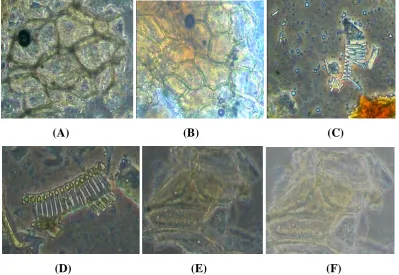
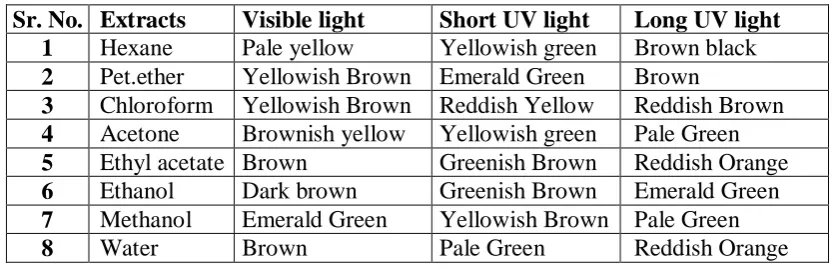
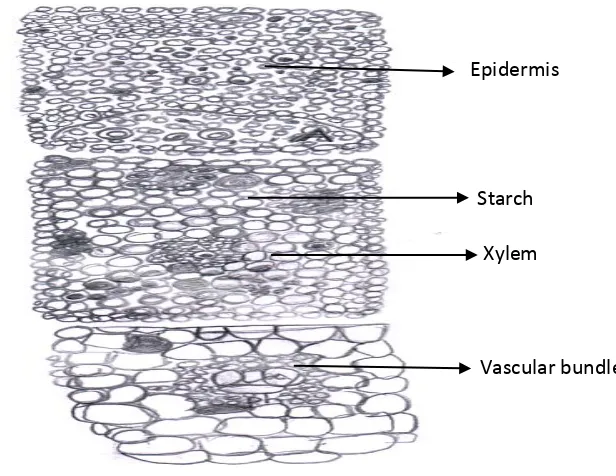
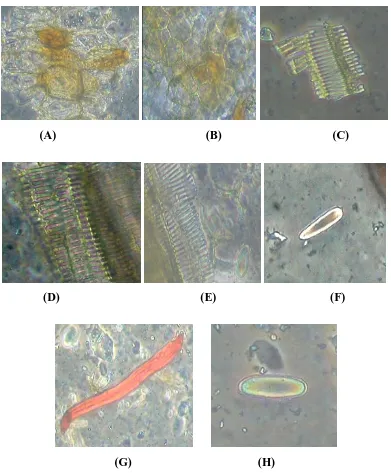
COMPARATIVE PHARMACOGNOSTIC AND PHYTOCHEMICAL INVESTIGATION OF TWO ALPINIA SPECIES FROM ZINGIBERACEAE FAMILY
Full text
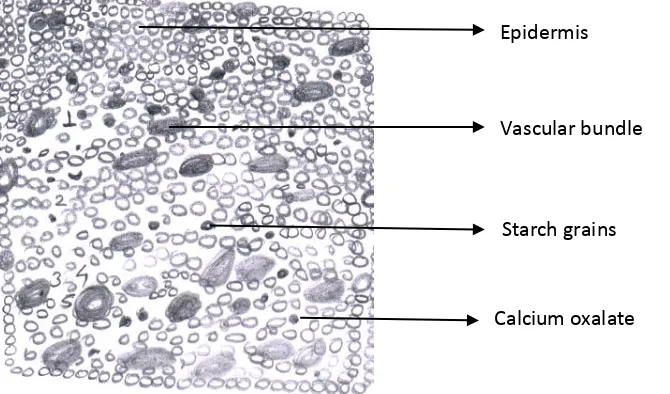
Figure

Related documents
In this section we compare our analysis with an alternative couched within a frame- work that treats metalinguistic comparison not as comparing degrees of imprecision, but rather
Issues in Assessing Vocationally Relevant Personality Factors of Issues in Assessing Vocationally Relevant Personality Factors of Prelingually Deaf Adults Utilizing the
There is an urgent need to establish statistical theories and methods that can deal with the test of stress-life relationship with censored data and non-normal distribution,
Malleable face cues including facial adiposity, skin colour and texture may offer more current and relevant information with regard to health than more stable aspects of
Although pul- monary fibrosis can be present at the time of MPA diagnosis, or manifest months to years prior to or after MPA diagnosis, cases in our cohort all had pulmonary fibrosis
Our study is the first to report the footwear habits in boys attending a secondary school in New Zealand. However, despite sampling from the intended popula- tion, our sample still
The formation of discrete lignin droplets deposited on the surface of the fibers was observed in all pretreatments, with a higher frequency in organosolv followed by steam
Further studies underway include a comparison of the safety, efficacy, and tolerability of rilpivirine in adolescents (Clinical trials.gov identifier: NCT0799864), a Phase III trial