IJSM
Simulation of austenitic stainless steel oxidation
containing nitrogen at temperature range 500
o
C
– 800
o
C
S. Ghali
1*, M. Eissa
2, H. El-Faramawy
31,2,3
Central Metallurgical Research and Development Institute (CMRDI), Cairo, Egypt
Postal Address: P.O Box 87, Helwan, Egypt
*Corresponding author: Dr. S. Ghali, Associate Professor, Department of Steel Technology, Central, Metallurgical Research and Development Institute (CMRDI), Postal Address: P.O Box 87, Helwan, Egypt. Email: a3708052@yahoo.com, Tel: +202-25010642, Fax: +202-25010639
This article aims at estimation the contribution effect of nickel, nitrogen, time and temperature on the oxidation behavior of austenitic stainless steels. Therefore, 24 factorial design and MatLab program were used to the derived regression model. According to the model the mass gain (mg/cm2) can be calculated by equation:
𝒎𝒂𝒔𝒔 𝒈𝒂𝒊𝒏
= −𝟎. 𝟎𝟑𝟐𝟑𝟒 + 𝟎. 𝟎𝟏𝟔𝟐𝟏𝟔 𝑵% + 𝟎. 𝟎𝟎𝟔𝟏𝟗𝟓 𝑵𝒊% + 𝟎. 𝟎𝟎𝟎𝟗𝟓𝟗 ∗ 𝒕 + 𝟎. 𝟎𝟎𝟎𝟏𝟏𝟑𝑻
− 𝟎. 𝟎𝟏𝟕𝟎𝟒 𝑵% 𝑵𝒊% − 𝟎. 𝟎𝟓𝟓𝟔𝟔 𝑵% ∗ 𝒕 + 𝟓. 𝟕𝟔 ∗ 𝟏𝟎−𝟓 𝑵% 𝑻 − 𝟎. 𝟎𝟎𝟒𝟐𝟗 𝑵𝒊% ∗ 𝒕
− 𝟏. 𝟏 ∗ 𝟏𝟎−𝟓 𝑵𝒊% 𝑻 + 𝟑. 𝟐𝟔 ∗ 𝟏𝟎−𝟔𝑻 ∗ 𝒕 + 𝟎. 𝟎𝟏𝟐𝟓𝟐𝟑 𝑵% 𝑵𝒊% ∗ 𝒕 + 𝟖. 𝟐𝟔
∗ 𝟏𝟎−𝟔 𝑵% 𝑵𝒊% 𝑻 + 𝟎. 𝟎𝟎𝟎𝟏𝟏𝟑 𝑵% 𝑻 ∗ 𝒕 + 𝟖. 𝟓𝟏 ∗ 𝟏𝟎−𝟔 𝑵𝒊% 𝑻 ∗ 𝒕 − 𝟐. 𝟔
∗ 𝟏𝟎−𝟓 𝑵% 𝑵𝒊% 𝑻 ∗ 𝒕
Where, [N%] & [Ni%] are nitrogen and nickel concentration in weight percent, T is temperature in Celsius, and t is time in hour.
The factorial design was based and applied for previous published work. The model showed that nickel has highest positive effect in retardation mass gain, while time and temperature enhance mass gain. Nitrogen has a little positive effect in increasing mass gain. The combination effect of nickel with nitrogen, time, and / or temperature has negative effect on mass gain with certain extent. The current regression model was used to predict the mass gain within temperature range 500 oC – 800 oC up to 8 hours. Interpretation was carried out between calculated and experimental results. The results of the derived mathematical model were found to be in a good agreement with the experimental data. The model is tested against oxidation behavior of steel grade 304L AISI at 800 oC in dry air.
Keywords: Factorial design, oxidation, Ni-N stainless, high temperature, MatLab.
INTRODUCTION
The austenitic stainless steels are more expensive than the steels just mentioned due to the increased alloying additions. They have poor thermal conductivity and larger thermal expansion coefficients which restricts their
Vol. 1(3), pp. 024-032, August, 2014. © www.premierpublishers.org,ISSN: 2375-0499x position the text box anywhere in the document. Use the Text Box Tools tab to change the formatting of the pull quote text box.]
position the text box anywhere in the document. Use the Text Box Tools tab to change the formatting of the pull quote text box.]
Ghali et al 024
applications, and so they are mainly recommended to be used at the high temperature applications which supposed to oxidation (Fry, A. et al., 2002).
Wood et al. (1964) presented two different theories on how nodules of iron - rich oxides form on stainless steels. The first one (the diffusion theory) proposes that iron and possibly nickel enter the Cr2O3 film and transform it to a spinal
structure. The other one (the cracking theory) deals with the stresses that can develop during oxide growth. These stresses can cause the oxide to crack so the underlying matrix is exposed, allows iron- rich oxides to grow. Basu et al. (1991) presented a third mechanism for the formation of nodules. They found that iron- rich nodules appear more often on large grained samples than on those with a small grain size. They emphasized the fact that Cr2O3 is
thermodynamically the most stable oxide, but that iron oxides have a higher growth rate. To attain protective oxidation behavior, the faster growing iron oxides have to be stopped by the more stable chromium oxide. In the initial stage of oxidation Cr2O3 will form as internal precipitates, while the faster-growing iron oxide nucleates on the surface. To get a
continuous layer of Cr2O3 there has to be a minimum flux of Cr from the underlying alloy. High diffusivity paths such as
grain boundaries can supply enough Cr to develop a protective layer where the grain boundary reaches the surface. However, if the distance to the next grain boundary is large, there might be a region that does not develop a protective layer below the fast growing iron oxide. In these intra-granular regions the iron oxides will continue to grow into iron-rich nodules.
The chromium content in the alloy is extremely important for improving the oxidation / corrosion resistance of stainless steel (Klueh, 2005; Lepingle et al., 2008; West, 1986), by the formation of a protective, adherent, slow growing Cr2O3
(chromia) oxide layer. This oxide is slow growing and blocks the outward diffusion of other alloy elements and the inward diffusion of gaseous impurities (Young, 2008) as transport processes through this scale are generally slow (Stott et al., 1995). The outward diffusion of chromium (Cr3+) along grain boundaries has shown to be faster than the inward diffusion of oxygen by a factor of three and so the chromia scale usually grows outward (Hussey et al., 1987) and can contain small amounts of iron, nickel and manganese (Sedriks, 1996) as seen on high chromium steels such as 310. Chromium content is important in dictating the oxide formed, lower chromium concentrations e.g. type 304, form the spinel oxide FeCr2O4 which can be protective to a lesser extent (Sedriks, 1996). The greater the chromium content in the alloy the
greater its oxidation resistance is (Lepingle et al., 2008). Steels with a chromium content of over 13 wt% show very low oxidation rates and their scales consist of Cr2O3, (Cr, Fe)2O3 or Cr rich (Cr, Fe Mn)3O4 with an outer layer of Fe2O3
(Zurek, 2004). However, at temperatures exceeding 900oC, chromia scales can react further with oxygen to form CrO3
which is a volatile species (Stott et al., 1995).
Silicon additions are also well known for enhancing the oxidation resistance of stainless steels with the lower silicon concentrations having the highest corrosion rates (Lepingle et al., 2008; Sedriks, 1996; Zurek, 2004; Gray et al., 2004). This effect has been explained by suggestions such as the formation of the initial chromia layer being facilitated by silicon, or the formation of silica particles beneath the chromia layer which increase the adhesion of the chromia layer, or the formation of a continuous silica layer beneath the chromia preventing outward diffusion of chromium ions (Sedriks, 1996). The additions of both chromium and silicon at optimum levels will improve oxidation resistance without adversely affecting the creep strength (Lepingle et al., 2008). The influence of silicon is enhanced at higher temperatures, i.e. above 700°C (Lepingle et al., 2008).
Manganese is also an alloying addition seen to affect oxidation resistance, but its effect has been measured both favourably and adversely (Lepingle et al., 2008, Francis, 1966). In some cases it has been found to be damaging to the oxidation resistance of austenitic stainless steels due to it forming a spinel oxide of MnO·Cr2O3 rather than the protective
chromia oxide (Francis, 1966). Nickel has also been shown to enhance the oxidation resistance by reducing the cation diffusion in the Cr2O3 scale and preventing the formation of FeCr2O4 + Fe2O3, having an influence on the adhesion and
mechanical properties of the scale (Sedriks, 1996). It is very difficult to consider these alloying elements separately due to their complex interactions. Taishi Moroishi et al (1979) reported that carbon deteriorate the oxidation resistance of stainless steel. M23C6 is the main carbide precipitated in austenitic Cr Ni steels, Cr being its main metallic constituent.
First of all M23C6 deteriorates the resistance to corrosion by depleting the chromium content at grain boundaries where it
mainly precipitates. A time needed for M23C6 precipitation at different interfaces increases in the following sequence: at
grain boundaries, at non-coherent twin boundaries, at coherent twin boundaries and within grains (Cihali, 1968). Nitrogen shifts the precipitation of M23C6 carbides to longer time. Nitrogen accelerates the chromium diffusion (Their et
al., 1969). This is a reason for the suppression of M23C6 precipitation by nitrogen i.e. in the retardation of M23C6
nucleation because these chromium carbides do not contain nitrogen. Chromium nitrides are less effective than chromium carbides in retardation of oxidation resistance.
Vanini, 1996) found that the presence of nitrogen in austenitic stainless steels improve oxidation resistance, corrosion behavior and mechanical properties.
The previous survey summarizes some studies that carried out experimentally on the oxidation behavior of stainless steel and the effect of Ni, Mn, Si, C. In addition, the influence of replacement of nickel by nitrogen on the properties has been studied. However, the effective and magnitude of nickel, nitrogen, at different temperatures is still required. Although the factorial design has several advantages such as prediction of process yield and process performance but its application is seems very limited in steel research field (Gao et al., 2012; Mesquita et al., 2011; Robinson, 2008; Ghali, & Mousa, 2014). The present work aims at investigation the contribution of nitrogen, nickel, time and temperature on oxidation behavior of stainless steel (Ghali et al., 2011) containing 0.30 – 0.41%C, 2.42 – 3.07 %Si, 1.16 – 1.61 % Mn, 17.8 – 20.33%Cr, and 1.14 – 1.22%W. Moreover the influence of the interaction combination between two, three and four parameters on the oxidation behavior was investigated.
Experiments
The factorial design was applied on the published work (Ghali et al., 2011). At this previous study, the influence of replacement nickel by nitrogen on oxidation behavior was studied from the point of kinetics but the contribution of nitrogen and nickel on oxidation behavior was not investigated at different temperatures and / or time. A 24 factorial design was used to estimate the contribution of nitrogen, nickel, time, and temperature on oxidation behavior. As well as the interaction effect i.e. combination effect of nitrogen with nickel, time, or temperature was deduced. Moreover, the interaction combination influence of three parameters (i.e. nitrogen and nickel with time or temperature) was determined. Regression model was formulated to calculate the mass gain in terms of N%, Ni%, time (hour), and temperature (oC). Interpretation between the predicted values and published work was carried out.
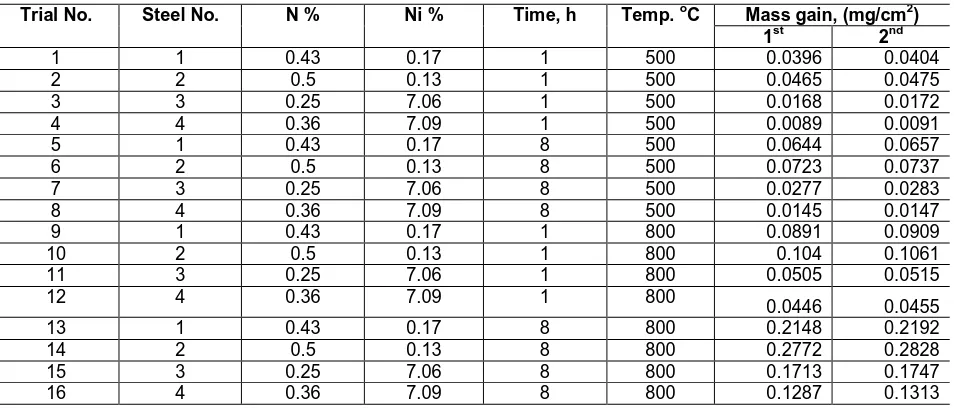
A factorial design 24 was built up based on mass gain (mg/cm2) of four stainless steels after 1 and 8 hours at temperatures 500 oC and 800 oC. Table 1 shows the influence of nitrogen, nickel content at different time and temperatures on mass gain.
The regression model is tested against previous work (Vangeli et al., 2012), in which the oxidation behavior of steel grade 304L AISI was investigated at 800 oC in dry air.
Table 1: Conditions (Ghali et al., 2011) of experiments and mass gain in mg/cm2.
Trial No. Steel No. N % Ni % Time, h Temp. oC Mass gain, (mg/cm2)
1st 2nd
1 1 0.43 0.17 1 500 0.0396 0.0404
2 2 0.5 0.13 1 500 0.0465 0.0475
3 3 0.25 7.06 1 500 0.0168 0.0172
4 4 0.36 7.09 1 500 0.0089 0.0091
5 1 0.43 0.17 8 500 0.0644 0.0657
6 2 0.5 0.13 8 500 0.0723 0.0737
7 3 0.25 7.06 8 500 0.0277 0.0283
8 4 0.36 7.09 8 500 0.0145 0.0147
9 1 0.43 0.17 1 800 0.0891 0.0909
10 2 0.5 0.13 1 800 0.104 0.1061
11 3 0.25 7.06 1 800 0.0505 0.0515
12 4 0.36 7.09 1 800 0.0446 0.0455
13 1 0.43 0.17 8 800 0.2148 0.2192
14 2 0.5 0.13 8 800 0.2772 0.2828
15 3 0.25 7.06 8 800 0.1713 0.1747
16 4 0.36 7.09 8 800 0.1287 0.1313
RESULTS AND DISCUSSION
Definition of affecting factors
Ghali et al 026
effect, “BD” refers to Ni and temperature interaction effect, “CD” refers to time and temperature interaction effect, “ABC” refers to N, Ni and time interaction effect, “ABD” N, Ni, and temperature interaction effect, “ACD” refers to N, time and temperature interaction effect, “BCD” refers to Ni, time and temperature interaction effect, “ABCD” refers to N, Ni, time and temperature interaction effect.
The low and high levels of A, B, C and D are denoted by “– “and “+” respectively. The sixteen treatment combinations in the design are usually represented by lowercase letters. The high level of any factor in the treatment combination is denoted by the corresponding lowercase letter and that the low level of a factor in the treatment combination is denoted by the absence of the corresponding letter. Thus, “a” represents the treatment combination of A at high level with B, C and D at low levels, “b” represents B at high level with A, C and D at low levels, “c” represents C at high level with A, B and D at low levels, “d” represents D at high level with A, B and C at low levels, “ab” represents A and B factors at the high levels with C and D at low levels, “ac” represents A and C factors at the high levels with B and D at low levels, “ad” represents A and D factors at the high levels with B and C at low levels, “bc” represents B and C factors at the high levels with A and D at low levels, “bd” represents B and D factors at the high levels with A and C at low levels, “cd” represents C and D factors at the high levels with A and B at low levels, “abc” represents A, B and C factors at the high levels with D at low level, “abd” represents A, B and D factors at the high levels with C at low level, “acd” represents A, C and D factors at the high levels with B at low level, “bcd” represents B, C and D factors at the high levels with A at low level, “abcd” represents A, B, C, and D at high levels, finally (1) is used to denote all factors at low level.
Calculation affecting factors
Based on the 24 factorial design, the average effect of a factor can be defined as the change in response produced by a change in the level of that factor averaged over the levels of the other factors. The effect of A at low levels of B, C and D is [a-(1)]/n, the effect of A at high levels of B, C and D is [abcd-bcd]/n, the effect of A at low level of B and high levels of C and D is [acd-cd]/n, the effect of A at low level of C and high levels of B and D is [abd-bd]/n, the effect of A at low level of D and high levels of B and C is [abc-bc]/n, the effect of A at high level of B and low levels of C and D is [ab-b]/n, the effect of A at high level of C and low levels of B and D is [ac-c]/n, and the effect of A at high level of D and low levels of B and C is [ad-d]/n. The main effect of A is the average quantities of its effect at low and high levels of B, C and D. The average effect of “A” factor is given by Equation (1).
𝐴 = 1
8𝑛[ 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑐 + 𝑎𝑏𝑑 + 𝑎𝑐𝑑 + 𝑎𝑏 + 𝑎𝑐 + 𝑎𝑑 + 𝑎 − 𝑏𝑐𝑑 + 𝑏𝑐 + 𝑏𝑑 + 𝑐𝑑 + 𝑏 + 𝑐 + 𝑑 + (1) ] (1)
Where, n is the repeat times
Similar way, the average main effect of one, two, three and the four factors and their interactions can be calculated. The averages main effecting factors are given in Equations (2-15).
𝐵 = 1
8𝑛[ 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑐 + 𝑎𝑏𝑑 + 𝑏𝑐𝑑 + 𝑎𝑏 + 𝑏𝑐 + 𝑏𝑑 + 𝑏 − 𝑎𝑐𝑑 + 𝑎𝑐 + 𝑎𝑑 + 𝑐𝑑 + 𝑎 + 𝑐 + 𝑑 + (1) ] (2)
𝐶 = 1
8𝑛[ 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑐 + 𝑐𝑏𝑑 + 𝑎𝑐𝑑 + 𝑐𝑏 + 𝑎𝑐 + 𝑐𝑑 + 𝑐 − 𝑎𝑏𝑑 + 𝑎𝑏 + 𝑏𝑑 + 𝑎𝑑 + 𝑏 + 𝑎 + 𝑑 + (1) ] (3)
𝐷 = 1
8𝑛[ 𝑎𝑏𝑐𝑑 + 𝑏𝑐𝑑 + 𝑎𝑏𝑑 + 𝑎𝑐𝑑 + 𝑏𝑑 + 𝑐𝑑 + 𝑎𝑑 + 𝑑 − 𝑎𝑏𝑐 + 𝑏𝑐 + 𝑎𝑏 + 𝑎𝑐 + 𝑏 + 𝑐 + 𝑎 + (1) ] (4)
𝐴𝐵 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑐𝑑 + 𝑏𝑐𝑑 + 𝑐𝑑 + 𝑎𝑏 + 𝑎 + 𝑏 + (1) − 𝑎𝑏𝑐 + 𝑎𝑐 + 𝑏𝑐 + 𝑐 + 𝑎𝑏𝑑 + 𝑎𝑑 + 𝑏𝑑 + 𝑑 (5)
𝐴𝐶 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑑 + 𝑏𝑐𝑑 + 𝑏𝑑 + 𝑎𝑐 + 𝑎 + 𝑐 + (1) − 𝑎𝑏𝑐 + 𝑎𝑏 + 𝑏𝑐 + 𝑏 + 𝑎𝑐𝑑 + 𝑎𝑑 + 𝑐𝑑 + 𝑑 (6)
𝐴𝐷 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑐 + 𝑏𝑐𝑑 + 𝑏𝑐 + 𝑎𝑑 + 𝑎 + 𝑑 + (1) − 𝑎𝑐𝑑 + 𝑎𝑐 + 𝑐𝑑 + 𝑐 + 𝑎𝑏𝑑 + 𝑎𝑏 + 𝑏𝑑 + 𝑏 (7)
𝐵𝐶 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑑 + 𝑎𝑐𝑑 + 𝑎𝑑 + 𝑏𝑐 + 𝑏 + 𝑐 + (1) − 𝑎𝑏𝑐 + 𝑎𝑏 + 𝑎𝑐 + 𝑎 + 𝑏𝑐𝑑 + 𝑏𝑑 + 𝑐𝑑 + 𝑑 (8)
𝐵𝐷 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑐 + 𝑎𝑐𝑑 + 𝑎𝑐 + 𝑏𝑑 + 𝑏 + 𝑑 + (1) − 𝑏𝑐𝑑 + 𝑏𝑐 + 𝑐𝑑 + 𝑐 + 𝑎𝑏𝑑 + 𝑎𝑏 + 𝑎𝑑 + 𝑎 (9)
𝐶𝐷 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑐 + 𝑎𝑏𝑑 + 𝑎𝑏 + 𝑐𝑑 + 𝑐 + 𝑑 + (1) − 𝑎𝑐𝑑 + 𝑎𝑐 + 𝑎𝑑 + 𝑎 + 𝑏𝑐𝑑 + 𝑏𝑐 + 𝑏𝑑 + 𝑏 (10)
𝐴𝐵𝐶 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑐 + 𝑎𝑑 + 𝑐𝑑 + 𝑏𝑑 + 𝑎 + 𝑏 + 𝑐 − 𝑎𝑏𝑑 + 𝑎𝑐𝑑 + 𝑏𝑐𝑑 + 𝑎𝑏 + 𝑎𝑐 + 𝑏𝑐 + 𝑑 + (1 (11)
𝐴𝐵𝐷 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑏𝑑 + 𝑎𝑐 + 𝑐𝑑 + 𝑏𝑐 + 𝑎 + 𝑏 + 𝑑 − 𝑎𝑏𝑐 + 𝑎𝑐𝑑 + 𝑏𝑐𝑑 + 𝑎𝑏 + 𝑎𝑑 + 𝑏𝑑 + 𝑐 + (1 (12)
𝐴𝐶𝐷 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑎𝑐𝑑 + 𝑎𝑏 + 𝑏𝑑 + 𝑏𝑐 + 𝑎 + 𝑐 + 𝑑 − 𝑎𝑏𝑐 + 𝑎𝑏𝑑 + 𝑏𝑐𝑑 + 𝑎𝑐 + 𝑎𝑑 + 𝑐𝑑 + 𝑏 + (1 (13)
𝐵𝐶𝐷 = 1
8𝑛 𝑎𝑏𝑐𝑑 + 𝑏𝑐𝑑 + 𝑎𝑏 + 𝑎𝑑 + 𝑎𝑐 + 𝑏 + 𝑐 + 𝑑 − 𝑎𝑏𝑐 + 𝑎𝑏𝑑 + 𝑎𝑐𝑑 + 𝑏𝑐 + 𝑏𝑑 + 𝑐𝑑 + 𝑎 + (1 (14)
𝐴𝐵𝐶𝐷 = 1
Application and validation of regression model
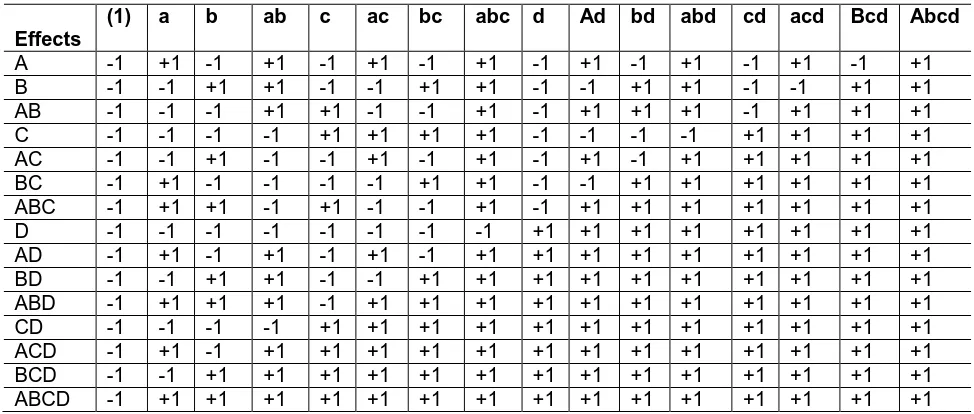
The symbols (1), a, b, ab, c, ac, bc, abc, d, ad, bd, abd, cd, acd, bcd, and abcd represent the total of all 2 replicates (n=2) taken at the treatment combination. Table 2 summarizes the plus and minus signs can be developed from the contrasts. Where, the high level is referred by plus sign and low level is referred by minus sign. The signs of identity element are plus.
Sum of squares for the effects in the 24 design with “n” replicates is SS=(Contrast)2/16n. The total sum of squares (SST)
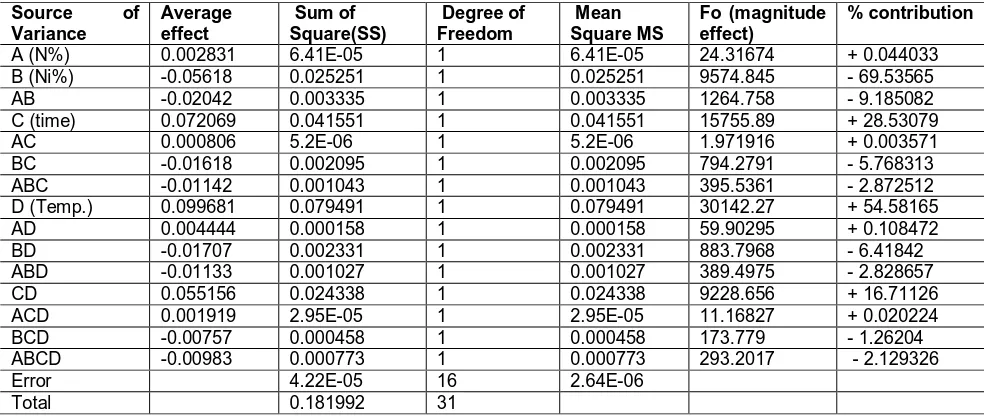
has (abcdn-1) degrees of freedom and the error sum of squares (SSE) has abcd(n-1) degrees of freedom. Table 3
summarizes the main effects of variables, sum of squares, and mean squares
Table 2. Contrast constants for the 24 Design
Treatment combination
Factorial Effect
I A B AB C AC BC ABC D AD BD ABD CD ACD BCD ABCD
(1) + - - + - + + - - + + - + - - +
a + + - - - - + + - - + + + + - -
b + - + - - + - + - + - + + - + -
ab + + + + - - - - - - - - + + + +
c + - - + + - - + - + + - - + + +
ac + + - - + + - - - - + + - - + -
bc + - + - + - + - - + - + - + - -
abc + + + + + + + + - - - - - - - +
d + - - + - + + + + - - + - + + +
ad + + - - - - + + + + - - - - + -
bd + - + - - + - - + - + - - + - -
abd + + + + - - - - + + + + - - - +
cd + - - + + - - + + - - + + - - +
acd + + - - + + - - + + - - + + - -
bcd + - + - + - + - + - + - + - + -
abcd + + + + + + + + + + + + + + + +
Table 3. Analysis of variances
Source of
Variance
Average effect
Sum of Square(SS)
Degree of Freedom
Mean Square MS
Fo (magnitude effect)
% contribution
A (N%) 0.002831 6.41E-05 1 6.41E-05 24.31674 + 0.044033
B (Ni%) -0.05618 0.025251 1 0.025251 9574.845 - 69.53565
AB -0.02042 0.003335 1 0.003335 1264.758 - 9.185082
C (time) 0.072069 0.041551 1 0.041551 15755.89 + 28.53079
AC 0.000806 5.2E-06 1 5.2E-06 1.971916 + 0.003571
BC -0.01618 0.002095 1 0.002095 794.2791 - 5.768313
ABC -0.01142 0.001043 1 0.001043 395.5361 - 2.872512
D (Temp.) 0.099681 0.079491 1 0.079491 30142.27 + 54.58165
AD 0.004444 0.000158 1 0.000158 59.90295 + 0.108472
BD -0.01707 0.002331 1 0.002331 883.7968 - 6.41842
ABD -0.01133 0.001027 1 0.001027 389.4975 - 2.828657
CD 0.055156 0.024338 1 0.024338 9228.656 + 16.71126
ACD 0.001919 2.95E-05 1 2.95E-05 11.16827 + 0.020224
BCD -0.00757 0.000458 1 0.000458 173.779 - 1.26204
ABCD -0.00983 0.000773 1 0.000773 293.2017 - 2.129326
Error 4.22E-05 16 2.64E-06
Total 0.181992 31
Ghali et al 028
positive value i.e. the mass gain increases by time but it less effective than temperature. It is clear that the effect of time is about half effect of temperature. A (nitrogen) has a little positive effect on mass gain i.e. increasing the nitrogen content, the mass gain will increase but by a very small extent. The interaction combination effect of nickel with nitrogen (AB), or with time (BC), or with temperature (BD) has relative high negative effect i.e. these interaction combination affecting factors retard oxidation process i.e. retard mass gain process. While, the interaction combination effect of nitrogen with time (AC) or with temperature (AD) has small positive effect i.e. they have a little enhancing effect on mass gain. The interaction combination effect of time with temperature (CD) has relatively high positive effect i.e. they will enhance oxidation process. Moreover, the interaction combination effect of nitrogen with nickel and time (ABC) or with temperature (ABD), or with both (ABCD) has moderate negative effect i.e these interaction combination affecting factors will cause decreasing in mass gain i.e. the oxidation process will be retarded by a certain extent.
The contrast coefficients used in estimation the effects are summerized in Table 4. Note that the contrast coefficient is always either (+1) or (-1) referring to the maximum and minimum level for the affecting factor.
Table 4. Contrast coefficients of effects
Effects
(1) a b ab c ac bc abc d Ad bd abd cd acd Bcd Abcd
A -1 +1 -1 +1 -1 +1 -1 +1 -1 +1 -1 +1 -1 +1 -1 +1 B -1 -1 +1 +1 -1 -1 +1 +1 -1 -1 +1 +1 -1 -1 +1 +1 AB -1 -1 -1 +1 +1 -1 -1 +1 -1 +1 +1 +1 -1 +1 +1 +1 C -1 -1 -1 -1 +1 +1 +1 +1 -1 -1 -1 -1 +1 +1 +1 +1 AC -1 -1 +1 -1 -1 +1 -1 +1 -1 +1 -1 +1 +1 +1 +1 +1 BC -1 +1 -1 -1 -1 -1 +1 +1 -1 -1 +1 +1 +1 +1 +1 +1 ABC -1 +1 +1 -1 +1 -1 -1 +1 -1 +1 +1 +1 +1 +1 +1 +1 D -1 -1 -1 -1 -1 -1 -1 -1 +1 +1 +1 +1 +1 +1 +1 +1 AD -1 +1 -1 +1 -1 +1 -1 +1 +1 +1 +1 +1 +1 +1 +1 +1 BD -1 -1 +1 +1 -1 -1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 ABD -1 +1 +1 +1 -1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 CD -1 -1 -1 -1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 ACD -1 +1 -1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 BCD -1 -1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 ABCD -1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1 +1
The mass gain (mg/cm2) can be predicted by using factorial design in terms of regression model. The regression model is given in Equation 16.
𝑀𝑎𝑠𝑠 𝑔𝑎𝑖𝑛 (𝑚𝑔
𝑐𝑚2) = 𝛽0+ 𝛽1𝑥1+ 𝛽2𝑥2+ 𝛽3𝑥3+ 𝛽4𝑥4+ 𝛽12𝑥1𝑥2+ 𝛽13𝑥1𝑥3+ 𝛽14𝑥1𝑥4+ 𝛽23𝑥2𝑥3+ 𝛽24𝑥2𝑥4+ 𝛽34𝑥3𝑥4+
𝛽123𝑥1𝑥2𝑥3+ 𝛽124𝑥1𝑥2𝑥4+ 𝛽134𝑥1𝑥3𝑥4+ 𝛽234𝑥2𝑥3𝑥4+ 𝛽1234𝑥1𝑥2𝑥3𝑥4+ 𝜖 (16)
Where x1, x2, x3 and x4 are coded variables that represent the N%, Ni% , time and temperature at high or low level (its
value +1 or -1) respectivelly, 𝛽𝑠, are regression coefficients. 𝛽0 is the intercept which is the grand average of all 32
observations (i.e. 𝛽0=0.086547), the regression coefficients 𝛽1, 𝛽2 , 𝛽3 and 𝛽4 are one-half the corresponding factor
effect A, B, C and D respectively (𝛽1= 0.001416, 𝛽2= -0.02809, 𝛽3= 0.036034, & 𝛽4= 0.049841), the regression
coefficients 𝛽12, 𝛽13, 𝛽14, 𝛽23, 𝛽24,and 𝛽34 are one-half the corresponding factor effect AB, AC, AD, BC, BD and CD (𝛽12=
-0.01021, 𝛽13= 0.000403, 𝛽14= 0.002222, 𝛽23= -0.00809, 𝛽24= -0.00853, and 𝛽34= 0.027578 ) and 𝛽123, 𝛽124, 𝛽134, 𝛽234,
and 𝛽1234 are one-half the corresponding factor effect ABC, ABD, ACD, BCD, and ABCD respectively which are (𝛽123
=-0.00571, 𝛽124= -0.00567, 𝛽134= 0.000959, 𝛽234= -0.00378, and 𝛽1234= -0.00492) and 𝜖 is the residual ( the difference
between observed and fitted point of the design).
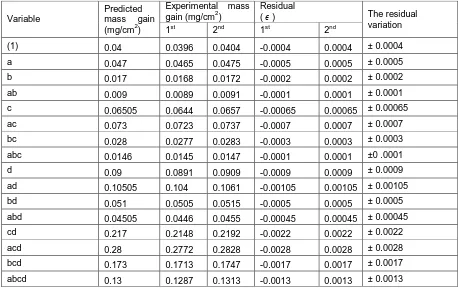
To estimate the residual (𝜖) and calculate the predicted mass gain at (1), a, b, ab, c, ac, bc, abc, d, ad, bd, abd, cd, acd, bcd, and abcd. The sign of coded variables can taken from table (2). The resuts are presented in Table 5. The average residual is ± 0.000866 which can be neglicted. The relation between the natural variable and the coded variable is given in Equation 17. The mass gain (mg/cm2) can be determined as a function in N%, Ni%, time and temperature as given in Equation 18.
𝐶𝑜𝑑𝑒𝑑 𝑣𝑎𝑟𝑖𝑎𝑏𝑙𝑒 = 𝑛𝑎 𝑡𝑢𝑟𝑎𝑙 𝑣𝑎𝑟𝑖𝑎𝑏𝑙𝑒 −(𝑣𝑎𝑟𝑖𝑎𝑏𝑙𝑒 𝑎𝑡 ℎ𝑖𝑔ℎ 𝑙𝑒𝑣𝑒𝑙 +𝑣𝑎𝑟𝑖𝑎𝑏𝑙𝑒 𝑎𝑡 𝑙𝑜𝑤 𝑙𝑒𝑣𝑒𝑙 )/2
(𝑣𝑎𝑟𝑖𝑎𝑏𝑙𝑒 𝑎𝑡 ℎ𝑖𝑔ℎ 𝑙𝑒𝑣𝑒𝑙 −𝑣𝑎𝑟𝑖𝑎𝑏𝑙𝑒 𝑎𝑡 𝑙𝑜𝑤 𝑙𝑒𝑣𝑒𝑙 )/2 (17)
𝑀𝑎𝑠𝑠 𝑔𝑎𝑖𝑛 (𝑚𝑔
𝑐𝑚2) = −0.03234 + 0.016216 𝑁% + 0.006195 𝑁𝑖% + 0.000959 ∗ 𝑡 + 0.000113𝑇 − 0.01704 𝑁% 𝑁𝑖% −
0.05566 𝑁% ∗ 𝑡 + 5.76 ∗ 10−5 𝑁% 𝑇 − 0.00429 𝑁𝑖% ∗ 𝑡 − 1.1 ∗ 10−5 𝑁𝑖% 𝑇 + 3.26 ∗ 10−6𝑇 ∗ 𝑡 + 0.012523 𝑁% 𝑁𝑖% ∗
Figure 1 shows the variation between the predicted and exprimental results of mass gain for each stainless steel at time 1 and 8 hours and at temperature 500 oC and 800 oC. It is clear that the predicted values are very close to the experimental ones.
Table 5: Actual and predicted mass gain at different conditions (variables)
Number of experiment
(1) a b ab c ac bc abc d ad bd abd cd acd bcd abcd
M
as
s
ga
in
, (
m
g/
cm
^2
)
0.00 0.05 0.10 0.15 0.20 0.25 0.30
Predicted Actual
Figure 1. Variation between predicted and actual mass gain at difference conditions
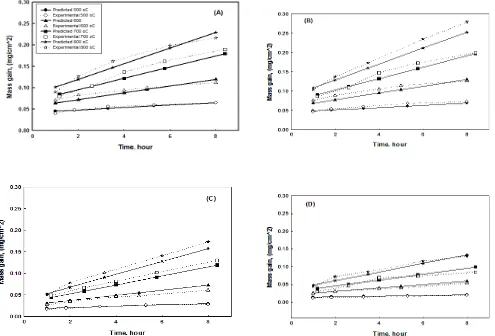
The derived model was used to predict the mass gain at different oxidation temperatures ( 500 oC, 600 oC, 700 oC and 800 oC) in time range 1 – 8 hours for each steel grades. The comparison between the predicted and experimental mass gain of different stainless steels grades is presented in Figure 2. It is clear that the predicted values are close to the experimental values for four stainless steels at temperature range between 500 oC – 800 oC within time range 1 – 8 hours. This means that factorial design is a good technique to analyze the data. Beside to, it successes to predict the
Variable
Predicted
mass gain
(mg/cm2)
Experimental mass
gain (mg/cm2)
Residual
( 𝜖 ) The residual
variation
1st 2nd 1st 2nd
(1) 0.04 0.0396 0.0404 -0.0004 0.0004 ± 0.0004
a 0.047 0.0465 0.0475 -0.0005 0.0005 ± 0.0005
b 0.017 0.0168 0.0172 -0.0002 0.0002 ± 0.0002
ab 0.009 0.0089 0.0091 -0.0001 0.0001 ± 0.0001
c 0.06505 0.0644 0.0657 -0.00065 0.00065 ± 0.00065
ac 0.073 0.0723 0.0737 -0.0007 0.0007 ± 0.0007
bc 0.028 0.0277 0.0283 -0.0003 0.0003 ± 0.0003
abc 0.0146 0.0145 0.0147 -0.0001 0.0001 ±0 .0001
d 0.09 0.0891 0.0909 -0.0009 0.0009 ± 0.0009
ad 0.10505 0.104 0.1061 -0.00105 0.00105 ± 0.00105
bd 0.051 0.0505 0.0515 -0.0005 0.0005 ± 0.0005
abd 0.04505 0.0446 0.0455 -0.00045 0.00045 ± 0.00045
cd 0.217 0.2148 0.2192 -0.0022 0.0022 ± 0.0022
acd 0.28 0.2772 0.2828 -0.0028 0.0028 ± 0.0028
bcd 0.173 0.1713 0.1747 -0.0017 0.0017 ± 0.0017
Ghali et al 030
oxidation behavior of stainless steels grades within the boundary conditions (Ni & N contents and temperature & time ranges).
Figure 2. The variation between experimental and predicted mass gain (mg/cm2) at temperatures 500 oC, 600 oC, 700 oC and 800
o
C within time duration from 1 to 8 hours for (A) steel number 1, (B) steel number 2, (C) steel number 3 and (D) steel number 4.
The derived model is tested against the previous experimental results (Vangeli et al., 2012) of oxidation steel grade 304L AISI (0.03%C, 0.3%Si, 1.5%Mn, 18.1%Cr, 8.2%Ni, and 0.06%N) in dry air at 800 oC. The comparison between the predicted and actual mass gain is illustrated in Figure 3. It is clear that the predicted mass gain is in agreement with the experimental results to a certain extent. The difference between the predicted and experimental results can be attributed to the following; the model is based on high carbon stainless steels (carbon content ranging from 0.30% to 0,41%), while the carbon content of experimental stainless steel is 0.03%. Where, carbon enhances the oxidation behavior of stainless steel as reported by Taishi Moroishi et al(1979). Also, this experiment was carried out in dry air i.e. less water vapor which causes decreasing in oxidation rate and hence the mass gain is reduced.
Time, hour
1.
11
7
1.
27
66
1.
43
62
1.
75
53
2.
55
32
3.
19
15
3.
82
98
4.
62
77
5.
42
55
6.
22
34
7.
02
13
7.
81
92
M
as
s
ga
in
, (
m
g/
cm
^2
)
1e-4 1e-3 1e-2 1e-1
Actual Predicted
Conlcusions
A 24 factorial design is implemented to analyse the oxidation behavior of stainless steel containing different nitrogen and nickel content at interval time 1 – 8 hour at temperatures 500 oC and 800 oC. The regression model was built up to predict the mass gain in terms of nitrogen content, nickel content, time and temperature as it is given in the following equations:
𝑀𝑎𝑠𝑠 𝑔𝑎𝑖𝑛 (𝑚𝑔
𝑐𝑚2)
= −0.03234 + 0.016216 𝑁% + 0.006195 𝑁𝑖% + 0.000959 ∗ 𝑡 + 0.000113𝑇 − 0.01704 𝑁% 𝑁𝑖%
− 0.05566 𝑁% ∗ 𝑡 + 5.76 ∗ 10−5 𝑁% 𝑇 − 0.00429 𝑁𝑖% ∗ 𝑡 − 1.1 ∗ 10−5 𝑁𝑖% 𝑇 + 3.26 ∗ 10−6𝑇 ∗ 𝑡
+ 0.012523 𝑁% 𝑁𝑖% ∗ 𝑡 + 8.26 ∗ 10−6 𝑁% 𝑁𝑖% 𝑇 + 0.000113 𝑁% 𝑇 ∗ 𝑡 + 8.51 ∗ 10−6 𝑁𝑖% 𝑇 ∗ 𝑡 − 2.6
∗ 10−5 𝑁% 𝑁𝑖% 𝑇 ∗ 𝑡
Factorial design demonstrated the contribution effect of nitrogen concentration, nickel concentration, time and temperature on oxidation behavior of austenitic stainless steel containing nitrogen.
Factorial design analysis showed that nickel has large significant effect on retardation the oxidation process, temperature enhances the oxidation process, time increase oxidation process but less than effect of temperature, nitrogen enhance oxidation process to a very small extent. The interaction combination effect of nickel with nitrogen or with time or temperature retard oxidation process. While, the interaction combination effect of nitrogen with time or temperature has a little enhancing effect on oxidation process. The interaction combination effect of time with temperature enhances oxidation process. Moreover, the interaction combination effect of nitrogen with nickel and time and/or temperature cause retardation in oxidation process to a certain extent. The predicted mass gain using the derived regression model was close and fitted with the experimental data.
The model is tested against the experimental oxidation of 304L in dry air at 800 oC. The predicted mass gain is in agreement with the experimental to certain extent. The difference between predicted and experimental results is attributed to the difference in carbon contents. The factorial design can be successfully used in the determination of mass gain especially when the boundary conditions are fitted with the model.
REFERENCES
Ahmed A, Ghali SN, Eissa M, El Badry SA (2011). Influence of partial replacement of nickel by nitrogen on microstructure and mechanical properties of austenitic stainless steel. J. of Metallurgy. Vol. 2011, article ID 639283, 6 pages, doi: 10.1155/2011/639283
Baba H, Kodarwa T, Katada Y (2002). Role of nitrogen on the corrosion behaviour of austenitic stainless steels. Corrosion Science. 44(10): 2393-2407.
Basu SN, Yurek GJ (1991). 'Effect of alloy grain size and silicon content on the oxidation of austenitic Fe-Cr-Ni-Mn-Si alloys in pure O2. Oxid. Met. Vol.36. No. 3/4: 281-315.
Cihal V (1968). Protect Met. (in Russian). 4(6): 637-655.
Francis JM (1966). Influence of minor alloying elements on structure of oxides formed during high-temperature oxidation of an austenitic steel. J. of the Iron and Steel Institute. 204: 910.
Fry A, Osgerby S, Wright M, Wright M (2002). Oxidation of Alloys in Steam Environments. A Review. in NPL Report MATC(A)90.
Gao Y, Kim HG, Sohn HY, Kim CW (2012). Gaseous pre-reduction for the magnetic beneficiation of ferruginous low-grade Mn Ore. ISIJ International. 52(5): 759-763.
Ghali S, Baiomy F, Eissa M (2011). 7th European Stainless Steel Conference Science and Market. Como (Italy), 21-23 Sept.
Ghali SN (2013a). Low carbon high nitrogen low nickel stainless steel. steel research int. 84(5): 450-456.
Ghali S, Eissa M, El-Faramawy H, Mishreky M, Mattar T (2013b). New Grades of Stainless Steel. International Conference on Advances in Refractories and Clean Steel Making (ARCS13), Ranchi, India, 26-28 Jun.
Ghali SN, Ahmed A, Eissa M, El-Faramawy H, Mishreky M, Mattar T. (2013c). Production and Application of Advanced High Nitrogen steel. International Conference on Science and Technology of Ironmaking and Steelmaking, Jamshedpur, India, 16-18 Dec.
Ghali S, Mousa EA (2014). Analysis of the reduction yield of synthetic iron oxide sinter reduced by H2 at 900-1100oC using factorial design approach. Steel Grips. 26 Aug.: 11 – 17
Ghali et al 032
Embiez, FRANCE: Trans Tech Publications Ltd.
Hussey RJ, Mitchell DF, Graham MJ. (1987). The growth and structure of oxide-films formed on single-crystal (100) and polycrystalline Cr between 550 and 900 degrees-C. Werkstoffe Und Korrosion Materials and Corrosion. 38(10) 575-583.
Klueh RL. (2005). Elevated temperature ferritic and martensitic steels and their application to future nuclear reactors. Int. Materials Reviews. 50(5): 287-310
Lepingle V, Louis G, Allue D, Lefebvre B, Vandenberghe B (2008). Steam oxidation resistance of new 12%Cr steels: Comparison with some other ferritic steels. Corrosion Science. 50(4): 1011-1019.
Lim YS, Kim JS, AIn SJ, Known HS, Katada Y (2001). The influences of microstructure and nitrogen alloying on pitting corrosion of type 3 16L and 20% Mn-subtituted type 3 16L stainless steels. Corrosion Science. 43(1): 53-68.
Mesquita TJ, Nogueira RP, Bastos IN (2011). Factorial design applied to corrosion of super duplex stainless steel. Latin America Applied Research. 41: 311-315.
Moroishi Taishi, Fujikawa Hisao, Makiura H (1979). The Effect of Carbon, Zirconium, Niobium, and Titanium on the Oxidation Resistance of Chromium Stainless Steel. J. Electrochem. Soc., 126: 12. doi: 10.1149/1.2128918: 2173-2182
Robinson R (2008). Studies in low temperature self-reduction of by-products from integrated iron and steel making. PhD thesis. Luleå University of Technology. ISSN: 1402-1544.
Sagara M (2002). Effect of alloying elements on localized corrosion characteristics of nitrogen-bearing stainless steels and evaluation of crevice corrosion in seawater environment. ISIJ Bulletin. 7(11) 858-859.
Sedriks AJ (1996). Corrosion of Stainless Steels. 2nd edn. Canada: John Wiley and Sons, Inc.
Stott FH, Wood GC, Stringer J (1995). The influence of alloying elements on the development and maintenance of protective scales. Oxidation of Metals. 44(1/2): 113-145.
Thier H, Baumel A, Schmidtmann E (1969). EinfluB von Stickstoff auf das Aus- scheidungsverhalten des Stahles X 5 CrNiMo 17 13. Arch Eisenhuttenw. 40(4): 333-339.
Vangeli PS, Ivarsson B, Pettersson R (2012). A corrosion management and applications engineering magazine from Outokumpu, Outokumpu Stainless AB. Avesta Research Centre. Avesta. Sweden 3: 2-9
Vanini AS, Audouard JP, Marcus P (1996). Corrosion Science. 36: 1825-1834.
West JM (1986). Basic Corrosion and Oxidation. 2rd edn. Chichester: Ellis Horwood Limited.
Wood GC, Hobby MG, Vaszko B (1964). Electron – probe microanalysis of a nodular scale growth on an austenitic stainless steel. J. Iron Steel Inst.: 685-695
Young D (2008). High Temperature Oxidation and Corrosion of Metals. 1st edn. Oxford: Elsevier Ltd.
Zurek J, Hierro LN, Piron Abellan J, Niewolak L, Singheiser L, Quadakkers WJ (2004). Effect of alloying additions in ferritic 9‐12%Cr steels on the temperature dependence of the steam oxidation resistance. 6th International Symposium on High Temperature Corrosion and Protection of Materials. Les Embiez, FRANCE: Trans Tech Publications Ltd. Accepted 24 August, 2014.
Citation: Ghali S, Eissa M, El-Faramawy H (2014). Simulation of austenitic stainless steel oxidation containing nitrogen at temperature range 500 oC – 800 oC. International Journal of Statistics and Mathematics, 1(3): 023-032.