Acta Cryst.(2002). E58, o1119±o1120 DOI: 10.1107/S1600536802016513 Nazan Ocaket al. C19H23NO
o1119
organic papers
Acta Crystallographica Section E Structure Reports
Online
ISSN 1600-5368
erythro
-2-Piperidinyl-1,2-diphenylethanol
Nazan Ocak,a* Canan Kazak,a Sema OÈ ztuÈrk,bCaliskan Zerrin,c Nergis Arsu,cHoong-Kun Fund and Ahmet ErdoÈnmeza
aDepartment of Physics, Faculty of Arts and Sciences, Ondokuz MayõÂs University, TR-55139, Kurupelit-Samsun, Turkey,bDepartment of Physics, Erciyes University, TR-38039, Kayseri, Turkey,cDepartment of Chemistry, YõÂldõÂz Technical University, TR-34210, Istanbul, Turkey, anddX-ray Crystallography Unit, School of Physics, Universiti Sains Malaysia, 11800 USM, Penang, Malaysia
Correspondence e-mail: nocak@omu.edu.tr
Key indicators
Single-crystal X-ray study T= 183 K
Mean(C±C) = 0.002 AÊ Rfactor = 0.058 wRfactor = 0.136
Data-to-parameter ratio = 20.1
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
The molecule of the title compound, C19H23NO, contains two
phenyl rings and a piperidinyl ring. The dihedral angle between the phenyl rings is 40.99 (5). The piperidine ring has a chair conformation. There is an intramolecular OÐH N hydrogen bond.
Comment
During the process of UV-radiation curing, oxygen is present as a free radical in the medium. To overcome the negative effect caused by the oxygen, we synthesizederythro -2-piper-idinyl-1,2 diphenylethanol to use it as a hydrogen donor for Type II initiators (Davidson, 1999). In this paper we report the structure oferythro-2-piperidinyl-1,2 diphenylethanol, (I). An
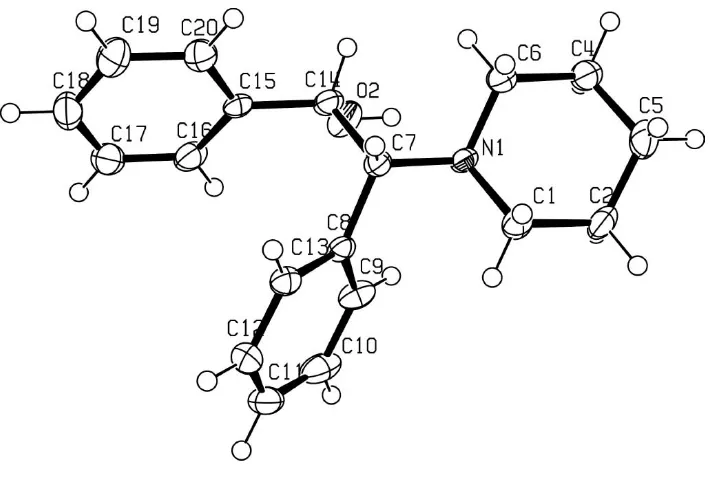
ORTEPIII (Burnett & Johnson, 1996) plot of the structure is shown in Fig. 1.
The C6ÐN1 and C1ÐN1 bond distances are 1.4749 (19) and 1.4730 (18) AÊ, respectively, and are similar to the corresponding bond lengths in ethyl 4-{2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]ethoxy}benzoate [1.467 (5) and 1.472 (4) AÊ; Jottier et al., 1991] and 3-{2-[4-(6-¯uoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl}-2,9-dimethyl]-4H -pyrido[1,2-a]pyrimidin-4-one (Ocaperidone) [1.477 (9) and 1.463 (9) AÊ; Jottier et al., 1992]. The C7ÐN1, C7ÐC14 and C14ÐO2 bond distances are 1.4872 (17) 1.5563 (19) and 1.4286 (17) AÊ, respectively, similar to the corresponding bond lengths in N-(2-hydroxyethyl)-piperidine, N -(2-hydroxyethyl)morpholine and N-(2-hydroxyethyl)piperazine [1.495 (4), 1.493 (4) and 1.397 (4) AÊ, respectively; Castellari & Sabatino, 1996]. The erythro-2-piperidinyl-1,2-diphenyl ethanol molecule contains three rings: two phenyl rings and a piperidinyl ring. For the piperidinyl ring we calculated, following the method of Cremer & Pople (1975), phase angle
2= 2.15 (17) and'2= 34 (5), indicating a chair
conform-ation, and a puckering amplitudeQ= 0.5814 (17) AÊ.
There is an intramolecular O2ÐH2A N1 hydrogen bond (Table 1).
Experimental
1 g oftrans-stilbene oxide and 1 molar equivalent of distilled piper-idine were re¯uxed for 12 h with vigorous stirring. The product was extracted with diethyl ether and excess morpholine was separated by the addition of 5 ml of distilled water. The combined organic layers were washed with distilled water several times. The solution was dried over anhydrous magnesium sulfate. The crude product was recrys-tallized from ethanol. M.p: 373 K Analysis calculated for C19H23NO: C, 81.14; H, 8.18; N, 4.98. Found C, 81.22; H, 8.19; N, 5.03.
Crystal data
C19H23NO
Mr= 281.38 Monoclinic,P21=n
a= 13.6624 (10) AÊ
b= 5.6452 (10) AÊ
c= 20.678 (4) AÊ
= 93.46 (4)
V= 1591.9 (4) AÊ3
Z= 4
Dx= 1.174 Mg mÿ3 MoKradiation Cell parameters from 5874
re¯ections
= 3.0±28.3
= 0.07 mmÿ1
T= 183 (2) K Block, colourless 0.720.500.36 mm
Data collection
Siemens SMART CCD area-detector diffractometer
!scans
Absorption correction: none 9095 measured re¯ections 3833 independent re¯ections
2344 re¯ections withI> 2(I)
Rint= 0.080
max= 28.3
h=ÿ15!18
k=ÿ7!7
l=ÿ27!25
Re®nement
Re®nement onF2
R[F2> 2(F2)] = 0.058
wR(F2) = 0.136
S= 0.81 3833 re¯ections 191 parameters
H-atom parameters constrained
w= 1/[2(F o2)]
whereP= (Fo2+ 2Fc2)/3 (/)max< 0.001
max= 0.23 e AÊÿ3 min=ÿ0.33 e AÊÿ3
Extinction correction:SHELXL
Extinction coef®cient: 0.044 (4)
Table 1
Selected geometric parameters (AÊ,).
O2ÐC14 1.4286 (17)
N1ÐC1 1.4730 (18)
N1ÐC6 1.4749 (19)
N1ÐC7 1.4872 (17)
C7ÐC14 1.5563 (19)
C1ÐN1ÐC6 108.77 (12)
C1ÐN1ÐC7 111.98 (11)
C6ÐN1ÐC7 111.87 (11)
N1ÐC6ÐC4 111.82 (13)
N1ÐC7ÐC8 112.18 (11)
N1ÐC7ÐC14 108.24 (11)
C8ÐC7ÐC14 110.26 (11)
C9ÐC8ÐC13 117.81 (14)
C9ÐC8ÐC7 122.51 (13)
O2ÐC14ÐC15 109.76 (12)
O2ÐC14ÐC7 109.39 (11)
Table 2
Hydrogen-bonding geometry (AÊ,).
DÐH A DÐH H A D A DÐH A
O2ÐH2A N1 0.82 2.39 2.774 (2) 109
All H atoms, located in a difference Fourier map, were positioned geometrically and constrained with a riding model. The CÐH bond distances range from 0.93 to 0.98 AÊ.
Data collection:SMART(Siemens, 1996); cell re®nement:SAINT
(Siemens, 1996); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 1997); program(s) used to re®ne structure:SHELXTL; molecular graphics:SHELXTL; software used to prepare material for publication: SHELXTL, PARST(Nardelli, 1995) andPLATON(Spek, 1990).
The authors thank the Malaysian Government and Universiti Sains Malaysia for research grant R&D No. 190-9609-2801. SOÈ thanks the Universiti Sains Malaysia for a Visiting Post Doctoral Fellowship.
References
Burnett, M. N. & Johnson, C. K. (1996).ORTEPIII. Report ORNL-6895. Oak Ridge National Laboratory, Tennessee, USA.
Castellari, C. & Sabatino, P. (1996).Acta Cryst.C52, 1708±1712. Cremer, D. & Pople, J. A. (1975).J. Am. Chem. Soc.97, 1354±1358. Davidson, R. S. (1999).Exploring the science, technology and applications of
UV EB curing.London: SITA Technology Ltd.
Jottier, W. I., De Winter, H. L., Blaton, N. M., Peeters, O. M. & De Ranter, C. J. (1991).Acta Cryst.C47, 1517±1520.
Jottier, W. I., De Winter, H. L., Peeters, O. M., Blaton, N. M. & De Ranter, C. J. (1992).Acta Cryst.C48, 1827±1830.
Nardelli, M. (1995).J. Appl. Cryst.28, 659.
Sheldrick, G. M. (1997).SHELXL97 andSHELXTL.University of GoÈttingen, Germany.
Siemens (1996).SMARTandSAINT. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
Spek, A. L. (1990).PLATON.University of Utrecht, The Netherlands. Figure 1
supporting information
sup-1
Acta Cryst. (2002). E58, o1119–o1120supporting information
Acta Cryst. (2002). E58, o1119–o1120 [doi:10.1107/S1600536802016513]
erythro
-2-Piperidinyl-1,2-diphenylethanol
Nazan Ocak, Canan Kazak, Sema
Ö
zt
ü
rk, Caliskan Zerrin, Nergis Arsu, Hoong-Kun Fun and
Ahmet Erd
ö
nmez
S1. Comment
During the process of UV-radiation curing there is an oxygen as a free radical in the medium. To overcome the negative
effect caused by the oxygen, we synthesized erythro-2-piperidinyl-1,2 diphenyl ethanol to use it as a hydrogen donor for
Type II initiators (Davidson, 1999). In this paper we report the structure of erythro-2-piperidinyl-1,2 diphenyl ethanol, (I).
An ORTEPIII (Burnett & Johnson, 1996) plot of compound is shown in Fig.1.
The C6—N1 and C1—N1 bond distances are 1.4749 (19) and 1.4730 (18) Å, respectively, which are similar to the
corresponding bond lengths in ethyl 4-{2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]ethoxy}benzoate [1.467 (5) and
1.472 (4) Å; Jottier et al., 1991] and 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl} −2,9-dimethyl]-4H
-pyrido[1,2-alpyrimidin-4-one (Ocaperidone) [1.477 (9) and 1.463 (9) Å; Jottier et al., 1992]. The C7—N1, C7—C14 and
C14—O2 bond distances are 1.4872 (17) 1.5563 (19) and 1.4286 (17) Å, respectively, which are similar to the
corresponding bond lengths in N-(2-Hydroxyethyl)-piperidine, N-(2-Hydroxyethyl)morpholine and N
-(2-Hydroxyethyl)-piperazine [1.495 (4), 1.493 (4) and 1.397 (4) Å, respectively; Castellari & Sabatino, 1996]. The erythro-2-piperidinyl-1,2
diphenyl ethanol molecule contains three ring systems: two phenyl rings and a piperidinyl ring. For the piperidinyl ring
we calculated, following the method of Cremer & Pople (1975), a phase angle θ2 =2.15 (17)° and φ2 =34 (5)°, indicating a
chair conformation, and a puckering amplitude Q=0.5814 (17) Å.
There is an intramolecular O2—H2A···N1 hydrogen bond (Table 1).
S2. Experimental
Erythro-2-piperidinyl-1,2-diphenyl ethanol: 1 g of trans-stilbene oxide and 1 molar equivalent of distilled piperidine was
refluxed for 12 h with vigorous stirring. The product was extracted with diethyl ether and excess morpholine was
separated by the addition of 5 ml of distilled water. The combined organic layers were washed with distilled water several
times. The solution was dried over anhydrous magnesium sulfate. The crude product was recrystallized from ethanol.
M.p:373 K Analysis calculated for C19H23NO: C,81.14; H, 8.18; N, 4.98. Found C, 81.22; H, 8.19; N,5.03.
S3. Refinement
The structure was solved by direct methods and refined by full-matrix least squares. The H atoms were located from
Figure 1
An ORTEPIII (Burnett & Johnson, 1996). drawing of the title compound showing the atom-numbering scheme.
Displacement ellipsoids of non-H atoms are shown at the 50% probability level.
Erythro-2-piperidinyl-1,2-diphenyl ethanol
Crystal data
C19H23NO Mr = 281.38
Monoclinic, P21/n Hall symbol: -P 2yn a = 13.6624 (10) Å b = 5.6452 (10) Å c = 20.678 (4) Å β = 93.46 (4)° V = 1591.9 (4) Å3 Z = 4
F(000) = 608 Dx = 1.174 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 5874 reflections θ = 3.0–28.3°
µ = 0.07 mm−1 T = 183 K Block, colourless 0.72 × 0.50 × 0.36 mm
Data collection
Siemens SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 8.33 pixels mm-1 ω scans
9095 measured reflections
3833 independent reflections 2344 reflections with I > 2σ(I) Rint = 0.080
θmax = 28.3°, θmin = 3.0° h = −15→18
k = −7→7 l = −27→25
Refinement
Refinement on F2 Least-squares matrix: full R[F2 > 2σ(F2)] = 0.058 wR(F2) = 0.136 S = 0.81 3833 reflections
191 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
supporting information
sup-3
Acta Cryst. (2002). E58, o1119–o1120Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2)]
where P = (Fo2 + 2Fc2)/3 (Δ/σ)max < 0.001
Δρmax = 0.23 e Å−3 Δρmin = −0.33 e Å−3
Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 Extinction coefficient: 0.044 (4)
Special details
Experimental. The data collection covered over a hemisphere of reciprocal space by a combination of three sets of exposures; each set had a different φ angle (0, 88 and 180°) for the crystal and each exposure of 10 s covered 0.3° in ω. The crystal-to-detector distance was 5 cm and the detector swing angle was −35°. Crystal decay was monitored by repeating fifty initial frames at the end of data collection and analysing the intensity of duplicate reflections, and was found to be negligible.
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C11 1.21075 (13) 0.6807 (4) 0.15911 (8) 0.0434 (5) H11A 1.2727 0.6154 0.1556 0.052* C12 1.20065 (12) 0.8918 (3) 0.19044 (8) 0.0389 (4) H12A 1.2557 0.9697 0.2085 0.047* C13 1.10811 (11) 0.9896 (3) 0.19525 (7) 0.0264 (4) H13A 1.1020 1.1342 0.2162 0.032* C14 0.87706 (11) 0.8576 (3) 0.23715 (7) 0.0248 (3) H14A 0.8178 0.9455 0.2467 0.030* C15 0.94437 (10) 0.8513 (3) 0.29803 (7) 0.0227 (3) C16 1.00775 (12) 0.6646 (3) 0.31171 (7) 0.0311 (4) H16A 1.0090 0.5369 0.2834 0.037* C17 1.06956 (12) 0.6666 (3) 0.36747 (8) 0.0367 (4) H17A 1.1121 0.5403 0.3761 0.044* C18 1.06850 (12) 0.8532 (3) 0.40996 (8) 0.0369 (4) H18A 1.1100 0.8532 0.4473 0.044* C19 1.00555 (12) 1.0408 (3) 0.39707 (8) 0.0367 (4) H19A 1.0045 1.1679 0.4256 0.044* C20 0.94356 (11) 1.0388 (3) 0.34096 (7) 0.0303 (4) H20A 0.9011 1.1652 0.3323 0.036*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-5
Acta Cryst. (2002). E58, o1119–o1120Geometric parameters (Å, º)
O2—C14 1.4286 (17) C8—C13 1.390 (2) O2—H2A 0.8200 C9—C10 1.386 (2) N1—C1 1.4730 (18) C9—H9A 0.9300 N1—C6 1.4749 (19) C10—C11 1.385 (3) N1—C7 1.4872 (17) C10—H10A 0.9300 C1—C2 1.518 (2) C11—C12 1.367 (2) C1—H1A 0.9700 C11—H11A 0.9300 C1—H1B 0.9700 C12—C13 1.389 (2) C2—C5 1.518 (2) C12—H12A 0.9300 C2—H2B 0.9700 C13—H13A 0.9300 C2—H2C 0.9700 C14—C15 1.5139 (19) C4—C5 1.516 (2) C14—H14A 0.9800 C4—C6 1.517 (2) C15—C20 1.382 (2) C4—H4A 0.9700 C15—C16 1.383 (2) C4—H4B 0.9700 C16—C17 1.388 (2) C5—H5A 0.9700 C16—H16A 0.9300 C5—H5B 0.9700 C17—C18 1.372 (2) C6—H6C 0.9700 C17—H17A 0.9300 C6—H6D 0.9700 C18—C19 1.380 (2) C7—C8 1.5187 (19) C18—H18A 0.9300 C7—C14 1.5563 (19) C19—C20 1.395 (2) C7—H7A 0.9800 C19—H19A 0.9300 C8—C9 1.387 (2) C20—H20A 0.9300
C2—C5—C4 108.80 (14) C7—C14—H14A 108.2 C2—C5—H5A 109.9 C20—C15—C16 118.80 (13) C4—C5—H5A 109.9 C20—C15—C14 119.23 (13) C2—C5—H5B 109.9 C16—C15—C14 121.97 (13) C4—C5—H5B 109.9 C15—C16—C17 120.40 (15) H5A—C5—H5B 108.3 C15—C16—H16A 119.8 N1—C6—C4 111.82 (13) C17—C16—H16A 119.8 N1—C6—H6C 109.3 C18—C17—C16 120.58 (15) C4—C6—H6C 109.3 C18—C17—H17A 119.7 N1—C6—H6D 109.3 C16—C17—H17A 119.7 C4—C6—H6D 109.3 C17—C18—C19 119.73 (15) H6C—C6—H6D 107.9 C17—C18—H18A 120.1 N1—C7—C8 112.18 (11) C19—C18—H18A 120.1 N1—C7—C14 108.24 (11) C18—C19—C20 119.64 (16) C8—C7—C14 110.26 (11) C18—C19—H19A 120.2 N1—C7—H7A 108.7 C20—C19—H19A 120.2 C8—C7—H7A 108.7 C15—C20—C19 120.84 (14) C14—C7—H7A 108.7 C15—C20—H20A 119.6 C9—C8—C13 117.81 (14) C19—C20—H20A 119.6
C6—N1—C1—C2 −59.83 (17) C10—C11—C12—C13 −0.5 (3) C7—N1—C1—C2 176.02 (13) C11—C12—C13—C8 0.8 (2) N1—C1—C2—C5 59.36 (19) C9—C8—C13—C12 −0.8 (2) C1—C2—C5—C4 −55.07 (18) C7—C8—C13—C12 176.19 (13) C6—C4—C5—C2 54.21 (19) N1—C7—C14—O2 51.90 (15) C1—N1—C6—C4 58.89 (17) C8—C7—C14—O2 −71.15 (14) C7—N1—C6—C4 −176.89 (13) N1—C7—C14—C15 174.47 (12) C5—C4—C6—N1 −57.5 (2) C8—C7—C14—C15 51.43 (16) C1—N1—C7—C8 −52.85 (16) O2—C14—C15—C20 −149.17 (13) C6—N1—C7—C8 −175.26 (12) C7—C14—C15—C20 88.47 (16) C1—N1—C7—C14 −174.72 (12) O2—C14—C15—C16 31.64 (19) C6—N1—C7—C14 62.87 (15) C7—C14—C15—C16 −90.73 (17) N1—C7—C8—C9 −41.60 (18) C20—C15—C16—C17 −0.2 (2) C14—C7—C8—C9 79.10 (16) C14—C15—C16—C17 178.95 (14) N1—C7—C8—C13 141.55 (13) C15—C16—C17—C18 0.2 (2) C14—C7—C8—C13 −97.74 (15) C16—C17—C18—C19 −0.1 (3) C13—C8—C9—C10 0.6 (2) C17—C18—C19—C20 0.0 (3) C7—C8—C9—C10 −176.35 (14) C16—C15—C20—C19 0.2 (2) C8—C9—C10—C11 −0.3 (2) C14—C15—C20—C19 −179.06 (14) C9—C10—C11—C12 0.2 (3) C18—C19—C20—C15 0.0 (2)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A