Neonatal Encephalopathy, and Intrapartum Asphyxia
Michael I. Shevell, MD, CM, FRCPC
The terms “cerebral palsy,” “neonatal encephalopathy,” and “intrapartum asphyxia” are frequently used in pediatric neurology. This article presents concise, verifiable definitions for each of these entities based on our current understanding and formulates the nature of the interrelationships between them. The aim is to provide a level of clarity that will enhance diagnostic and pathogenetic precision and minimize conceptual misunderstand-ing. This should aid future therapeutic and research efforts in this important area.
© 2004 Elsevier Inc. All rights reserved.
T
HE TERM “Bermuda triangle” has entered our lexicon as an eponymous synonym for mystery or confusion. Nominally denoting a largely open stretch of the Atlantic Ocean with its apices on Florida, Bermuda, and Puerto Rico, its lore is a seemingly disproportionate share of un-explained disappearances of ships and airplanes. Careful scrutiny of the actual record has revealed that the triangle is no more dangerous than any other equivalent stretch of open ocean and that a rational explanation (most commonly weather or human error) can explain the misfortunes that have occurred there.1Cerebral palsy (CP), neonatal encephalopathy, and intrapartum asphyxia are all concepts integral to an understanding of neonatal neurology. How-ever, these terms are often used imprecisely, sow-ing confusion that can take on the appearance of a quagmire. This confusion hampers communication among health professionals and may convey erro-neous impressions regarding clinical evolution and causality. However, like the actual Bermuda trian-gle of popular mythology, careful scrutiny can help clarify the relationships among these entities.
Epidemiologic prevalence data highlight the scope of the problem. CP occurs in 1.5 to 2.5/1000 live births;2neonatal encephalopathy, in 1.8 to 7.7/1000 live births;3and intrapartum asphyxia, in 1.5 to 3.0/ 1000 live births.4These numbers must be considered in the context of an overall frequency of the full
spectrum of neurodevelopmental disabilities of 5% to 7% of the pediatric population.5
This article provides a systematic review of the consensus definitions of these 3 concepts, together with a brief survey of what we know about them. It then systematically explores the relationships between these concepts.
CEREBRAL PALSY
“Cerebral palsy” is a historical term first intro-duced into the medical literature in the latter half of the nineteenth century.6 It is a clinically defined symptom complex that functions as a “term of convenience” and provides a shorthand way to communicate about a group of commonly encoun-tered children with developmental disability.2 Va-lidity for the concept is imparted by a commonality of core features shared by the members of this group, including impairments, challenges, medical requirements, and rehabilitation needs.7 Persons with CP display considerable variability in terms of severity8 and comorbid conditions.9 CP is but one type of childhood neurodevelopmental disabil-ity encountered in practice.
A consensus definition for CP was not offered until 1958.10The most recent consensus definition defines CP as “an umbrella term covering a group of non-progressive but often changing motor im-pairment syndromes secondary to lesions or anom-alies of the brain arising in the early stages of its development.”11Stated more simplistically, CP is a static, nonprogressive motor impairment of early onset that is cerebral in origin. Here, “static and nonprogressive” means that the process responsi-ble for the neurologic deficits cannot be ongoing, with the infliction of additional injury or damage on the brain as time proceeds.12 This effectively excludes neoplastic, neurodegenerative, and meta-bolic processes from the rubric of CP.13However, although the pathological lesions are nonprogres-sive, it is well recognized that the apparent clinical From the Departments of Neurology/Neurosurgery and
Pe-diatrics, McGill University and Division of Pediatric Neurol-ogy, Montreal Children’s Hospital, Montreal, Quebec, Canada. Address reprint requests to Michael Shevell, CM, FRCPC, Montreal Children’s Hospital, Room A-514, 2300 Tupper Street, Montreal, Quebec, Canada H3H 1P3.
© 2004 Elsevier Inc. All rights reserved.
1071-9091/04/1101-0000$30.00/0 doi:10.1016/j.spen.2004.01.005
manifestations may change against the backdrop of a maturing nervous system.7
All individuals with CP have motor impairment characterized by objective, reproducible abnormal-ities on systematic examination.14 This includes alterations in tone, posture, response to muscle stretch, and reflexes.15 Clinically, this motor im-pairment may manifest as delayed acquisition of motor skills, clumsiness, and gait/ambulatory dif-ficulties. Other neurologic disabilities (eg, epi-lepsy, mental retardation) often co-occur with CP yet are not necessary features for diagnosis or an invariable accompaniment.
Universal agreement does not yet exist on the “early onset” component of the definition of CP.7 The upper limit for inclusion of postnatal cases of acquired CP can vary considerably.16In addition, the initial age at which the diagnosis can be enter-tained is also not uniformly agreed on, given the potential for transitory early neurologic abnormal-ities that resolve on a second evaluation at a later date.17Symptomatically, early onset is manifested by early hand preference, motor delay, stiffness, or seizures.
Traditionally, a long list of syndromic disorders or chromosomal abnormalities have been excluded from the concept of CP.13It is acknowledged that local idiosyncrasies may influence the precise con-text of this approach. What is agreed on is that neural tube defects and neuromuscular disorders, which may in themselves result in early-onset motor impairment syndromes, are excluded from the CP diagnostic label.
From the foregoing, it is apparent that CP is conceptualized as a possible outcome. It is a quite heterogeneous entity with respect to pathogenesis, clinical manifestations, and evolution.18Although there may be a multiplicity of possible causes,19 from a pathogenetic perspective what is shared is a congenital aberration or acquired injury to the maturing, not yet fully formed, central nervous system. The onset of this aberration or injury may be prenatal, perinatal, or postnatal in timing.
Etiologically heterogeneous, clinical research in CP has traditionally focused on the identification of prenatal and perinatal risk factors for the later identification of CP.20-22 This has permitted the elucidation of possible pathogenetic mechanisms. Uncertainty exists regarding the actual causal spec-trum of CP and the relative contribution of the various causes.5Traditionally, the role and contri-bution of intrapartum asphyxia as an etiologic
cause has been emphasized23-25 with considerable obstetrical and medicolegal implications.26Recent advances in imaging technology,27 the molecular understanding of neuroembryology,28 and identi-fying specific deficits in the coagulation cascade29 that exert a prothrombotic effect have worked jointly to increase etiologic yield. Understanding the causal spectrum of CP requires the systematic assessment of affected individuals together with the application of these advances to properly ad-dress the question of why a particular individual is so affected.19
Recent studies have documented that the causal spectrum for CP is a function of the type of CP and gestational age.19 Detailed investigations reveal a cause in most cases, with consensus that intrapar-tum asphyxia is a cause in only a minority of cases (certainly ⬍20%, perhaps even as little as ⬍10%).19 Consensus has also been reached that intrapartum asphyxia of sufficient severity to cause later CP must produce evidence of a significant acute neonatal neurologic dysfunction (ie, neonatal encephalopathy).30 Thus neonatal encephalopathy is a “way station” through which intrapartum as-phyxia passes to yield later CP and is invariably present if intrapartum asphyxia is causal for this eventual outcome.
NEONATAL ENCEPHALOPATHY Like CP, neonatal encephalopathy is also a clin-ically defined symptom complex. Neonatal en-cephalopathy is essentially a constellation of neu-rologic signs noted within the first 7 days after birth. It has been defined as a “syndrome of dis-turbed neurologic function in the earliest days of life in the term infant manifested by difficulty with initiating and maintaining respiration, depression of tone and reflexes, sub-normal levels of con-sciousness and often by seizures.”31 It is mani-fested by acute neonatal neurologic dysfunction that remains the single best early clue that a new-born is potentially neurologically compromised and potentially at increased risk for later neurode-velopmental sequelae.32
The severity of observed neonatal encephalopa-thy varies and can be graded as mild, moderate, or severe at the bedside according to the classification scheme of Sarnat and Sarnat first developed in 1976 (Table 1).32The grade is based on behavioral observations, response to handling, tone changes, the presence and frequency of seizures, and any evidence of brainstem dysfunction. Although
largely lacking operationalization and validation,33 this grading system for neonatal encephalopathy has proven useful as a predictor of later potential of survival and neurodevelopmental sequelae in both the intermediate and long term.34,35 As a useful predictor, clinicians have relied on neonatal en-cephalopathy as a mechanism of influencing acute treatment intervention in conjunction with other objective markers, such as electroencephalography and neuroimaging.36
Neonatal encephalopathy and CP also share an etiologically heterogeneous character.31There are a multiplicity of potential causes. Frequently, the term “hypoxic ischemic encephalopathy” is used synonymously with neonatal encephalopathy; however, this is inappropriate in the absence of certainty that intrapartum asphyxia is causal for the observed neonatal encephalopathy.37,38 Little is actually known regarding the precise etiologic spectrum of neonatal encephalopathy; however, recent studies have identified significant antepar-tum and intraparantepar-tum risk factors, including small gestational age, maternal fever or viral illness, placental abnormalities, and severe preeclamp-sia.39,40 The presence of neonatal encephalopathy suggests, but does not necessarily imply, a rela-tively recent antepartum or intrapartum compro-mise. Although the severity of neonatal encepha-lopathy relates directly to the risk of later adverse outcome,41 observed improvement in the actual grade of neonatal encephalopathy acutely suggests
enhanced individual resiliency and is a favorable prognostic indicator.36
It is recognized that intrapartum asphyxia is but one cause of neonatal encephalopathy. Indeed, there is strong evidence to suggest that, as for CP, it is only an infrequent cause.19,31 However, as noted earlier, neonatal encephalopathy at a moder-ate or severe level is a necessary antecedent to lmoder-ater CP if intrapartum asphyxia is causally responsible for the CP.
INTRAPARTUM ASPHYXIA
The definition of intrapartum asphyxia has two components. Asphyxia is defined as impaired re-spiratory gas exchange accompanied by the devel-opment of acidosis. Biochemically, the hallmarks are hypoxemia, hypercapnea, and, most important, metabolic acidosis, characterized by reduced bicar-bonate and elevated negative base excess. Intrapar-tum refers to occurrence during the process of labor and parturition. The importance of intrapar-tum asphyxia is its potential, if sufficiently sus-tained and severe, to result in end organ (ie, central nervous system) injury.42
Thus intrapartum asphyxia is conceptualized as a mechanism of acquired injury or pathogenesis. It is a process that can be incited by various events that then triggers a cascade of cellular and patho-physiologic responses that can then yield various possible short-term (eg, neonatal encephalopathy) and eventual (eg, CP) outcomes.42The significance of intrapartum asphyxia lies in the potential for its prevention and possible intervention to either avoid triggering the asphyxial cascade or modify it once it begins, thus reducing the risk of eventual neurodevelopmental sequelae.42 These neurode-velopmental sequelae often have significant life-long morbidity implications and attendant care costs at individual, familial, and societal levels.43 Pragmatically, there are substantial medicolegal implications with respect to the 2 often-linked questions of (1) whether the inciting events them-selves were foreseeable and perhaps preventable, and (2) whether the cause and its outcome could have been avoided by timely obstetrical interven-tion (ie, rapid delivery by one of various meth-ods).44
A particular challenge has been the ability to accurately and reliably diagnose intrapartum as-phyxia. Simply put, at present there is no single gold standard for either clinical or laboratory di-agnosis.31 It is readily apparent that the single Table 1. Neonatal Encephalopathy
Mild
●Increased irritability
●Hyperexcitability
●Jitteriness
●Exaggerated Moro and tendon reflexes
●Sympathetic overreactivity
●Transient changes in tone (⬍6 hours) Moderate
●Lethargy
●Hypotonia
●Diminished reflexes
●With or without associated seizures Severe
●Profound obtundation/coma
●Flaccid muscle tone
●Brainstem dysfunction
●Apnea
●Skew deviation, nystagmus, sucking and swallowing abnormalities
●Increased intracranial tension
markers that have been identified and evaluated— including fetal heart rate changes, meconium pas-sage, Apgar scores, pH/base deficit, time to first breath or need for resuscitation, neonatal enceph-alopathy, other organ (ie, non– central nervous sys-tem) dysfunction, electrophysiologic changes (ie, electroencephalography, evoked potentials), and imaging changes (eg, computed tomography, mag-netic resonance imaging, magmag-netic resonance spec-troscopy, diffusion-weighted imaging)— have rel-atively low sensitivity and specificity for accurate diagnosis.36,45 Many pathophysiologic processes other than intrapartum asphyxia may result in ab-normalities in any of these single markers.
To respond to this diagnostic challenge, empha-sis has been placed on a constellation of signs (ie, the presence of multiple markers) to diagnose in-trapartum asphyxia. Some of these signs are deemed essential; others, supportive. Some are diagnostic of asphyxia, and others are diagnostic of timing. Since 1992, 3 different consensus state-ments have addressed the diagnosis of intrapartum asphyxia (Tables 2, 3, and 4).46-48Experience has also shown that adverse events occurring during the intrapartum period do not occur in isolation and often reflect an antepartum predisposition for a fetus to respond inappropriately to the physiolog-ical stresses of normal labor and delivery.49
All 3 consensus statements elaborated thus far share an emphasis on the concurrent observation and documentation of multiple markers to make a diagnosis of intrapartum asphyxia. These markers
include neonatal encephalopathy, profound meta-bolic acidosis with precise cutoffs specified (ie, pH, base excess), multiple systemic involvement (typically renal), and depressed Apgar scores at and beyond 5 minutes of age.46-48 The 2 more recent consensus criteria47,48 have restricted the diagnosis of intrapartum asphyxia to only certain types of CP and provided additional supportive features of intrapartum timing and objective mark-ers (ie, electroencephalography and/or imaging) of asphyxiation. The last consensus criterion48 also calls for the careful search for and exclusion of other possible etiologies. The extent of this search and its mechanism is not specified, however.
The restriction of intrapartum asphyxia to cer-tain types of CP47,48is problematic in that it is put forward without the possibility of absolute verifi-cation. That is, a single case of intrapartum as-phyxia resulting in an outcome other than spastic quadriparesis, dyskinetic, or mixed CP will render the scheme erroneous. Also it requires an eventual outcome (ie, a specific type of CP),49which may not be apparent for several years, as a means of diagnosing what is an acute process. Subsequent to intrapartum asphyxia, a range of possible outcomes exists, from normal through the entire spectrum of neurodevelopmental disability (eg, sensorineural hearing loss, developmental coordination disorder, attention deficit hyperactivity disorder, learning disability, global developmental delay, mental re-tardation, and CP), largely reflecting the dynamic interplay between the severity of asphyxia and the resiliency of the individual.50
With respect to intrapartum asphyxia, it is ap-parent that the presence of multiple markers is a necessary precondition for diagnosis in the scheme of neonatal encephalopathy. For some infants with Table 2. American Academy of Pediatrics/American
College of Obstetrics and Gynecology (1992)
●Profound metabolic acidosis (pH⬍7.0)
●Apgar 3 or lower beyond 5 minutes
●Neonatal encephalopathy
●Multiorgan system dysfunction
Table 3. International Cerebral Palsy Task Force (1999) Essential
●Moderate to severe neonatal encephalopathy
●pH⬍7.0
●CP: spastic, quadraparetic, dyskinetic, or mixed Supportive
●Sentinel event
●Severe fetal heart rate changes
●Apgar lower than 6 beyond 5 minutes
●Multisystem dysfunction
●Evidence of acute cerebral involvement (electroencephalography/imaging)
Table 4. American College of Obstetrics and Gynecology (2002)
Asphyxia
●pH⬍7.0; base deficit⬎12 mmol/L
●Moderate to severe neonatal encephalopathy
●CP: spastic, quadriparesis, dyskinetic, or mixed
●Exclusion of other etiologies Intrapartum
●Sentinel event associated with labor
●Fetal heart rate changes: bradycardia, loss of variability, decelerations
●Apgar 3 or lower beyond 5 minutes
●Multisystem involvement
intrapartum asphyxia, an antepartum precondition may predispose to intrapartum asphyxia occur-rence. The threat of injury initiates a cellular and physiological cascade that reflects individual vari-ation in resiliency and the capacity to adapt to this threat. Eventual outcomes are variable; CP is but one type of potential outcome of intrapartum as-phyxia.
RELATIONSHIPS
From the foregoing, based on our present knowl-edge and understanding, it is possible to elaborate a number of statements regarding the interrelation-ships between CP, neonatal encephalopathy, and intrapartum asphyxia within the context of children encountered in practice (ie, those with and without neurodevelopmental disability). There relation-ships are shown schematically in Figure 1 and are listed below:
1. Some children with neonatal encephalopathy will have intrapartum asphyxia.
2. All children with intrapartum asphyxia will have neonatal encephalopathy.
3. Some children with intrapartum asphyxia will develop later CP.
4. Some children with neonatal encephalopathy and no intrapartum asphyxia will develop later CP.
5. Some children with later CP will have had previous neonatal encephalopathy and no
in-trapartum asphyxia. (Note that 4 and 5 are equivalent statements.)
6. Most children with later CP will not have previous intrapartum asphyxia.
7. Most children with later CP will not have previous neonatal encephalopathy.
8. All children with CP will have a neurodevel-opmental disability.
9. Some children with a later non-CP neurode-velopmental disability will have had previous intrapartum asphyxia.
10. Some children with a later non-CP neurode-velopmental disability will have had previous neonatal encephalopathy but no intrapartum asphyxia.
11. Most children with a later non-CP neurodevel-opmental disability will not have had previous neonatal encephalopathy.
12. Some normal children will have had previous intrapartum asphyxia.
13. Some normal children will have had previous neonatal encephalopathy but no intrapartum asphyxia.
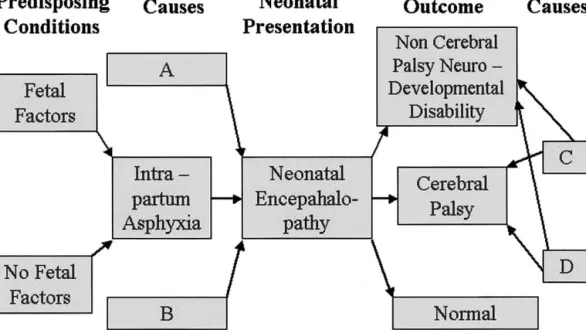
The relationships between causes, neonatal pre-sentation, and outcome are also shown schemati-cally in Figure 2. This figure highlights that fetal predisposing conditions may or may not exist as an antecedent of possible intrapartum asphyxia. A multitude of possible causes other than intrapartum asphyxia exists to explain neonatal encephalopa-Fig 1. Venn diagram illustrating relationships between neonatal encephalopathy, cerebral palsy, intrapartum asphyxia, non-cerebral palsy neurodevelopmental disability, and normality.
thy. There also exists a multitude of possible out-comes subsequent to neonatal encephalopathy, ranging from normality to CP to a non-CP neuro-developmental disability. Causes other than those operating through intrapartum asphyxia and/or neonatal encephalopathy may also result in CP and non-CP neurodevelopmental disability.
CONCLUSION
Improving the clarity and understanding of key terminology and concepts, together with their in-terrelationships, will help us focus our future ef-forts more sharply. Better means and mechanisms (ie, markers) for reliable, earlier, and more certain diagnosis are needed. Clarification of interrelation-ships will depend on the application of these mark-ers to the clinical situation and on the performance of longitudinal studies that more sharply define possible outcomes. Factors that predispose to the
occurrence of asphyxia and modify eventual out-comes need to be identified. Identification of such predisposing conditions, reliable diagnostic mark-ers, and mechanisms of resiliency, coupled with elaboration of the cellular and physiological cas-cade in response to injury, will provide potential means for intervention both acutely and in the long term. Ancillary to this will be improvement in maternal/fetal health promotion and health service delivery to the affected population, which will both reduce the number of those affected and minimize the impacts at multiple levels of neurodevelopmen-tal disability.
ACKNOWLEDGMENTS
The author thanks Alba Rinaldi for secretarial assistance in preparing this manuscript and the Montreal Children’s Hospital Foundation for support during the writing of this manuscript. The author is a Chercheur Boursier Clinicien (Clinical Research Scholar) of the Fonds de Recherche en Sante du Quebec. REFERENCES
1. http://www.unmuseum.org/triangle.htm
2. Kuban KCK, Leviton A: Cerebral palsy. N Engl J Med 330:188-195, 1994
3. Badawi N, Keogh JM, Dixon G, et al: Developmental outcomes of newborn encephalopathy in the term infant. Indian J Pediatr 8:527-530, 2001
4. Robertson NJ, Edwards AD: Recent advances in develop-ing neuroprotective strategies for perinatal asphyxia. Curr Opin Pediatr 10:575-580, 1998
5. Canadian Institute of Child Health: The Health of Cana-da’s Children (ed 3). Ottawa, Ontario, author, 2000
6. Ingram TTS: A historical view of the definition and classification of the cerebral palsies, in Stanley F, Alberman F (eds): The Epidemiology of the Cerebral Palsies. London, Spastics International Medical Publications, 1984, pp 1-11
7. Stanley F, Blair D, Alberman E: What are the cerebral palsies? in Cerebral Palsies: Epidemiology & Causal Pathways. London, MacKeith, 2000, pp 8-13
Fig 2. Schematic diagram illustrating relationships between fetal status, intrapartum asphyxia, neonatal encephalopathy, and eventual outcome.
8. Paneth N: Etiologic factors in cerebral palsy. Pediatr Ann 15:191-201, 1986
9. Evans P, Elliott M, Alberman E, et al: Prevalence and disabilities in 4 to 8 year olds with cerebral palsy. Arch Dis Child 60:940-945, 1985
10. MacKeith RC, Polani PE: Cerebral palsy. Lancet 1:61, 1958
11. Mutch L, Alberman E, Hagberg B, et al: Cerebral palsy epidemiology: Where are we now and where are we going? Dev Med Child Neurol 34:547-551, 1992
12. Ferriero DH: Cerebral palsy: Diagnosing something that is not one thing. Curr Opin Pediatr 11:485-486, 1999
13. Badawi N, Watson L, Petterson B, et al: What constitutes cerebral palsy? Dev Med Child Neurol 40:520-527, 1998
14. Minear WL: A classification of cerebral palsy. Pediatrics 18:841-852, 1956
15. Bax MCO: Terminology and classification of cerebral palsy. Dev Med Child Neurol 6:295-307, 1964
16. Surveillance of Cerebral Palsy in Europe (SCPE): Sur-veillance of cerebral palsy in Europe: A collaboration of cere-bral palsy surveys and registers. Dev Med Child Neurol 42:816-824, 2000
17. Taudorf K, Hansen FJ, Melchior JC, et al: Spontaneous remission of cerebral palsy. Neuropediatrics 17:19-22, 1986
18. Nelson KB, Grether JK: Causes of cerebral palsy. Curr Opin Pediatr 11:487-491, 1999
19. Shevell MI, Majnemer A, Morin I: Etiologic yield of cerebral palsy: A contemporary case series. Pediatr Neurol 28:352-359, 2003
20. Nelson KB, Ellenberg JH: Antecedents of cerebral palsy: Multivariate analysis of risk. N Engl J Med 315:81-86, 1986
21. Torfs CP, van den Berg B, Oechsli FW, et al: Prenatal and perinatal factors in the etiology of cerebral palsy. J Pediatr 116:615-619, 1990
22. Stanley FJ, Blair E, Hockey A, et al: Spastic quadriplegia in Western Australia: A genetic epidemiological study. I: Case population and perinatal risk factors. Dev Med Child Neurol 35:191-201, 1993
23. Blair E, Stanley FJ: Intrapartum asphyxia: A rare cause of cerebral palsy. J Pediatr 112:515-519, 1988
24. Freeman JM, Nelson KB: Intrapartum asphyxia and cerebral palsy. Pediatrics 82:240-249, 1988
25. Nelson KB: What proportion of cerebral palsy is related to birth asphyxia? J Pediatr 112:572-574, 1988
26. Bedrick AD: Perinatal asphyxia and cerebral palsy: Fact, fiction, or legal prediction? Am J Dis Child 143:1139-1140, 1989
27. Grant PE, Berkovich AJ: Neuroimaging in CP: Issues in pathogenesis and diagnosis. Mental Retard Dev Dis Res Rev 3:118-128, 1997
28. Lequin MH, Barkovich AJ: Current concepts of cerebral malformation syndromes. Curr Opin Pediatr 11:492-496, 1999 29. Nelson KB, Dambrosia JM, Grether JK, et al: Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol 44:665-675, 1998
30. Nelson KB: The neurologically impaired child and al-leged malpractice at birth. Neurol Clin 17:283-293, 1999
31. Nelson KB, Leviton A: How much of neonatal enceph-alopathy is due to birth asphyxia? Am J Dis Child 145:1325-1331, 1991
32. Sarnat HB, Sarnat MS: Neonatal encephalopathy follow-ing fetal distress. A clinical and electroencephalographic study. Arch Neurol 33:696-705, 1976
33. Leviton A, Nelson KB: Problems with definitions and classifications of newborn encephalopathy. Pediatr Neurol 8:85-90, 1992
34. Robertson CMT, Finer NN, Grace MGA: School perfor-mance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr 114:753-760, 1989
35. Robertson CMT, Grace MGA: Validation of prediction of kindergarten-age school-readiness scores of nondisabled sur-vivors of moderate neonatal encephalopathy in term infants. Can J Public Health 83:S51-S57, 1992
36. Shevell MI, Majnemer A, Miller SP: Neonatal neurolog-ical prognostication: The term asphyxiated newborn. Pediatr Neurol 21:776-784, 1999
37. Ellis M, Costello A: Birth asphyxia, Apgar score and neonatal encephalopathy. Indian Pediatr 34:975-978, 1997
38. Low JA, Galbraith RS, Muir DW, et al: The relationship between perinatal hypoxia and newborn encephalopathy. Am J Obstet Gynecol 152:256-260, 1985
39. Badawi N, Jurinczuk JJ, Keogh JM, et al: Antepartum risk factors for newborn encephalopathy: The Western Austra-lian case control study. BMJ 317:1549-1553, 1998
40. Badawi N, Kurinczuk JJ, Keogh JM, et al: Intrapartum risk factors for newborn encephalopathy: The Western Austra-lian case control study. BMJ 317:1554-1558, 1998
41. Volpe JJ: Hypoxic-ischemic encephalopathy: Clinical aspects, in Neurology of the Newborn (ed 4). Philadelphia, PA, Saunders, 2001, pp 331-394
42. Volpe JJ: Hypoxic-ischemic encephalopathy: Biochem-ical and physiologBiochem-ical aspects, in Neurology of the Newborn (ed 4). Philadelphia, PA, Saunders, 2001, pp 217-276
43. Grether JK, Cummins SK, Nelson KB: The California Cerebral Palsy Project. Pediatr Perinat Epidemiol 6:339-351, 1992
44. Mello MM, Studdert DM, Brennan TA: The new medi-cal malpractice crisis. N Engl J Med 348:2281-2284, 2003
45. Nelson KB, Emergy ES: Birth asphyxia and the neonatal brain: What do we know and when do we know it? Clin Perinatal 20:327-344, 1993
46. American Academy of Pediatrics Committee on the Fetus and Newborn, and American College of Obstetricians & Gynecologists Committee on Obstetric Practice: Use and abuse of the Apgar score. Pediatrics 98:141-146, 1996
47. MacLennan A (for the International Cerebral Palsy Task Force): A template for defining a causal relationship between acute intrapartum events and cerebral palsy: International con-sensus statement. BMJ 319:1054-1059, 1999
48. American College of Obstetrics and Gynecology Task Force on Neonatal Encephalopathy and Cerebral Palsy: Neona-tal Encephalopathy and Cerebral Palsy: Defining the Pathogen-esis and Pathophysiology. Washington, DC, American College of Obstetrics and Gynecology, 2003, pp XVII-XIX
49. Low JA, Galbraith RS, Muir DW, et al: Motor and cognitive deficits after intrapartum asphyxia in the mature fetus. Am J Obstet Gynecol 158:356-361, 1988
50. Lebeer J: How much brain does a mind need? Scientific, clinical, and educational implications of ecological plasticity. Dev Med Child Neurol 40:352-357, 1998