Difference in incidence rates between Māori or Pacific compared to non-Māori/Pacific
Source: Milne, R., D. Lennon, et al. (2010). Burden and cost of rheumatic fever and rheumatic heart disease in New Zealand: focus on school age children. A report to the Ministry of Health. Auckland, New Zealand, Health Outcomes Associates Limited.
Deaths associated with chronic RHD have increased from an average of 123 deaths per annum between 1971 and 1980 to 186 reported deaths in 2006.13 For Māori this equates to a prevalence rate for mortality of 8.5/100,000 population (95%CI 7.0 to 10.3) and for non-Māori 1.4/100,000 population (95%CI 1.2 to 1.5). Rheumatic heart disease mortality was over six times greater in Māori than non-Māori (relative risk (RR) 6.27 [95%CI 4.95 to 7.94]).13
Māori experience of rheumatic fever prevention and management
It is important to point out that the susceptibility of both Māori and Pacific children to rheumatic fever is most likely attributable to economic deprivation (and associated factors) experienced by Māori and Pacific people in New Zealand (ie, overcrowding, poor housing conditions, rural locations and decreased access to and utilisation of health care services)13. However, while a World Health Organization report into global burden of GAS-related disease states that ‘The burden of GAS diseases and the association of these diseases with poverty cannot be ignored’,11 the evidence to date has not been designed to reliably indicate which particular factors contribute to the high rates of rheumatic fever in New Zealand.
NZGG could not locate any specific data that explored Māori or Pacific people’s
experiences of, or access to, care for rheumatic fever. However, given that the majority of sore throats are managed in primary care settings, research relating to Māori
experiences of primary care and general practice is relevant.19 In a qualitative
investigation into Māori experience of health care in New Zealand, themes to emerge from hui with 86 Māori regarding general practice care is encapsulated in the following statement:
Participants’ experiences of general practice were, in the main, related to how they had been treated by health staff, and their hesitancy about seeking treatment. This hesitancy, or ‘wait and see’ attitude, described by many participants was associated with their financial concerns and their values and beliefs, as well as with their knowledge of how general practice staff were likely to treat them based on their previous experiences (Jansen et al). 19
Further surveying of a larger group of Māori (n=651), the majority of whom had either school- or pre-school aged children (54.2%), revealed, in general, a satisfaction with health services. However, clustering of the survey results found that that those in the younger age bracket (aged 39 years or less) reported a greater reluctance to use health and disability services, and a greater dissatisfaction with the interactions they had with these services. Of particular concern in relation to the management of sore throats in primary care is that a significantly-higher proportion of the younger
respondents agreed that:
they had to be quite sick and usually waited until the last minute before going to the doctor
it was too expensive to go every time they were sick the doctor was not good value for money
they do not like taking drugs for their illnesses.
Further reporting on the same study, but comparing Māori and non-Māori experiences of access to primary care,20 found differences in reported access to general practice care. For example, there were significant differences between Māori and non-Māori participants in terms of being: seen in the timeframe needed (93% of Māori 96.5% of non-Māori); given a suitable time (93.8% of Māori 98.3% of non-Māori); given a choice of times (68.3% of Māori 77.8% of non-Māori); and being seen on time (64.2% of Māori 75.1% of non-Māori).
The authors state that there may be a number of issues that explain the discrepancies, including non-medical staff attitudes to Māori patients, Māori cultural beliefs (including the tendency to noho whakaiti – to not cause a ruckus), and self-selection bias into the study. However, in relation to treatment of sore throat, timely access to a medical practitioner when required is very important. Once a sore throat is recognised as a serious issue by individuals and whānau living in high risk communities, a responsive primary care service upon presentation is no doubt critical to both treatment success and further developing those individual’s and community’s confidence in an equitable and responsive healthcare system.20
In terms of use of and access to treatments specifically relevant to the prevention of rheumatic fever, a study of antibiotic use in Te Tairawhiti between 2005 and 2006, revealed that Māori are dispensed fewer antibiotics than non-Māori, and the differences increase for Māori living in rural areas. Forty-eight percent of Māori people and 55% of non-Māori received one or more antibiotic prescriptions during the study period. Both Māori and non-Māori living in rural areas received fewer prescriptions for antibiotics, but the difference was much larger for Māori than for non-Māori. There was very low prevalence for antibiotic prescriptions for rural Māori children (aged <6 years) (43%) compared with that for rural non- Māori (68%) or urban dwellers (80% and 85% for Māori and non- Māori, respectively). Unfortunately no statistical analysis was
completed to determine if the differences were significant. However, given that in the Tairawhiti DHB area rates of rheumatic fever in 2010 were the highest in the country at 15.1 per 100 000 population, the report highlights a serious issue that warrants further exploration and certainly consideration in the context of the prevention of ARF in young Māori.21
Messages from research with Māori are clear; their experiences with primary healthcare services could be improved. For the New Zealand health systems and individual practitioners within that system it is important to consider how such experiences may impact upon the effective management of sore throats and the prevention of ARF.
Indigenous populations’ experience of rheumatic fever care
Given the lack of data identified specific to Māori experiences of ARF prevention and management, research with indigenous Aboriginal Australians may be useful to consider in the context of sore throat management approaches with both Māori and Pacific people, until more specific research is conducted.
Qualitative research on patient’s experiences of rheumatic fever programmes in Aboriginal communities in the Northern Territories provides useful insight for the implementation of rheumatic fever prevention programmes.
In a study of Aboriginal people in the Kimberly region of Australia with a diagnosis of rheumatic fever or rheumatic heart disease there was a varied understanding of either disease or its management. The findings highlighted the need for culturally-appropriate access to information about the disease, and the importance of the relationship
between patient and healthcare workers – compliance with medication was closely linked with positive patient-staff interactions.22 Although the study was mainly about secondary prophylaxis, the findings may equally apply in the prevention of rheumatic fever and GAS throat infection prevention.
A second qualitative study exploring the experiences of 15 patients with RHD or a history of rheumatic fever, 18 relatives and 18 health care workers in a remote
Aboriginal community, found a mix of staff and patient factors influence the success of the programme in terms of compliance to a secondary prophylaxis regime.23 Staffing factors that influence compliance included: appropriately trained, socially and culturally competent staff, staff willingness to treat patients at home, and an active recall system. Individual and family factors that encouraged uptake of regimes were an enhanced belief that the disease is chronic and serious, confidence in the health service and a feeling of holistic care, and family support for the treatment and belief in the efficacy of the treatment.
The same study found that staff factors that inhibited uptake included: negative perception of the secondary prophylaxis programme, conflicting priorities for staff, no effective strategy for dealing with absent patients, staff fatigue and frustration.23 Individual and family factors inhibiting uptake included: conscientious refusal of treatment, inconvenience to the patient, not ‘belonging’ to the health service, lack of family support and lack of confidence in the treatment.
Specific issues relating to primary care workforce requirements that have been noted during rheumatic fever work with aboriginal communities in Australia may also apply to New Zealand.24 Examples include: a lack of trained health professionals willing to stay for extended periods of time in remote communities to provide co-ordinated care, and a high turnover of nursing staff (in remote communities). There is also a scarcity of appropriately-trained Aboriginal health workers (these people are often considered the key players of the primary health service in remote settings), who are often pulled in many directions at the community level. This leads to a high burden of work and responsibility, with associated high rates of burnout.24
Signs and symptoms of GAS throat infection
Signs and symptoms of GAS throat Infection
Sore throat is one of the common signs and symptoms of streptococcal pharyngitis.6 Four guidelines were identified that summarised data on signs and symptoms of GAS throat infection;25-28 all agree that the cardinal symptoms suggestive of streptococcal pharyngitis include:
history of fever
tender anterior cervical adenopathy exudative tonsillitis
lack of cough.
A systematic review found that the most useful findings for evaluating the likelihood of streptococcal pharyngitis are the presence of tonsillar exudate, pharyngeal exudate, or exposure to streptococcal pharyngitis in the previous two weeks (positive likelihood ratios, 3.4, 2.1, and 1.9 respectively) and the absence of tender anterior cervical nodes, tonsillar enlargement or exudate (negative likelihood ratios, 0.60, 0.63, and 0.74,
respectively).3
GAS throat infection: timing, length
The Ministry of Health asked the research question below in an attempt to gain a better understanding of the window of opportunity for throat swabbing in people with
suspected GAS throat infection. NZGG undertook a literature review to answer the question.
Research question: When do sore throats occur in the natural course of streptococcal pharyngitis and how long they tend to last?
Body of evidence
Two guidelines from the United States agree that patients are more likely to present with GAS throat infection in the colder months of winter and spring.25, 26 The New Zealand Heart Foundation guideline found that evidence was sparse in relation to other climatic conditions and cite no clear seasonal peak in Auckland over a four-year period.
The natural history is for symptoms to subside within 3 to 5 days unless suppurative complications intervene.7, 25 Children are most infectious during the acute phase of the illness;5, 7 however, they may remain infectious for more than two weeks.5 Transmission is by inhalation of large droplets or direct contact with respiratory secretions.
Summary of findings
No evidence was found to suggest seasonal variation in GAS throat infection in New Zealand. Evidence from narrative reviews reported the incubation period to be 2 to 5 days and for symptoms to subside within 3 to 5 days from onset. Narrative reviews also report that children are most infectious during the acute phase of the illness. However, they may remain infectious for more than two weeks.
2
Rapid Antigen Diagnostic Tests
This chapter addresses diagnostic testing for people with suspected Streptococcal A infection of the throat, specifically, the accuracy of the Rapid Antigen Diagnostic Test (RADT). The chapter includes the following topics:
the accuracy of the RADT in people with a current sore throat the accuracy of the RADT in people with a resolved sore throat timing of testing.
Rapid Antigen Diagnostic Test in people with a current sore
throat
Research question: In children and adults with sore throats, what is the accuracy of the Rapid Antigen Diagnostic (RAD) testing compared to culture to confirm GAS?
We did not identify any existing English language systematic reviews investigating RADT for GAS throat infection. We undertook a systematic review and outline the specific methodology here, as it differs to the other sections in this report. Methodology for the remaining chapters can be found in Appendix 1.
Methods
Selection of studies for inclusion Study design
This review included diagnostic accuracy studies of which there are two basic types, defined by the Centre for Reviews and Dissemination; single-gate design and two-gate design. Full details of the designs of these studies is reported elsewhere.29 Single- and two-gate studies were eligible for inclusion if they compared a RADT/s with culture in a primary or secondary care setting. Studies were included only if they provided sufficient data to construct a 2x2 contingency table which displays numbers of true positive cases, false positive cases, false negative cases, and true negative cases.
Participants
Studies in adults and children who presented to a healthcare facility (primary or secondary care setting) with symptoms suggestive of streptococcal A throat infection were eligible for inclusion.
Studies in animals and studies with fewer than 10 participants were excluded. Studies where RADTs were done to assess outcomes or disease progression after treatment was started were also excluded.
Index test
Rapid antigen tests for diagnosing Streptococcal A pharyngitis were the index tests considered in this review. Any rapid antigen test was considered, including:
optical immunoassay
immunochromatographic detection double sandwich immunoassay latex particle agglutination
Polymerase chain reaction (PCR) assays.
Reference standard
Culture for diagnosing Streptococcal A pharyngitis was the reference standard
considered in this review. Studies carrying out throat swab culture carried out on blood agar at the same time as the index RAD test (or with minimal gap) were eligible for inclusion.
Data extraction and management
For each included study, we used standard evidence tables to extract characteristics of participants, data about the index tests and reference standard, and aspects of study methods. We extracted indices of diagnostic performance from data presented in each primary study by constructing 2x2 contingency tables of true positive cases, false positive cases, false negative cases, and true negative cases. If these were not reported, we reconstructed the contingency table using the available information on relevant parameters (sensitivity, specificity or predictive values). In cases of studies where only a subgroup of participants met the review inclusion criteria, data was extracted and presented only for that particular subgroup.
There were some studies where patients had undergone two different index tests with throat swab culture as the reference standard. In such studies, pooled analysis was done utilising data from the more common type of index test so as to avoid double counting.
Assessing study quality
Study quality was assessed using the QUADAS checklist,30 with each item scored as a yes/no response, or noted as unclear if insufficient information was reported to allow a judgment to be made; the reasons for the judgment made were documented. Results of the quality assessment are presented in the text, and in graphs using the Cochrane Collaboration’s Review Manager 5 software.31
A summary score estimating the overall quality of an article was not calculated since the interpretation of such summary scores is problematic and potentially misleading.32, 33
Data analysis and synthesis
Sensitivity, specificity, positive and negative predictive values, and likelihood ratios (with 95% confidence intervals) were calculated for each test using the methods described by the Centre for Reviews and Dissemination and are presented in tables. Efforts were made to identify common threshold points for each test so as to enable
calculation of pooled estimates of sensitivity and specificity. Coupled forest plots and summary receiver operator curves (sROCs) were generated (with 95% confidence intervals), giving graphical representations of sensitivity and specificity of a test in each study and allowing for assessment of diagnostic threshold and the area under the curve (AUC). Significant heterogeneity was considered where I2 was greater than 50%. Threshold effect was assessed by visual inspection of the sROC curve and by
computing Spearmans correlation coefficient between the logit of sensitivity and logit of 1-specificity.
In order to explore heterogeneity, we carried out predefined subgroup analysis for adults and children, and also for the different groups of rapid antigen tests identified in the literature. Where >10 studies were included in any pooled group, regression analyses were undertaken to investigate potential sources of observed heterogeneity. Additionally, we conducted sensitivity analysis excluding two-gate studies. All analyses were conducted using MetaDiSc software.34
Interpreting the results Diagnostic threshold
Threshold effects are common in diagnostic studies and occur when the included studies use different thresholds (explicitly or implicitly) to define positive and negative test results; this can be the reason for detectable differences in sensitivity and
specificity (heterogeneity). RAD tests utilise specific antibodies to detect the disease causing organisms and their results come as positive or negative only. However, threshold variability is expected since the results are based on visual inspection rather than a standardised measurement. In this analysis, threshold effects have been investigated in two ways:
a) by visual inspection of the relationship between pairs of accuracy estimates in ROC curves. If threshold effect is present, the ROC curve will show increasing
sensitivities with decreasing specificities, or vice versa, and is often described as a ‘shoulder-arm’ pattern or a ‘smooth curve’
b) by statistical computation of Spearmans correlation where a strong positive correlation suggests a threshold effect.
Summary measures
In a ROC curve the true positive rate (sensitivity) is plotted in function of the false positive rate (100-specificity) for different cut-off points of a parameter. Each point on the ROC curve represents a sensitivity/specificity pair corresponding to a particular study. The area under the ROC curve is a measure of how well a parameter can distinguish between two diagnostic groups (diseased/normal). The value for the area under the ROC curve can be interpreted as follows: an area of 0.84, for example, means that a randomly-selected individual from the positive group has a test value larger than that for a randomly-selected individual from the negative group in 84% of the time. When the variable under study cannot distinguish between the two groups, that is, where there is no difference between the two distributions, the area will be equal to 0.5 (the ROC curve will coincide with the diagonal).
When there is a perfect separation of the values of the two groups, ie, there no overlapping of the distributions, the area under the ROC curve equals 1 (the ROC curve will reach the upper left corner of the graph).
The area under the curve was interpreted using the following: 0.9 – 1 = excellent 0.8 – 0.9 = good 0.7 – 0.8 = fair 0.6 – 0.7 = poor 0.5 – 0.6 = very poor.35 Meta-regression
If substantial heterogeneity was identified, the reasons for variability were explored by meta-regression using the Littenberg and Moses Linear model36 weighted by the inverse of the variance where there were more than 10 studies in any pooled group. Estimations of coefficients of the model were performed by least squares method. The outputs from meta-regression modelling are the coefficients of the model, as well as the relative diagnostic odds ratio (rdOR) with respective confidence intervals. If a particular study level co-variate is significantly associated with diagnostic accuracy, then its coefficient will have a low p-value and the rdOR will give a measure of magnitude of the association.34
Body of evidence
Thirty-one studies were identified investigating the use of RAD tests in people with suspected GAS throat infection and are presented in Table 2.1. Studies were conducted in several countries across the world – 10 studies in the USA, four in
Canada, four in Western Europe (Sweden, Switzerland, Spain and Norway) three each in the UAE, Brazil and Turkey, three in Asia (Philippines, Hong Kong and Korea), one in Southern Europe (Cyprus) and one multicentre study spanning Brazil, Croatia, Latvia and Egypt (see Table 2.1). Except for a single two-gate study (diagnostic case control), all other studies were single-gate in design. The sample size in the studies ranged from 50 to 2472 patients (mean 587).
Of the 31 included studies, 19 studies reported data in children, nine reported data in adults, four studies reported data in both children and adults (three reported as a single data set, one reported as two separate data sets), and in one study age was unclear.
15 commercial brands employing four main types of RAD tests were identified in the included studies. These were:
nine brands employing chromatographic immunoassay tests: (QuickVue In-Line Strep A [Quidel Corporation]; Acceava Strep A [Inverness Medical Professional Diagnostics, Princeton, NJ, USA]; Genzyme OSOM Strep A [Genzyme Diagnostics, Street, San Diego, CA]; Abbott TestPack Plus Strep A [Abbott Laboratories];
Beckton-Dickinson Link 2 Strep A Rapid Test; Accustrip [Jant Pharmacutical Corportation, USA]; SD Bioline Strep A RAT [SD, Korea]; Detector strep A direct [Immunostics] and the Step A Rapid Test Device [SARTD] [Nova Century Scientific Inc.])
three brands employing sandwich immunoassays Tests: (Diaquick [DIALAB, Austria]; Kodak SureCell Strep A test [Kodak, USA]; INTEX Strep A Test II [INTEX Diagnostic Pharmazeutische Produkte, AG])
single brand employing optical immunoassay: (Strep A OIA MAX [Thermo Biostar/Inverness Medical Professional Diagnostics, Princeton, NJ, USA]) two brands using latex particle agglutination tests: (PathoDx Strep A kit [Inter
Medico]; Reveal color step A test [Murex]).
We did not identify any studies investigating immune-PCR assays.
Twenty-six of the included studies investigated a single index test compared to culture; five studies used two or more index tests of which only one (the most common) was included in the pooled results to avoid double counting.
Fourteen of the included studies used sheep blood agar as the reference standard, four used horse blood agar, one used goat blood agar, ten used blood agar but did not specify type and two studies did not report the culture medium.
Summary of findings
Table 2.1: Characteristics of included studies
Reference (study design)
Country Participants Age Reference
standard Type of RAD test Sens Spec PPV NPV LR+ LR-
Prevalence Rogo et al Single-gate37 USA n=228 90% w ere children Culture (5% sheep blood agar) Acceava 98.4% 98.8% 96.9% 99.4% 81 (95%CI 20, 320)* 0.02 (0.00, 0.11)* 28.1%
OSOM 98.5% 99.4% 98.5% 99.4% 160 (95%CI 23, 1126)* 0.02 (95%CI 0.00, 0.11)* 28.9% QuickVue 92.3% 96.3% 90.9% 96.9% 25 (95%CI 11, 55)* 0.08 (0.03, 0.19)* 28.5% Gurol et al
Single-gate38 Turkey n=453 All age groups Culture (5% sheep blood agar) QuickVue 64.6% 96.8% 81.0% 92.8% 81 (95%CI 20, 320)* 0.02 (0.00, 0.11)* 28.1% 0 to 9 years 70% 97.8% 90.3% 91.8% 32 (95%CI 10, 100)* 0.31 (0.19, 0.49)* 22.5% 20+ years 59.4% 96.1% 70.4% 93.8% 15 (95%CI 7.31, 32)* 0.42 (0.28, 0.64)* 13.4% Sarikaya et al Single-gate39 Turkey n=100 Adults aged 18 to 64 Culture (5% sheep blood agar) QuickVue 68.2% 89.7% 65.2% 90.9% 6.65 (95%CI 3.25, 14) 0.02 (0.19, 0.66) Rimoin et al Single-gate40 Brazil Croatia Egypt Latvia n=2472 Children 2 to 12 years Culture (5% sheep blood agar)
OIA MAX 79% 92% 80% 92% 10 (95%CI 8.67, 12) 0.23 (0.20, 0.26)
28.7% Kim Single-gate41 Korea n=293 Children (age not specified) Culture (no
detail) SD Bioline Strep A 95.9% 91.8% 95.9% 91.8%
11.75 (95%CI 6.04, 22.84) 0.04 (95%CI 0.02, 0.09) 66.5% Llor et al Single-gate42 Spain n=222 Adults over 14 years Culture (5%
blood agar) OSOM 94.5% 91.6% 78.8% 98.1%
11.28 (95%CI 6.8, 18.69)
0.06 (95%CI 0.02, 0.18)
Reference (study design)
Country Participants Age Reference
standard Type of RAD test Sens Spec PPV NPV LR+ LR-
Prevalence Tanz et al Single-gate43 USA n= 1848 Children 3 to 18 years Culture (5% sheep blood agar) QuickVue 71% 97% 91.65% 88.85% 26 (95%CI 19, 36) 0.29 (95%CI 0.26, 0.34) 29.9% Al-Najjar and Uduman Single-gate44 UAE n=425 Children (80% under 5)
Culture Diaquick 96% 99% 96% 99% 136 (95%CI 44, 419) 0.04 (0.01, 0.13)
14.3% Camardan et al Single-gate45 Turkey n=1248 Children Overall Culture (7% sheep blood agar)
INTEX Strep A Test
II 89.7% 97.2% 95.1% 93.88% 32 (95%CI 21, 49)
0.11 (95%CI 0.08, 0.14)
38.1%
0 to 6years 89.7% 96.9% 90.8% 96.54% 29 (95%CI 18, 48) 0.11 (95%CI 0.07, 0.17) 25.2% 7 to 12 years 90% 97.5% 97.67% 89.27% 36 (95%CI 16, 80) 0.10 (95%CI 0.07, 0.15) 53.9% 13+ years 87.1% 97.7% 96.43% 91.49% 38 (95%CI 5.5, 261) 0.13 (95%CI 0.05,
0.33) 41.3% Maltezou et al Single-gate46 Cyprus n=451 Children 2 to 14 years Culture (5% blood agar) Beckton-Dickinson Link 2 Strep A Rapid Test 83.1% 93.3% 82.4% 93.6% 12 (7.82, 18) 0.18 (0.13, 0.26) 32.4% Fontes et al Single-gate47 Brazil n=229 Children 1 to 18 years Culture (5% lamb blood agar) Latex particle agglutination 90.7 89.1 72.1 96.9 8.36 (5.42, 13) 0.10 (0.04, 0.24) 23.6% Wright et al Single-gate48 USA n=350 Children 0 to 18 years Culture (blood agar) OSOM 85.5% 97% 91% 95% 31 (95%CI 15, 65) 0.15 (95%CI 0.09, 0.25) 24.6% QuickVue 79.5% 95% 84.6% 93% 17 (95%CI 9.62, 30) 0.21 (95%CI 0.14, 0.33) 24.6%
Reference (study design)
Country Participants Age Reference
standard Type of RAD test Sens Spec PPV NPV LR+ LR-
Prevalence Abu Sabbah and Ghazi Single-gate49 Saudi Arabia n=355 Adults and children Culture (horse blood agar) Detector Strep A Direct 88% 91% 70% 97% 10 (95%CI 6.90, 15) 0.13 (95%CI 0.07, 0.25) 18.9% Children aged 4 to 14 81% 86% 67% 93% 5.93 (95%CI 3.33, 11) 0.21 (95%CI 0.10, 0.48) 25.2% Adults aged >15 93% 93% 73% 98% 14 (95%CI 8.22, 23) 0.08 (95%CI 0.03, 0.24) 16.1% Araujo Filho et al Single-gate50
Brazil n=81 Adults over 18 years Culture (5% goat blood agar) OIA MAX 93.9% 68.7% 67.4% 94.2% 3.01 (1.96, 4.61) 0.09 (0.02, 0.34) 40.7% Forw ard et al Single-gate51 Canada n=818 overall Culture (5% sheep blood agar)
Step A Rapid Test
Device (SARTD) 71.9% 94.3% 76.9% 92.7% 11 (95%CI 7.92, 14) 0.25 (95%CI 0.19, 0.33) 19.6% n=328 adults 67.8% 93.8% 77.7% 90.2% 11 (95%CI 7.24, 17) 0.34 (95%CI 0.26, 0.45) 24.1% n=490 children Children w ere <16 years 81.1% 94.9% 75.4% 96.3% 16 (95%CI 9.41, 27) 0.20 (95%CI 0.11, 0.35) 16.2% Humair et al Single-gate52 Sw itzer-land n=372 Patients age >15 years Culture (blood agar)
Testpack Plus Strep A w /OBC[On Board Controls] II (Abbott Laboratories) 91.4% 95.3% 92.1% 94.9% 19.3 (95%CI 11, 34) 0.09 (95%CI 0.05, 0.16) 37.6% Shaheen and Hamdan Single-gate53 Amman n=200 Adults 20 to 42 years (mean 28.3 years) Culture (blood agar) Latex particle agglutination 90.00% 98.22% 90.00% 98.22% 50.70 (95%CI 16.41, 156.61) 0.10 (0.03, 0.30) 15.1% Atlas et al Single-gate54
USA n=150 Adults over
18 years Culture Acceava 92.1% 100% 100% 98% Not estimable
0.08 (95%CI 0.03, 0.24)
Reference (study design)
Country Participants Age Reference
standard Type of RAD test Sens Spec PPV NPV LR+ LR-
Prevalence Ezike et al Single-gate55 USA n=363 Children 5 to 18 years Culture (5% sheep blood agar) OIA MAX 94.7%
100% 100% 96.2% Not estimable 0.05 (95%CI
0.02-0.14)* 42.4% Lindbaek et al Single-gate56 Norw ay n=306 Adults and children (<10 years) Culture (Columbia agar w ith horse blood)
TestPack Plus 96% 86% 79.7% 97.7% 7.0 (95%CI 4.92,9.95) 0.04 (95%CI 0.02,0.11) 36% Santos et al Single-gate57 Brazil n=50 Children age 1 to 12 years Culture (5%
blood agar) TestPack 73% 94% 85% 88%
12 (95%CI 3.14, 14.49) 0.28 (95%CI 0.12, 0.66) 34% Nerbrand et al Single-gate58
Sw eden n=536 All ages
Culture (6% defibrinised horse blood) QuickVue 73.9% 86.8% 59.4% 92.7% 5.60 (95%CI 4.28, 7.32) 0.30 (95%CI 0.21, 0.43) 15.3% n=615 All ages Culture (6% defibrinised horse blood)
TestPack 82.8% 96.1% 92.7% 94.2% 21 (95%CI 14, 33) 0.18 (95%CI 1.02, 0.26) 21.1% Chapin et al Single-gate59 USA n=520 Children (age not specified) Culture (5% sheep blood agar).
Thermo Biostar OIA 86.1% 97.1% 93.7% 93.4% 28 (95%CI 15, 52) 0.13 (95%CI 0.09, 0.18) 37.9% Gieseker et al Single-gate60 USA n=302 Children (age not specified)
Culture OSOM 97% 92% 82% 98% 12 (95%CI 7.4, 18) 0.04 (95%CI 0.01, 0.11) 28.8% OIA Max 79% 95% 84% 92% 15 (95%CI 8.29, 25) 0.252 (95%CI 0.14, 0.34) 27.2% Rosenberg et al Single-gate61
Canada n=126 All ages
Culture (5% sheep blood
agar)
Testpack 75% 99% 96% 92% 71(95%CI 9.93, 500) 0.25 (95%CI 0.14, 0.46)
Reference (study design)
Country Participants Age Reference
standard Type of RAD test Sens Spec PPV NPV LR+ LR-
Prevalence Keahey et al Single-gate62 Canada n=165 Children age 5 to 16 years Culture (Sheep blood agar)
PathoDx Strep A Kit 86.7% 80.1% 78.3% 87.8% 4.33 (95%CI 2.84, 6.61) 0.17 (95%CI 0.09, 0.30) 45.5% Gieseker et al Single-gate63 USA n=887 Children (age not specified) Culture (no details) OSOM 87.6% 96.2% 87.6% 96.2% 22.81 (95%CI 15.60, 33.37) 0.13 (95%CI 0.09, 0.18) 23.7% Sheeler et al
Tw o-gate64 USA n=211 cases All ages
Culture (5% sheep blood
agar)
Testpack Plus 91% 96% 96% 90% 9.92 (95%CI 5.5, 18) 0.04 (95%CI 0.02, 0.11)
50.2%
n=232 controls All ages
Culture (5% sheep blood
agar)
Testpack Plus 70% 98% 92% 90% 8.88 (95%CI 5.75, 14) 0.09 (95%CI 0.04, 0.24) 20.7% Wong and Chung Single-gate65 Hong
Kong n=1491 All ages
Culture (5% horse blood
agar)
Accustrip 52.6% 98.2% 52.6% 98.2% 28.9 (95%CI 13, 63) 0.48 (95%CI 0.30, 0.78) 37% Kurtz et al Single-gate66 USA n=537 Children age 4 to 15 years Culture (5% standard) Testpac Plus 80% 92.7% 83.1% 91.1% 10.89 (6.38, 18.59) 0.22 (95%CI 0.14, 0.34) 31.1% Alesna et al Single-gate67 Philip-pines n=233 All ages >3 years Culture (5% sheep blood agar)
Overall 94.12% 89.45% 60.38% 98.89% 8.92 (95%CI 5.90, 13) 0.07 (95%CI 0.02, 0.25)
14.6%
Testpack Plus 93.3% 94.7% 73.7% 98.9% 18 (95%CI 7.4, 42) 0.07 (95%CI 0.01, 0.47)
13.8% Kodak SureCell 94.7% 84.8% 52.9% 98.9% 6.22 (95%CI 3.91,
9.88)
0.06 (95%CI 0.01, 0.42)
15.3%
Sens = sensitivity; Spec = specificity; PPV = positive predictive value; NPV = negative predictive value; LR+ = positive likelihood ratio; LR - = negative likelihood ratio
Quality of included studies
The overall methodological quality is summarised in Figures 2.1 and 2.2.
Most studies reported representative spectrums of patients and explained selection criteria. Two studies did not recruit a representative spectrum of patients:55, 61 both studies used a convenience sample based on the availability of the lead investigator. Two studies did not clearly describe selection criteria.4.9, 53
Almost all the included studies reported avoidance of partial verification and differential verification, and all reported avoidance of incorporation bias. Only one study did not adequately describe the details or execution of the RAD test or culture.44 Blinding was not well reported, approximately 75% of studies reported blinding of the index test, but less than half of the included studies reported blinding of the reference standard. In one study it was unclear whether the same clinical information would be available in
practice.64
Withdrawals were not explained in three studies: in one study67 233/269 patients who completed both RAD test and culture were reported with no reason for withdrawals given, in another study54 two patients did not receive culture and in the third study45 it was not clear how many participants were included. Overall, the studies included were of high quality.
Figure 2.2 Summary of methodological quality
Overall results
The forest plots of sensitivities and specificities from all 31 studies are shown in Figure 2.3. Sensitivities of all tests ranged from 53% to 96%, specificities from 69% to 100%. Of the 31 included studies, 26 reported specificities greater than 90%. Eight of the 31 studies reported sensitivities greater than 80%. The pooled average sensitivity and specificity were 84.5% (95%CI 83.4 to 85.6) and 94.7% (95%CI 94.2 to 95.1),
respectively, but significant heterogeneity was noted between studies with I2 tests of 89.1% and 89.8%, respectively. Figure 2.4 shows the spread of studies on a ROC plane.
Figure 2.3 Forest plot of overall study results (sensitivity and specificity) Study Abu Sabbah 2006 Al Najjar 2008 Alesna 2000 Araujo Filho 2006 Atlas 2005 Camurdan 2008 Chapin 2002 Ezike 2005 Fontes 2007 Forward 2006 Gieseker 2002 Gieseker 2003 Gurol 2010 Humair 2006 Keahey 2002 Kim 2009 Kurtz 2000 Lindbaek 2004 Llor 2009 Maltezou 2008 Nerbrand 2002 Rimoin 2010 Rogo 2011 Rosenberg 2002 Santos 2003 Sarikaya 2010 Shaheen 2006 Sheeler 2002 Tanz 2009 Wong 2002 Wright 2007 TP 59 68 14 31 38 427 173 71 49 123 84 184 51 128 65 187 64 106 52 121 107 561 65 24 11 15 27 165 395 10 71 FP 25 3 5 15 0 22 10 0 19 48 18 26 12 11 18 8 13 27 14 21 19 136 1 1 2 8 3 19 36 9 7 FN 8 3 1 2 3 49 24 4 5 37 3 26 28 12 10 8 16 4 3 25 22 149 1 8 4 7 3 4 158 9 12 TN 263 422 89 33 112 751 313 102 156 610 197 651 362 221 72 90 164 169 153 284 466 1626 161 93 32 70 166 44 1259 486 248 Sensitivity 0.88 [0.78, 0.95] 0.96 [0.88, 0.99] 0.93 [0.68, 1.00] 0.94 [0.80, 0.99] 0.93 [0.80, 0.98] 0.90 [0.87, 0.92] 0.88 [0.82, 0.92] 0.95 [0.87, 0.99] 0.91 [0.80, 0.97] 0.77 [0.70, 0.83] 0.97 [0.90, 0.99] 0.88 [0.82, 0.92] 0.65 [0.53, 0.75] 0.91 [0.86, 0.95] 0.87 [0.77, 0.93] 0.96 [0.92, 0.98] 0.80 [0.70, 0.88] 0.96 [0.91, 0.99] 0.95 [0.85, 0.99] 0.83 [0.76, 0.89] 0.83 [0.75, 0.89] 0.79 [0.76, 0.82] 0.98 [0.92, 1.00] 0.75 [0.57, 0.89] 0.73 [0.45, 0.92] 0.68 [0.45, 0.86] 0.90 [0.73, 0.98] 0.98 [0.94, 0.99] 0.71 [0.67, 0.75] 0.53 [0.29, 0.76] 0.86 [0.76, 0.92] Specificity 0.91 [0.87, 0.94] 0.99 [0.98, 1.00] 0.95 [0.88, 0.98] 0.69 [0.54, 0.81] 1.00 [0.97, 1.00] 0.97 [0.96, 0.98] 0.97 [0.94, 0.99] 1.00 [0.96, 1.00] 0.89 [0.84, 0.93] 0.93 [0.90, 0.95] 0.92 [0.87, 0.95] 0.96 [0.94, 0.97] 0.97 [0.94, 0.98] 0.95 [0.92, 0.98] 0.80 [0.70, 0.88] 0.92 [0.85, 0.96] 0.93 [0.88, 0.96] 0.86 [0.81, 0.91] 0.92 [0.86, 0.95] 0.93 [0.90, 0.96] 0.96 [0.94, 0.98] 0.92 [0.91, 0.93] 0.99 [0.97, 1.00] 0.99 [0.94, 1.00] 0.94 [0.80, 0.99] 0.90 [0.81, 0.95] 0.98 [0.95, 1.00] 0.70 [0.57, 0.81] 0.97 [0.96, 0.98] 0.98 [0.97, 0.99] 0.97 [0.94, 0.99] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1
Figure 2.4 ROC of RAD tests
Sensitivity analysis excluding the two-gate study design did not significantly alter the pooled average sensitivity or specificity (84.0% [95%CI 82.8 to 85.1] and 94.8% [95%CI 94.4 to 95.2], respectively). Post-hoc sensitivity analysis excluding any study that scored a ‘no’ on the QUADAS checklist did not significantly alter the pooled average sensitivity or specificity (82.8% [95%CI 81.4% to 84.1%] and 94.5% [95%CI 94.0% to 95.0%], respectively). Significant heterogeneity was noted for all summary measures.
Chromatographic immunoassay tests
The most commonly-reported rapid antigen tests were chromatographic immunoassay tests of which nine different types were identified in the included studies. The forest plots of sensitivities and specificities are shown for 26 comparisons (21 studies) in Figure 2.5.
Sensitivities of all tests ranged from 53% to 98%, specificities from 70% to 100% with all but one study reporting specificity of more than 85% (Figure 2.6). The pooled overall sensitivity and specificity were 83.9% (95%CI 82.3 to 85.4) and 94.4% (95%CI 93.8 to 95.0), respectively. Tests of homogeneity for sensitivity and specificity reported I2 tests of 90.6% and 89.0%, respectively; indicating significant heterogeneity. Sensitivity analysis excluding the two-gate study design did not significantly alter the pooled average sensitivity or specificity (82.5% [95%CI 80.8 to 84.1] and 94.6% [95%CI 94.0 to 95.2], respectively).
Figure 2.5: Forest plot of study results (sensitivity and specificity) for chromatographic immunoassay tests
QuickVue Study Gurol 2010 Nerbrand 2002 Rogo 2011 Sarikaya 2010 Tanz 2009 Wright 2007 TP 51 61 60 15 395 66 FP 12 60 6 8 36 12 FN 28 21 5 7 158 17 TN 362 394 157 70 1259 243 Sensitivity 0.65 [0.53, 0.75] 0.74 [0.64, 0.83] 0.92 [0.83, 0.97] 0.68 [0.45, 0.86] 0.71 [0.67, 0.75] 0.80 [0.69, 0.88] Specificity 0.97 [0.94, 0.98] 0.87 [0.83, 0.90] 0.96 [0.92, 0.99] 0.90 [0.81, 0.95] 0.97 [0.96, 0.98] 0.95 [0.92, 0.98] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1 Acceava Study Atlas 2005 Rogo 2011 TP 38 63 FP 0 2 FN 3 1 TN 112 162 Sensitivity 0.93 [0.80, 0.98] 0.98 [0.92, 1.00] Specificity 1.00 [0.97, 1.00] 0.99 [0.96, 1.00] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1 OSOM Study Gieseker 2002 Gieseker 2003 Llor 2009 Rogo 2011 Wright 2007 TP 84 184 52 65 71 FP 18 26 14 1 7 FN 3 26 3 1 12 TN 197 651 153 161 248 Sensitivity 0.97 [0.90, 0.99] 0.88 [0.82, 0.92] 0.95 [0.85, 0.99] 0.98 [0.92, 1.00] 0.86 [0.76, 0.92] Specificity 0.92 [0.87, 0.95] 0.96 [0.94, 0.97] 0.92 [0.86, 0.95] 0.99 [0.97, 1.00] 0.97 [0.94, 0.99] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1
Detector Strep A Direct Kit Study Abu Sabbah 2006 TP 59 FP 25 FN 8 TN 263 Sensitivity 0.88 [0.78, 0.95] Specificity 0.91 [0.87, 0.94] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1
Abbott Test Pack Study Alesna 2000 Humair 2006 Kurtz 2000 Lindbaek 2004 Nerbrand 2002 Rosenberg 2002 Santos 2003 Sheeler 2002 TP 14 128 64 106 107 24 11 165 FP 5 11 13 27 19 1 2 19 FN 1 12 16 4 22 8 4 4 TN 89 221 164 169 466 93 32 44 Sensitivity 0.93 [0.68, 1.00] 0.91 [0.86, 0.95] 0.80 [0.70, 0.88] 0.96 [0.91, 0.99] 0.83 [0.75, 0.89] 0.75 [0.57, 0.89] 0.73 [0.45, 0.92] 0.98 [0.94, 0.99] Specificity 0.95 [0.88, 0.98] 0.95 [0.92, 0.98] 0.93 [0.88, 0.96] 0.86 [0.81, 0.91] 0.96 [0.94, 0.98] 0.99 [0.94, 1.00] 0.94 [0.80, 0.99] 0.70 [0.57, 0.81] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1
Strep A Rapid Test device Study Forward 2006 TP 123 FP 48 FN 37 TN 610 Sensitivity 0.77 [0.70, 0.83] Specificity 0.93 [0.90, 0.95] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1
SD Bioline Strep A RAT Study Kim 2009 TP 187 FP 8 FN 8 TN 90 Sensitivity 0.96 [0.92, 0.98] Specificity 0.92 [0.85, 0.96] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1
Link 2 Strep A Rapid Test Study Maltezou 2008 TP 121 FP 21 FN 25 TN 284 Sensitivity 0.83 [0.76, 0.89] Specificity 0.93 [0.90, 0.96] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1 Accustrip Study Wong 2002 TP 10 FP 9 FN 9 TN 486 Sensitivity 0.53 [0.29, 0.76] Specificity 0.98 [0.97, 0.99] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1
The pattern of the points on the summary ROC (sROC) in Figure 2.6 do not show a threshold effect and the Spearman correlation coefficient was 0.410 (p=0.065)
indicating borderline, but not significant presence of a threshold effect. The area under the sROC curve was 0.9672. Table 2.2 shows summary measures for chromatographic immunoassay tests in children and adults; the tests appear to be good at ruling in streptococcal A sore throat in both groups. The test appears to be better at ruling out streptococcal A sore throat in adults, however, significant heterogeneity was present in all summary measures.
Figure 2.6 Summary ROC plot for chromatographic immunoassay tests*
*Red circles indicate children, yellow circles indicate adults, green circles indicate studies that included all age groups.
Sensitivity sROC Curve
1-specificity 0 0.2 0.4 0.6 0.8 1 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 Symmetric sROC AUC = 0.9672 SE(AUC) = 0.0058 Q* = 0.9153 SE(Q*) = 0.0090
Table 2.2 Summary measures for children and adults Number of participants (number of studies) Pooled sensitivity (95%CI) Heterogeneity (I2) Pooled specificity (95%CI) Heterogeneity (I2) Total children n=5444 (11 studies) 81.1 (79.1, 83.0) 91.4% 95.4 (94.7, 96.0) 73.0% Total adults n=1153 (5 studies) 92.1 (88.9, 94.7) 72.4% 92.4 (90.3, 94.1) 86.8% Total mixed population (adults and children) n=1517 (5 studies) 86.4 (82.2, 90.0) 92.1% 92.2 (90.5, 93.6) 95.4% Total n=8131 (21 studies) 83.9 (82.3, 85.4) 90.6% 94.4 (93.8, 95.0) 89.0%
Pooled results for the most common chromatographic immunoassay tests were similar; the pooled sensitivity and specificity for the Quickvue test (n=3503, 6 studies), the OSOM test (n=1977, 5 studies) the Abbott test (n=2065, 8 studies) and the Acceava test (n=381, 2 studies) were comparable (Table 2.3). Sensitivity analysis excluding the two-gate study design from the Abbott test did not alter results.
Table 2.3 Summary measures by test brand
Name of test Number of participants (number of studies) Pooled sensitivity (95%CI) Heterogeneity (I2) Pooled specificity (95%CI) Heterogeneity (I2) QuickVue n=3503 (6 studies) 73.3 (70.3, 76.2) 76.4% 94.9 (94.0, 95.7) 92.7% Acceava n=381 (2 studies) 96.2 (90.5, 99.0) 55% 99.3 (97.4, 99.9) 52.2% OSOM n=1977 (5 studies) 91.0 (88.2, 93.4) 76.6% 95.5 (94.3, 96.5) 82.2% Detector Strep A Direct n=355 (1 study) 88 (78–95) - 91 (87, 94) - Abbott n=2065 (8 studies) 89.7 (87.2, 91.9) 84.3% 92.9 (91.5, 94.2) 88.3% Strep A Rapid test device n=818 (1 study) 77 (70–83) - 93 (90–95) - SD Bioline n=293 (1 study) 96 (92, 98) - 92 (85–96) - Link 2 n=451 (1 study) 83 (76–89) - 93 (90–96) - Accustrip n=514 (1 study) 53 (29–76) - 98 (97–99) - Total n=8131 (21 studies) 83.9 (82.3, 85.4) 90.6% 94.4 (93.8, 95.0) 89.0%
Double sandwich immunoassay tests
Three different types of double sandwich immunoassay tests were reported in three different studies. The forest plots of sensitivities and specificities are shown in Figure 2.7. Tests of homogeneity for sensitivity and specificity reported I2 tests of 45.0% and 94.9%, respectively; indicating no heterogeneity for sensitivity, and significant
heterogeneity for specificity.
Sensitivities ranged from 90% to 96%, specificities from 85% to 99% (Figure 2.8). The pooled sensitivity and specificity were 90.6% (95%CI 87.9 to 92.9) and 96.9% (95%CI 95.8 to 97.7), respectively. The area under the ROC curve was 0.9802. There are too few studies of double sandwich immunoassay tests to draw conclusions about their accuracy.
Figure 2.7 Forest plot of study results (se nsitivity and specificity) for double sandwich immunoassay tests Diaquick Study Al Najjar 2008 TP 68 FP 3 FN 3 TN 422 Sensitivity 0.96 [0.88, 0.99] Specificity 0.99 [0.98, 1.00] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1
INTEX Strep A test II
Study Camurdan 2008 TP 427 FP 22 FN 49 TN 751 Sensitivity 0.90 [0.87, 0.92] Specificity 0.97 [0.96, 0.98] Sensitivity 0 0.2 0.4 0.6 0.8 1 Specificity 0 0.2 0.4 0.6 0.8 1 Kodak Surecell Study Alesna 2000 TP 18 FP 16