Comparing the disease course of patients with seronegative and seropositive rheumatoid arthritis fulfilling the 2010 ACR/EULAR classification criteria in a treat to target setting: 2 year data from the ARCTIC trial
Full text
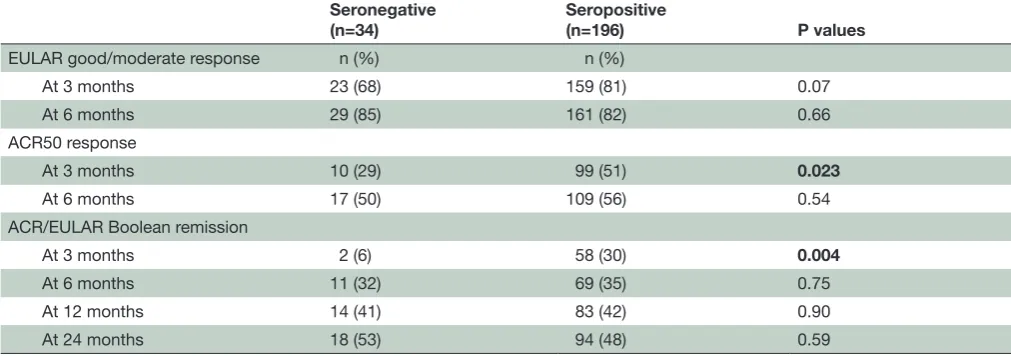
Figure


Related documents
Discussion topics covered in all client groups included the following: previous messages that have attracted participants ’ attention and influenced their knowledge or
ABSTRACT: To investigate the anti-arthritis effects of hydro-alcoholic stem bark extract (HSBE) of Plumeria rubra in Complete Freund’s adjuvant (CFA) induced
A number of changes in Russian economic policy during 2003–05 augur ill for both the further growth of its core resource-exporting sectors (especially oil and gas) and the
Variation in soil properties has been known and has been the subject of much research, as horizons may differ in organic matter content, structure, texture, pH, base
In this study, we have established two-dimensional quantitative structure propriety relationships (2D- QSPR) model, for a group of 78 molecules based on pyrazine, these molecules
Comparing the dynamic responses under persistent interest rate and productivity shocks es- tablishes the claim in the introduction of the paper that under either type of
The committee, based centrally in the Wits Research Office (WRO), was reorganised in 1998 when a secretariat for sponsored clinical trials was formed in the Wits