Total plasma magnesium, zinc, copper and selenium
concentrations in type-I and type-II diabetes
Ame´lie I. S. Sobczak.Fiona Stefanowicz.Samantha J. Pitt.Ramzi A. Ajjan . Alan J. Stewart
Received: 19 December 2018 / Accepted: 29 December 2018 ÓThe Author(s) 2019
Abstract Glycemia and insulin resistance are impor-tant regulators of multiple physiological processes and their dysregulation has wide-ranging consequences, including alterations in plasma concentrations of metal micronutrients. Here, magnesium, zinc, copper, sele-nium and glycated albumin (HbA1c) concentrations and quartile differences were examined in 45 subjects with type-I diabetes (T1DM), 54 subjects with type-II diabetes (T2DM) and 62 control subjects in order to assess potential differences between sexes and between T1DM and T2DM. Plasma magnesium con-centration was decreased in T1DM subjects, with the second, third and fourth quartiles of magnesium concentrations associated with the absence of T1DM. This effect was observed in females but not males. In T2DM, the highest quartile of selenium concentrations and the third quartile of copper concentrations
associated with the absence of diabetes in males. The highest quartile of magnesium concentrations was associated with the absence of T2DM in males but not females. HbA1c correlated with plasma concentrations of magnesium (negatively, in both sexes together in T1DM and T1DM males), copper (positively, in T1DM males and in both sexes together in T2DM), selenium (positively, in both sexes together in T1DM and T2DM, and T2DM females) and with zinc/copper ratio (negatively, in both sexes together in T1DM and T2DM). This study shows that plasma magnesium concentration is altered to the highest degree in T1DM, while in T2DM, plasma selenium and copper concen-trations are significantly affected. This work increases our understanding of how T1DM and T2DM affects plasma metal concentrations and may have future implications for diabetes management.
Keywords DiabetesHbA1cICP-MSMetal homeostasisZinc/copper ratio
Abbreviations
CI Confidence interval HbA1c Glycated haemoglobin
ICP-MS
Inductively-coupled plasma-mass spectrometry
OR Odds ratio
Q Quartile
T1DM Type-I diabetes mellitus T2DM Type-II diabetes mellitus A. I. S. SobczakS. J. PittA. J. Stewart (&)
School of Medicine, University of St Andrews, Medical and Biological Sciences Building, St Andrews, Fife KY16 9TF, UK
e-mail: ajs21@st-andrews.ac.uk
F. Stefanowicz
Scottish Trace Element and Micronutrient Diagnostic and Research Laboratory, Department of Clinical
Biochemistry, NHS Greater Glasgow and Clyde, Glasgow, UK
R. A. Ajjan
Introduction
Diabetes is mainly divided into two types. In type-I diabetes (T1DM), theb-cells of the islets of Langer-hans in the pancreas responsible for producing insulin are lost, typically from an attack from the immune system, causing an insulin deficiency in the body. In type-II diabetes (T2DM), cells become resistant to insulin signalling, failing to respond to it sufficiently. This is combined with either normal or increased insulin levels but can also develop into insulin deficiency through a relative loss of the insulin storage function of b-cells. Glucose levels in the body are important, as directly or indirectly they regulate many physiological processes, including glucose, glycogen metabolism. It also acts to control food intake (satiety) and maintaining long-term body weight but also regulates inflammation, vasodilatation and basic cell growth and replication. Thus, mishandling of glucose has wide-ranging consequences in the body. Among them is an alteration in the total plasma concentrations of micronutrients including some metal ions (Kaur and Henry 2014). This is thought to be caused by numerous factors, which include increased fluid loss from the body, an increased demand for micronutri-ents due to altered protein metabolism as well as defective metal transport due to oxidative stress (Kaur and Henry2014; Maret2017).
Variations in plasma metal concentrations in dia-betes have been extensively studied in different populations. However there is a great heterogeneity in the data, with both higher and lower levels of the same metals having been reported (e.g. zinc and selenium) (Wang et al. 2016; Sanjeevi et al. 2018). Several meta-analyses have been performed in order to find a consensus on whether levels of some metals are higher or lower in diabetes (Wang et al. 2016; Sanjeevi et al. 2018). Analyses of quartiles of selenium concentrations yielded interesting results, with both the lowest and highest quartiles being associated with T2DM (Wang et al. 2016). Sex differences have also been shown to be of some importance with higher levels of copper reported in females with T1DM but not males (Ruiz et al.1998). In addition, glycated haemoglobin (HbA1c) level as an indication of diabetes management was found to correlate with several plasma metal concentrations, including zinc, copper and magnesium and also to the
zinc/copper ratio (Naka et al. 2013; Ramadass et al.
2015; Atari-Hajipirloo et al.2016).
Here, we have examined the total plasma concen-trations of zinc, copper, magnesium and selenium in patients with T1DM and T2DM and in controls, assessing the correlation between quartiles of metal cations concentrations and diabetes, as well as the influence of sex and diabetes management through blood HbA1c levels. Plasma metal concentrations and potential sex differences in those concentrations have not been thoroughly examined both in T1DM and in T2DM in the same study before. The results indicate that magnesium deficiency is particularly important in T1DM while elevated copper and selenium deficiency in females are more important in T2DM. A better understanding of those effects could lead to a better understanding of metal micronutrient handling and to better management and treatment of T1DM and T2DM.
Methods
Samples collection and treatment
A total of 45 patients with T1DM, 54 patients with T2DM and 62 controls were recruited from Leeds Teaching Hospital Trust. Plasma samples from sub-jects with either T1DM or T2DM and controls were collected following approval by the local Research Ethics Committee in Leeds and after obtaining written informed consent. For all subjects, baseline fasting blood samples were collected in lithium heparin coated tubes. Plasma was then separated within 2 h of collection by centrifugation at 24009gfor 20 min at 4°C. Samples were snap frozen in liquid nitrogen and stored at-40°C until analysis.
Inductively-coupled plasma-mass spectrometry (ICP-MS)
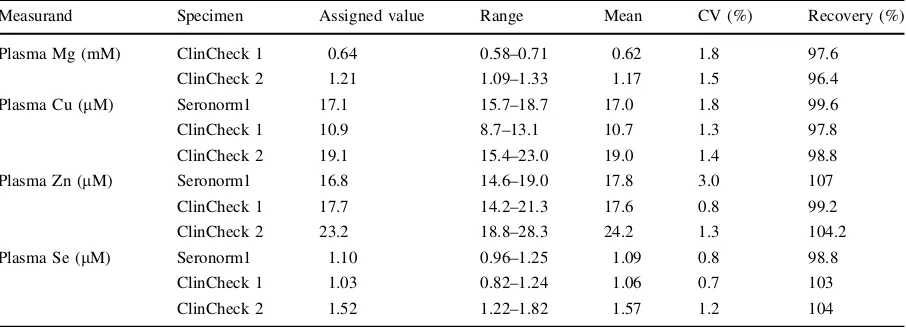
18.2 Mohm reverse osmosis deionised water (Elga Maxima High Wicombe, UK). Lithium heparin plasma samples were diluted 1 in 10 with the same solution used to prepare calibration standards with 25 ppb germanium. ClinChekÒ 1 & 2 (RECIPE, Germany) and SeronormTM1 (SERO, Norway) human serum certified reference materials were used to demonstrate accuracy. ClinChekÒ1 and 2 are materi-als with consensus values while SeronormTM is traceable to a National Institute of Standard Technol-ogy (NIST) certified reference material.
Copper, zinc, selenium and magnesium analyses were performed simultaneously using an Agilent 7900 ORS-ICP-MS (Agilent Technologies, Santa Clara, California, USA). The instrument was controlled using Mass Hunter software (version 4.1, Agilent Technologies). Argon was used to form the plasma (CryoServices Ltd, purirty: 99.9%). Polyatomic inter-ferences for copper, zinc and magnesium were removed through collision induced dissociation and kinetic energy discrimination using helium gas (Air Products and Chemicals, Inc, purity 99.9%). Poly-atomic and doubly charged interferences for selenium were removed using a charge exchange reaction by using the collision cell with hydrogen. The concen-tration of all elements was measured three times within a single run using the central 0.05 m/z of the peak. The ICP-MS was equipped with an CETAC ASX-500 series autosampler (Teledyne CETAC, Omaha, USA), an Integrated Sample Introduction System and Discrete Sampler—3 (ISIS-DS, Agilent Technologies) and a G32992A re-circulating chiller (Polyscience, Illinois, USA). The ISIS-DS was fitted with a Quartz Scott-Type, Double-Pass Spray Cham-ber (Agilent Technologies), a glass micromist nebu-liser (Agilent Technologies) and a sample loop. The sample loop was prepared in-house using 0.8 mm internal diameter and PTFE sample tubing (Agilent Technologies). A quartz torch with 2.5 mm internal diameter was used (Agilent Technologies). Nickel sampling and skimmer cones were used at all times (Agilent Technologies). All instrument parameters were optimised daily by performing an auto-tune while aspirating a tuning solution containing 10 ppb lithium, yttrium, cobalt, cerium and thallium. Typical instrument parameters are shown in Table 1. Three certified reference materials (Seronorm 1 and Clin-Check 1 and 2) with assigned values and ranges were used to demonstrate the accuracy of the method used
to determine the concentrations of Mg, Cu, Zn and Se in human plasma (Table2). The mean for all measur-ands were within the assigned range for all materials and had satisfactory recoveries ranging between 96.4 and 107%. The precision of the method, represented by coefficient of variation, was also satisfactory with values less than or equal to 3%. All metal concentra-tions in plasma were compared to the glycated haemoglobin concentrations which were measured in the samples as a routine test.
Statistical analysis and representation
Data are presented as mean±standard error of the mean (SEM). Graphs were generated and statistical analysis was performed using Prism 7.0 (GraphPad Software, La Jolla, CA). Differences between groups were analysed using multiple Student’s t-tests or analysis of variance, while correlations between linear variants were analysed with Pearson’s correlation. The data was also separated into quartiles and odd ratios were calculated. Significance threshold was set at p B0.05.
Results and discussion
Total plasma zinc, copper, magnesium, selenium concentrations and zinc/copper ratios in T1DM and T2DM
ICP-MS was used to determine the total plasma concentrations of zinc, copper, magnesium and sele-nium in subjects with T1DM or T2DM and in their respective age-matched controls. The zinc/copper ratio was also calculated in all samples. Table3
T1DM than in controls but not with T2DM, while the plasma zinc/copper ratio was significantly higher in T2DM subjects than in controls, but this was not the case in T1DM.
The data from each of the two diabetes groups, (T1DM and T2DM subjects; each with their respec-tive age-matched controls) were split into quartiles for each metal, defined as four groups delimited by 25%, median and 75% of highest values in each of the groups. The odd ratios (ORs) of a subject in a specific quartile having diabetes were calculated (Table4). No significant difference in total plasma zinc concentra-tions was observed between either the T1DM or the T2DM groups and their age-matched controls. This is contrary to two previous reports—a meta-analysis accumulating 20,183 T2DM subjects and a study with 51 T1DM subjects both showing that zinc concentra-tion is lower in diabetes (Li et al.2018; Sanjeevi et al.
[image:4.547.48.501.241.405.2]2018). Despite previous reports of both higher and lower zinc concentrations being associated with T2DM, no significance could be gained by examining quartiles of zinc concentrations (Sanjeevi et al.2018). In T2DM, the ORs of having diabetes in the second (Q2), third (Q3) and fourth (Q4) copper quartile groups were respectively 0.031 [95% confidence interval (CI) 0.080–1.275, p[0.05], 0.193 (95% CI 0.051–0.966, p\0.05) and 0.276 (95% CI 0.070–1.154, p[0.05) compared with the first (Q1) quartile group. The absence of a clear difference between T2DM subjects and controls (with only Q3 being significantly associated with the absence of diabetes) can be explained by the mean plasma copper concentration being very low in our T2DM group and high in our age-matched controls, almost enough to be significantly higher in the controls (p = 0.0698), whereas a meta-analysis involving 20,183 T2DM Table 1 ICP-MS
instrument parameters Parameter Setting
Isotopes monitored (m/z) Cu 63, Zn 66, Se 78 and Mg 24
RF power (W) 1550
RF matching 1.70
Sampling depth (mm) 10
Carrier gas (L/min) 1.05
Make up gas L/min) 0.0
Spray chamber temperature (°C) 2 He octopole reaction system flow (mL/min) 5.0
Nebuliser pump (rps) 0.1
Table 2 Accuracy of the ICP-MS method and details of the concentration of trace elements in each material (coefficient of variation and recovery)
Measurand Specimen Assigned value Range Mean CV (%) Recovery (%)
Plasma Mg (mM) ClinCheck 1 0.64 0.58–0.71 0.62 1.8 97.6
ClinCheck 2 1.21 1.09–1.33 1.17 1.5 96.4
Plasma Cu (lM) Seronorm1 17.1 15.7–18.7 17.0 1.8 99.6
ClinCheck 1 10.9 8.7–13.1 10.7 1.3 97.8
ClinCheck 2 19.1 15.4–23.0 19.0 1.4 98.8
Plasma Zn (lM) Seronorm1 16.8 14.6–19.0 17.8 3.0 107
ClinCheck 1 17.7 14.2–21.3 17.6 0.8 99.2
ClinCheck 2 23.2 18.8–28.3 24.2 1.3 104.2
Plasma Se (lM) Seronorm1 1.10 0.96–1.25 1.09 0.8 98.8
ClinCheck 1 1.03 0.82–1.24 1.06 0.7 103
individuals has shown that plasma copper is higher in T2DM (Sanjeevi et al. 2018). There were no differ-ences in plasma copper concentrations between quar-tiles in the T1DM group, contrary to a previous study (Ruiz et al. 1998). The ORs of having T1DM in the Q2, Q3, Q4 magnesium groups were 0.176 (95% CI 0.058–0.628, p\0.01), 0.1173 (95% CI 0.036–0.432, p\0.01) and, 0.086 (95% CI 0.028–0.325, p\0.001) compared with the Q1 group. Thus, T1DM is associated with lower plasma magnesium concentrations (Q2–Q4), as supported by a previous study (Brown et al.1999). In T2DM, no differences in magnesium concentration were observed between
quartiles. This is despite a meta-analysis involving 286,668 T2DM subjects having found an association between low dietary magnesium levels and T2DM, while another study following 12,128 non-diabetic subjects over 6 years reported an inverse relationship between serum magnesium concentrations and inci-dence of T2DM amongst white (but not black) participants (Kao et al. 1999; Larsson and Wolk
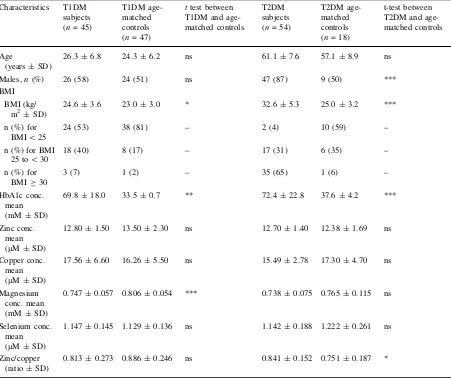
[image:5.547.48.499.73.452.2]2007). For plasma selenium concentrations, the ORs of having T2DM in the Q2, Q3 and Q4 selenium groups were respectively 0.850 (95% CI 0.221–3.292, p[0.05), 0.560 (95% CI 0.152–2.484, p[0.05) and 0.100 (95% CI 0.017–0.682, p\0.01) compared with Table 3 Demographic characteristics of the studied population and mean plasma HbA1c and metal concentrations in each group
Characteristics T1DM subjects (n= 45)
T1DM age-matched controls (n= 47)
ttest between T1DM and age-matched controls
T2DM subjects (n= 54)
T2DM age-matched controls (n= 18)
t-test between T2DM and age-matched controls
Age
(years±SD)
26.3±6.8 24.3±6.2 ns 61.1±7.6 57.1±8.9 ns
Males,n(%) 26 (58) 24 (51) ns 47 (87) 9 (50) ***
BMI
BMI (kg/ m2±SD)
24.6±3.6 23.0±3.0 * 32.6±5.3 25.0±3.2 ***
n (%) for BMI\25
24 (53) 38 (81) – 2 (4) 10 (59) –
n (%) for BMI 25 to\30
18 (40) 8 (17) – 17 (31) 6 (35) –
n (%) for BMIC30
3 (7) 1 (2) – 35 (65) 1 (6) –
HbA1c conc. mean (mM±SD)
69.8±18.0 33.5±0.7 ** 72.4±22.8 37.6±4.2 ***
Zinc conc. mean (lM±SD)
12.80±1.50 13.50±2.30 ns 12.70±1.40 12.38±1.69 ns
Copper conc. mean (lM±SD)
17.56±6.60 16.26±5.50 ns 15.49±2.78 17.30±4.70 ns
Magnesium conc. mean (mM±SD)
0.747±0.057 0.806±0.054 *** 0.738±0.075 0.765±0.115 ns
Selenium conc. mean (lM±SD)
1.147±0.145 1.129±0.136 ns 1.142±0.188 1.222±0.261 ns
Zinc/copper (ratio±SD)
0.813±0.273 0.886±0.246 ns 0.841±0.152 0.751±0.187 *
the Q1 group. Thus, low selenium concentrations were linked with T2DM. The fact that subjects in Q4 did not associate with T2DM is contrary to the findings of previously published meta-analysis based on 13,460 T2DM patients, where both Q1 and Q4 were associ-ated with T2DM (Wang et al.2016). No differences in plasma selenium concentrations were observed between quartiles in T1DM, although a previous study indicated that lower plasma selenium concentrations have been found in T1DM (Ruiz et al.1998). Here we were unable to see any differences between zinc/cop-per ratio quartiles in either T1DM or T2DM, contrary to the higher mean ratios we measured in T2DM. Some previous publications also indicated that lower plasma zinc/copper ratios have been observed in T1DM (Lin et al. 2014) and in T2DM (Atari-Hajipirloo et al.2016). The failure to detect some of these previously reported relationships in our study may be due to the number of subjects (and the resultant effect on statistical power). Thus larger cohort studies enable relatively subtle differences in plasma metal concentrations to be observed between individuals with diabetes and controls. However, those differences may not be as relevant as those that can be detected in smaller cohorts.
Differences in plasma metal concentrations between males and females with T1DM or T2DM
The influence of the sex of the subjects on plasma metal concentrations between the various groups was examined (Table5). In our age-matched controls for the T1DM group, males had significantly higher zinc concentrations and lower copper concentrations than females, while in the T1DM group males had signif-icantly higher zinc and magnesium concentrations and lower copper concentrations than females. The T2DM group was harder to study as there were few females in the group. However, copper concentrations were significantly lower and the zinc/copper ratio higher in males than in females. The T1DM and T2DM groups were then compared with their respective age-matched controls. Magnesium concentrations were lower in males and females with T1DM than in the respective controls, while only males with T2DM had a lower magnesium concentration than their respective controls. In addition, the zinc/copper ratio was higher in males with T2DM than in the male controls.
The plasma metal concentration quartile groups described above were split according to sex (Table6). The ORs of having T1DM in the Q2, Q3 and Q4 female magnesium quartile groups were respectively 0.000 (95% CI 0–0.480, p\0.01), 0.059 (95% CI 0.005–0.482, p\0.05) and 0.033 (95% CI 0.003–0.287, p\0.001) compared to the Q1 group. There were no differences between magnesium quar-tiles in T1DM males despite a lower mean having been observed compared to male controls. To our knowl-edge, no sex differences in mean plasma magnesium concentrations in T1DM subjects have been reported before. No significance differences in male copper quartiles or in female copper quartiles were observed in T1DM. This is contrary to a report, which found that plasma copper concentrations were higher in females with T1DM than in females controls but that this was not the case in males (Ruiz et al. 1998). However another study (with only 18 individuals and both T1DM and T2DM analysed together) reported the absence of any difference in plasma copper concen-tration between males and females with diabetes (Terres-Martos et al.1998). In addition, no significant differences in male or in female zinc or zinc/copper quartiles were identified in T1DM.
The low number of females among our T2DM cohort made accurate comparison between the sexes more difficult to perform and no statistically signifi-cant differences were found with any metal when examining female subjects in the quartiles. However, when looking at males only the copper Q3, magnesium Q4 and selenium Q4 groups were associated with the absence of T2DM. The ORs of having T2DM in the Q2, Q3 and Q4 male copper quartile groups were respectively 0.167 (95% CI 0.011–1.649, p[0.05), 0.074 (95% CI 0.006–0.625, p\0.05) and 0.074 (95% CI 0.005–0.842, p[0.05) compared to the Q1 group. In a meta-analysis of copper concentrations in T2DM, no sex differences were observed (Sanjeevi et al.2018). The ORs of having T2DM in the Q2, Q3 and Q4 male magnesium quartile groups were respec-tively 0.9444 (95% CI 0.04734–18.9, p[0.05), 0.1944 (95% CI 0.01285–2, p[0.05) and 0.056 (95% CI 0.004–0.582, p\0.05) compared to the Q1 group. Contrary to this, a previous study has found that females with T2DM were more likely to have lower magnesium concentrations than males (Kao et al.
selenium 0.938 (95% CI 0.047–18.89, p[0.05), 0.219 (95% CI 0.017–1.703, p[0.05), 0.031 (95% CI 0.002–0.601, p\0.05) compared to the Q1 group. Association between T2DM and selenium concentra-tions (too low or too high) in males but not females have been reported before (Bleys et al.2007; Laclaus-tra et al.2009; Akbaraly et al.2010).
Relationship between plasma metal concentrations and HbA1c concentration
Relationships between HbA1c concentration, as a proxy for glycemic control, and metal concentrations in subjects with T1DM (Fig.1) or T2DM (Fig.2) were examined. Plasma zinc concentration did not correlate with HbA1c concentration in either T1DM or T2DM, which is in accord with a previous finding in T1DM but contrary to a previous publication that has found a negative correlation (Luo et al.2015; Lin et al.
2016). In T1DM, plasma copper concentration posi-tively correlated with HbA1c concentration in males (p = 0.042, Fig.1e) but not with females or with both sexes together. In T2DM, the plasma copper concen-tration correlated with HbA1c concenconcen-tration only when considering both sexes together (p = 0.003, Fig.2d) but not in males or females only. Previous studies have found that plasma copper concentration do not correlate with HbA1c concentration in T1DM but positively correlates in T2DM (Ruiz et al.1998; Atari-Hajipirloo et al. 2016). Plasma magnesium concentration negatively correlated with HbA1c con-centration in T1DM when looking at both sexes
Table
6
continued
T1DM
and
age-matched
controls
T2DM
and
age-matched
controls
Q
1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
Males
n
4
14
14
17
4
7
21
24
OR
(95%
CI)
1
0.000 (0.000–2.397) 0.000 (0.000–1.316)
0.000
(0.000–1.408)
1
1.333 (0.138–12.360)
6
(0.662–45.600)
11
(1.069–89.650)
Females
n
2
5
9
5
3
5
3
4
3
OR
(95%
CI)
1
0.393 (0.094–1.827) 0.196 (0.015–1.616)
0.393
(0.026–3.839)
1
0.333 (0.018–5.167)
0.667
(0.062–7.580)
0.333 (0.018–5.166)
Data
is
presented
as
OR
(95%
CI)
Significance
is
indicated
as
*p
\
0.05,
**p
\
0.01
and
***p
\
0.001
[image:10.547.55.172.75.664.2]together (p = 0.004, Fig.1g) and in males (p = 0.007, Fig.1h), but not in females and it did not correlate with HbA1c concentration in T2DM. The lack of an observable relationship between plasma magnesium concentration and HbA1c concentration in females with T1DM is surprising considering that magnesium concentration was lower in females with T1DM compared to males with T1DM (while no difference between the sexes could be seen in controls). Previous studies have shown that HbA1c levels correlate with magnesium excretion in females but not males (Brown et al. 1999; Lin and Huang 2015). A negative correlation between plasma magnesium concentration and HbA1c concentration has also been reported in T2DM (Ramadass et al. 2015). Plasma selenium concentration correlated negatively with HbA1c in T1DM when looking at both sexes together (p = 0.031, Fig.1j), as in a previous study (Ruiz et al.1998), but there was no correlation when looking individually at males or females. In T2DM, plasma selenium concentration was correlated with HbA1c concentration when looking at both sexes together (p = 0.039, Fig.2j) and in females (p = 0.015, Fig.2l), contrary to a previous study that found a negative correlation with dysregulation of glucose and selenium concentration in males but not in females (Akbaraly et al.2010). The plasma zinc/copper ratio was negatively correlated with HbA1c in T1DM and T2DM when looking at both sexes together (p = 0.0258, Fig.1m and p = 0.0428, Fig. 2m respec-tively) but not in males or females only. In previous publications, the zinc/copper ratio was also negatively
correlated in T1DM and T2DM (Lin et al.2014; Atari-Hajipirloo et al.2016). Correlation of HbA1c concen-tration with total plasma metal concenconcen-trations could potentially be explained by glycation or oxidation-associated modifications of metal ion transport pro-teins (Abdelmagid et al. 2015), formation of glyco-cholate (anion of the bile acid glycholic acid) that can complex and excrete metals in bile (Atari-Hajipirloo et al.2016) or abnormal insulin signalling leading to dysregulated metal homeostasis (McNair et al.1982; Gommers et al.2016).
Implications for dysregulated metal homeostasis in diabetes
Changes in total plasma metal concentrations in subjects with diabetes have the potential to negatively affect metabolic processes in the body. Low magne-sium concentrations have been associated with an increased incidence of T1DM and T2DM and poor glycaemic control (Lin and Huang 2015; Ramadass et al. 2015). This may be due to the fact that magnesium ions (Mg2?) are an essential cofactor in several processes. Mg2? increases the affinity of insulin receptors for ATP and is thus essential for their auto-phosphorylation and tyrosine kinase activ-ity, which results in Mg2?sensitising cells to insulin (Guerrero-Romero and Rodriguez-Moran2011; Gom-mers et al. 2016; Al Alawi et al. 2018). Thus chronically low Mg2? concentrations can lead to insulin resistance (Guerrero-Romero and Rodriguez-Moran 2011; Gommers et al. 2016; Al Alawi et al.
2018). Furthermore, Mg2?can also block the entry of Ca2? into adipocytes through the L-type calcium channel. Therefore, when Mg2? concentrations are insufficient, increased Ca2?entry in adipocyte leads to inflammation, oxidative stress and increase insulin resistance (Nielsen et al.2007; Gommers et al.2016; Al Alawi et al. 2018). Mg2? is also involved in the transport of glucose across membranes, it is a cofactor of several enzymes essential for carbohydrate oxida-tion (e.g. phosphotransferases and phosphohydrolases such as ATPases) and also plays a role in the release of insulin (Saris et al.2000; Al Alawi et al.2018). On the other hand, insulin decreases tubular reabsorption of Mg2?and so high insulin levels (as in T2DM) could lead to reduced Mg2?concentrations (McNair et al.
1982; Gommers et al.2016). bFig. 2 Relationship between HbA1c concentration and plasma
Selenium is mostly found in selenoproteins (as selenomethionine), of which many are important anti-oxidants (Roman et al.2014). Therefore, high plasma concentrations of selenium may be due to oxidative stress mobilising high levels of selenoproteins. For example, the plasma concentration of selenoprotein P is known to be higher in T2DM subjects, a disease state associate with high levels of oxidative stress, and to be associated with insulin resistance (Misu et al.
2010; Ogawa-Wong et al.2016). However, expression of plasma glutathione peroxidase (another selenopro-tein) as well as serum albumin (which transports selenomethionine) are reduced in diabetes (Roman et al.2010). Expression of selenoprotein P is known to be reduced during inflammation (Nichol et al.1998; Hesse-Bahr et al.2000). Thus blood levels of selenium decrease during systemic inflammatory responses, which occur in T2DM (Wang et al.2013). It has been hypothesised that the stages of the disease (impaired glucose tolerance or well-established T2DM) and the associated level of oxidative stress influences plasma selenium concentration (Rayman and Stranges2013). Zinc participates in the synthesis, storage and release of insulin. Notably, zinc is present in secretory vesicles within b-cells of the pancreas where it participates in the crystallisation/storage of insulin and is thus released alongside insulin into the plasma (Scott1934; Chabosseau and Rutter2016). Deficiency of zinc disrupts insulin homeostasis, resulting in a reduction of insulin secretion byb cells (Fung et al.
2015). Zinc also stimulates lipogenesis and glucose uptake and reduced lipolysis in adipocytes (Coulston and Dandona1980; Nishide et al.2008). As zinc and copper homeostasis are closely linked, notably through competition during intestinal absorption and through shared transporter proteins such as serum albumin, a deficiency in one can affect the other (Osredkar and Sustar2011). For example, zinc over-supplementation can lead to copper deficiency (Duncan et al. 2015). Impaired metabolism of zinc and copper are associated with a higher sensitivity to oxidative damage, as both zinc and copper are needed for the activity of the antioxidant enzyme superoxide dismutase, whose activity is reduced in T2DM (Sundaram et al.1996). Thus, changes in the ratio of zinc/copper will affect enzyme catalysis and potentially further increase levels of free radicals, which are already increased in diabetes (Giacco and Brownlee 2010). In addition, copper is released from superoxide dismutase at high
glucose concentrations due to fragmentation of the protein during glycation (Ookawara et al. 1992). Elevated copper levels in T2DM correlate with formation of reactive oxygen species (Masad et al.
2007). Insulin also reduces copper concentration in the liver through the regulation of at least one copper-transporting ATPase, ATP7B (Hilario-Souza et al.
2016). A reduction in insulin concentrations may thus result in an accumulation of copper in the liver.
Deficiency in magnesium, selenium and zinc have been shown to increase the risks of developing complicates associated with diabetes. Therefore, the effect of supplementation of these metals has been assessed. Magnesium supplementation was found to both improve diabetes management and to reduce the development of T2DM in individuals at risk (Ro-driguez-Moran and Guerrero-Romero 2003; Hruby et al. 2014; Guerrero-Romero et al. 2015). Zinc supplementation has been shown to improve insulin and glucose levels in diabetes subjects and decrease the risk of developing T2DM (Jayawardena et al.
2012; Islam et al. 2016; Ranasinghe et al. 2018). However, selenium supplementation was not found to improve the risk of developing T2DM or the risk of developing complications in individuals with diabetes (Ogawa-Wong et al.2016). This could be because the association between selenium concentrations and diabetes is not linear and that only individuals with selenium deficiency would benefit from supplementa-tion, while individuals with sufficient selenium could increase their risk of developing T2DM if given an excess of this metal (Ogawa-Wong et al. 2016). Another explanation may be that the form of selenium used for supplementation in some studies offer variable bioavailability (Ogawa-Wong et al.2016).
Conclusion
likely to develop complications. In addition, plasma metal concentrations are also influenced by insulin and glucose plasma levels. Thus, if those are not properly controlled, plasma metal concentrations are likely to become further dysregulated. Here we show that plasma magnesium concentration is altered to the highest degree in T1DM. In T2DM, plasma selenium and copper concentrations were significantly affected. This work increases our understanding of T1DM and T2DM pathogenesis and may have future implications for the management of diabetes.
Acknowledgements This work was supported by the British Heart Foundation (Grant codes: PG/15/9/31270 and FS/15/42/ 31556).
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http:// creativecommons.org/licenses/by/4.0/), which permits unre-stricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Com-mons license, and indicate if changes were made.
References
Abdelmagid SA, Clarke SE, Nielsen DE, Badawi A, El-Sohemy A, Mutch DM, Ma DW (2015) Comprehensive profiling of plasma fatty acid concentrations in young healthy Cana-dian adults. PLoS ONE 10(2):e0116195
Akbaraly TN, Arnaud J, Rayman MP, Hininger-Favier I, Roussel AM, Berr C, Fontbonne A (2010) Plasma selenium and risk of dysglycemia in an elderly French population: results from the prospective Epidemiology of Vascular Ageing Study. Nutr Metab (Lond) 7:21
Al Alawi AM, Majoni SW, Falhammar H (2018) Magnesium and human health: perspectives and research directions. Int J Endocrinol 2018:9041694
Atari-Hajipirloo S, Valizadeh N, Khadem-Ansari MH, Rasmi Y, Kheradmand F (2016) Altered concentrations of copper, zinc, and iron are associated with increased levels of gly-cated hemoglobin in patients with type 2 diabetes mellitus and their first-degree relatives. Int J Endocrinol Metab 14(2):e33273
Bleys J, Navas-Acien A, Guallar E (2007) Serum selenium and diabetes in U.S. adults. Diabetes Care 30(4):829–834 Brown IR, McBain AM, Chalmers J, Campbell IW, Brown ER,
Lewis MJ (1999) Sex difference in the relationship of cal-cium and magnesium excretion to glycaemic control in type 1 diabetes mellitus. Clin Chim Acta 283(1–2):119–128 Chabosseau P, Rutter GA (2016) Zinc and diabetes. Arch
Bio-chem Biophys 611:79–85
Coulston L, Dandona P (1980) Insulin-like effect of zinc on adipocytes. Diabetes 29(8):665–667
Duncan A, Yacoubian C, Watson N, Morrison I (2015) The risk of copper deficiency in patients prescribed zinc supple-ments. J Clin Pathol 68(9):723–725
Fung EB, Gildengorin G, Talwar S, Hagar L, Lal A (2015) Zinc status affects glucose homeostasis and insulin secretion in patients with thalassemia. Nutrients 7(6):4296–4307 Giacco F, Brownlee M (2010) Oxidative stress and diabetic
complications. Circ Res 107(9):1058–1070
Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH (2016) Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes 65(1):3–13
Guerrero-Romero F, Rodriguez-Moran M (2011) Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: double-blind, randomized clinical trial. Eur J Clin Invest 41(4):405–410
Guerrero-Romero F, Simental-Mendia LE, Hernandez-Ron-quillo G, Rodriguez-Moran M (2015) Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: a double-blind placebo-controlled randomized trial. Diabetes Metab 41(3):202–207
Hesse-Bahr K, Dreher I, Kohrle J (2000) The influence of the cytokines Il-1beta and INFgamma on the expression of selenoproteins in the human hepatocarcinoma cell line HepG2. BioFactors 11(1–2):83–85
Hilario-Souza E, Cuillel M, Mintz E, Charbonnier P, Vieyra A, Cassio D, Lowe J (2016) Modulation of hepatic copper-ATPase activity by insulin and glucagon involves protein kinase A (PKA) signaling pathway. Biochim Biophys Acta 1862(11):2086–2097
Hruby A, Meigs JB, O’Donnell CJ, Jacques PF, McKeown NM (2014) Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged americans. Diabetes Care 37(2):419–427
Islam MR, Attia J, Ali L, McEvoy M, Selim S, Sibbritt D, Akhter A, Akter S, Peel R, Faruque O, Mona T, Lona H, Milton AH (2016) Zinc supplementation for improving glucose handling in pre-diabetes: a double blind random-ized placebo controlled pilot study. Diabetes Res Clin Pract 115:39–46
Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G, Katulanda P (2012) Effects of zinc sup-plementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr 4(1):13
Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL (1999) Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Commu-nities Study. Arch Intern Med 159(18):2151–2159 Kaur B, Henry J (2014) Micronutrient status in type 2 diabetes: a
review. Adv Food Nutr Res 71:55–100
Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E (2009) Serum selenium concentrations and diabetes in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect 117(9):1409–1413
Larsson SC, Wolk A (2007) Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med 262(2):208–214 Li Z, Wang C, Li L, Shao M, Wang L, Lv X, Gao C, Niu H, Li B
mineral elements and diabetes. Biol Trace Elem Res 183(2):226–232
Lin CC, Huang YL (2015) Chromium, zinc and magnesium status in type 1 diabetes. Curr Opin Clin Nutr Metab Care 18(6):588–592
Lin CC, Huang HH, Hu CW, Chen BH, Chong IW, Chao YY, Huang YL (2014) Trace elements, oxidative stress and glycemic control in young people with type 1 diabetes mellitus. J Trace Elem Med Biol 28(1):18–22
Lin CC, Tsweng GJ, Lee CF, Chen BH, Huang YL (2016) Magnesium, zinc, and chromium levels in children, ado-lescents, and young adults with type 1 diabetes. Clin Nutr 35(4):880–884
Luo YY, Zhao J, Han XY, Zhou XH, Wu J, Ji LN (2015) Relationship between serum zinc level and microvascular complications in patients with type 2 diabetes. Chin Med J (Engl) 128(24):3276–3282
Maret W (2017) Zinc in pancreatic islet biology, insulin sensi-tivity, and diabetes. Prev Nutr Food Sci 22(1):1–8 Masad A, Hayes L, Tabner BJ, Turnbull S, Cooper LJ, Fullwood
NJ, German MJ, Kametani F, El-Agnaf OM, Allsop D (2007) Copper-mediated formation of hydrogen peroxide from the amylin peptide: a novel mechanism for degener-ation of islet cells in type-2 diabetes mellitus? FEBS Lett 581(18):3489–3493
McNair P, Christensen MS, Christiansen C, Madsbad S, Transbol I (1982) Renal hypomagnesaemia in human dia-betes mellitus: its relation to glucose homeostasis. Eur J Clin Invest 12(1):81–85
Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, Sakurai M, Yamashita T, Mizukoshi E, Yamashita T, Honda M, Miyamoto K, Kubota T, Kubota N, Kadowaki T, Kim HJ, Lee IK, Minokoshi Y, Saito Y, Takahashi K, Yamada Y, Takakura N, Kaneko S (2010) A liver-derived secretory protein, selenoprotein P, causes insulin resis-tance. Cell Metab 12(5):483–495
Naka T, Kaneto H, Katakami N, Matsuoka TA, Harada A, Yamasaki Y, Matsuhisa M, Shimomura I (2013) Associa-tion of serum copper levels and glycemic control in patients with type 2 diabetes. Endocr J 60(3):393–396
Nichol C, Herdman J, Sattar N, O’Dwyer PJ, St JORD, Little-john D, Fell G (1998) Changes in the concentrations of plasma selenium and selenoproteins after minor elective surgery: further evidence for a negative acute phase response? Clin Chem 44(8 Pt 1):1764–1766
Nielsen FH, Milne DB, Gallagher S, Johnson L, Hoverson B (2007) Moderate magnesium deprivation results in calcium retention and altered potassium and phosphorus excretion by postmenopausal women. Magnes Res 20(1):19–31 Nishide M, Yoshikawa Y, Yoshikawa EU, Matsumoto K,
Sakurai H, Kajiwara NM (2008) Insulinomimetic Zn(II) complexes as evaluated by both glucose-uptake activity and inhibition of free fatty acids release in isolated rat adipocytes. Chem Pharm Bull (Tokyo) 56(8):1181–1183 Ogawa-Wong AN, Berry MJ, Seale LA (2016) Selenium and
metabolic disorders: an emphasis on type 2 diabetes risk. Nutrients 8(2):80
Ookawara T, Kawamura N, Kitagawa Y, Taniguchi N (1992) Site-specific and random fragmentation of Cu, Zn-superoxide
dismutase by glycation reaction. Implication of reactive oxygen species. J Biol Chem 267(26):18505–18510 Osredkar J, Sustar N (2011) Copper and zinc, biological role and
significance of copper/zinc imbalance. J Clinic Toxicol S3:001–018
Ramadass S, Basu S, Srinivasan AR (2015) SERUM magne-sium levels as an indicator of status of Diabetes Mellitus type 2. Diabetes Metab Syndr 9(1):42–45
Ranasinghe P, Wathurapatha WS, Galappatthy P, Katulanda P, Jayawardena R, Constantine GR (2018) Zinc supplemen-tation in prediabetes: a randomized double-blind placebo-controlled clinical trial. J Diabetes 10(5):386–397 Rayman MP, Stranges S (2013) Epidemiology of selenium and
type 2 diabetes: can we make sense of it? Free Radic Biol Med 65:1557–1564
Rodriguez-Moran M, Guerrero-Romero F (2003) Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care 26(4):1147–1152 Roman M, Lapolla A, Jitaru P, Sechi A, Cosma C, Cozzi G,
Cescon P, Barbante C (2010) Plasma selenoproteins con-centrations in type 2 diabetes mellitus–a pilot study. Transl Res 156(4):242–250
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54 Ruiz C, Alegria A, Barbera R, Farre R, Lagarda J (1998)
Sele-nium, zinc and copper in plasma of patients with type 1 diabetes mellitus in different metabolic control states. J Trace Elem Med Biol 12(2):91–95
Sanjeevi N, Freeland-Graves J, Beretvas SN, Sachdev PK (2018) Trace element status in type 2 diabetes: a meta-analysis. J Clin Diagn Res 12(5):OE01–OE08
Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A (2000) Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta 294(1–2):1–26 Scott DA (1934) Crystalline insulin. Biochem J
28(4):1592–1602
Sundaram RK, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R, Shanmugasundaram KR (1996) Antioxidant sta-tus and lipid peroxidation in type II diabetes mellista-tus with and without complications. Clin Sci (Lond) 90(4):255–260 Terres-Martos C, Navarro-Alarcon M, Martin-Lagos F, De La
Serrana HLG, Perez-Valero V, Lopez-Martinez MC (1998) Serum zinc and copper concentrations and Cu/Zn ratios in patients with hepatopathies or diabetes. J Trace Elem Med Biol 12(1):44–49
Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG (2013) Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36(1):166–175 Wang XL, Yang TB, Wei J, Lei GH, Zeng C (2016) Association
between serum selenium level and type 2 diabetes mellitus: a non-linear dose-response meta-analysis of observational studies. Nutr J 15(1):48